Anti-Bacterial and Microecosystem-Regulating Effects of Dental Implant Coated with Dimethylaminododecyl Methacrylate
Abstract
:1. Introduction
2. Results
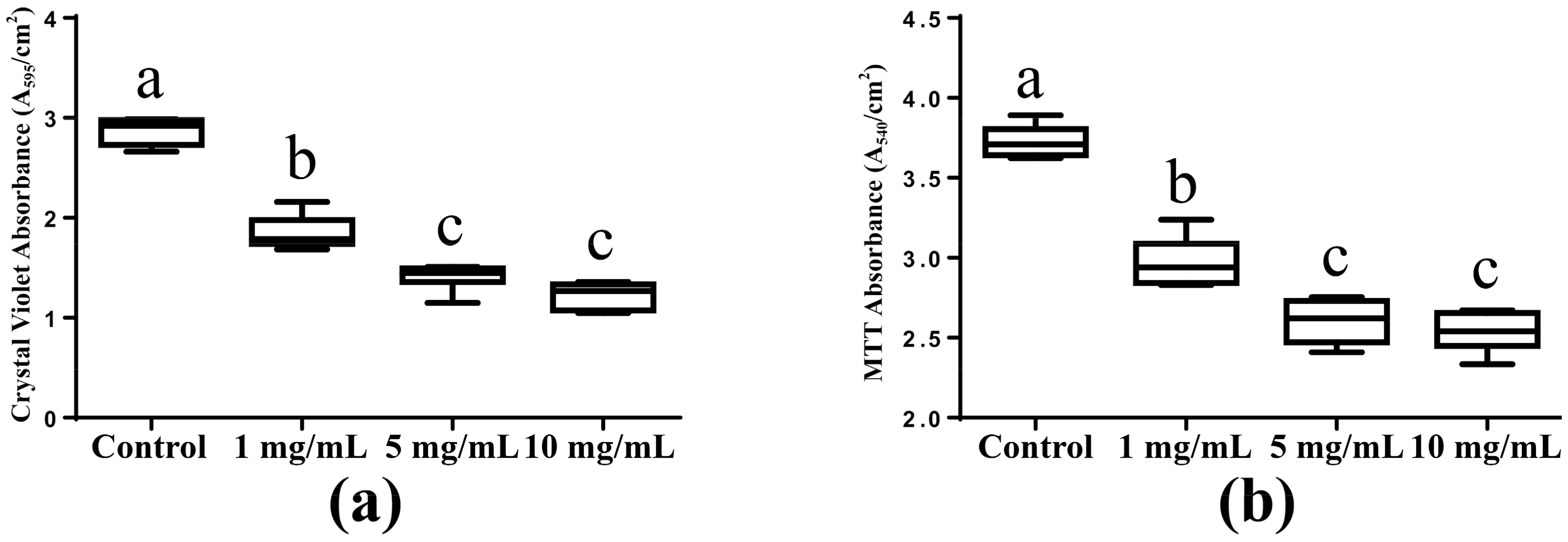
2.1. Biomass Accumulation and Metabolic Activity
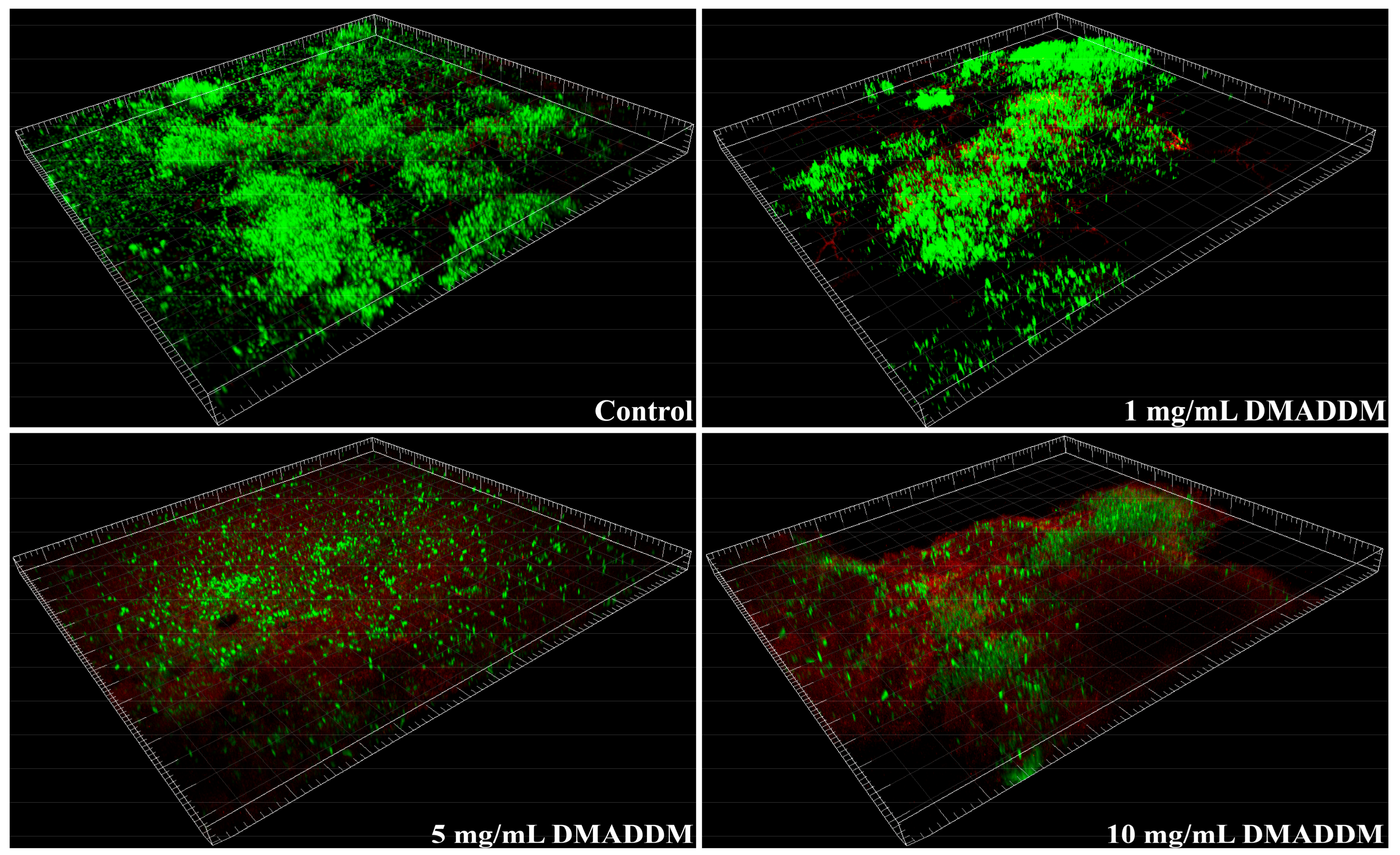
2.2. Live/Dead Bacteria Staining
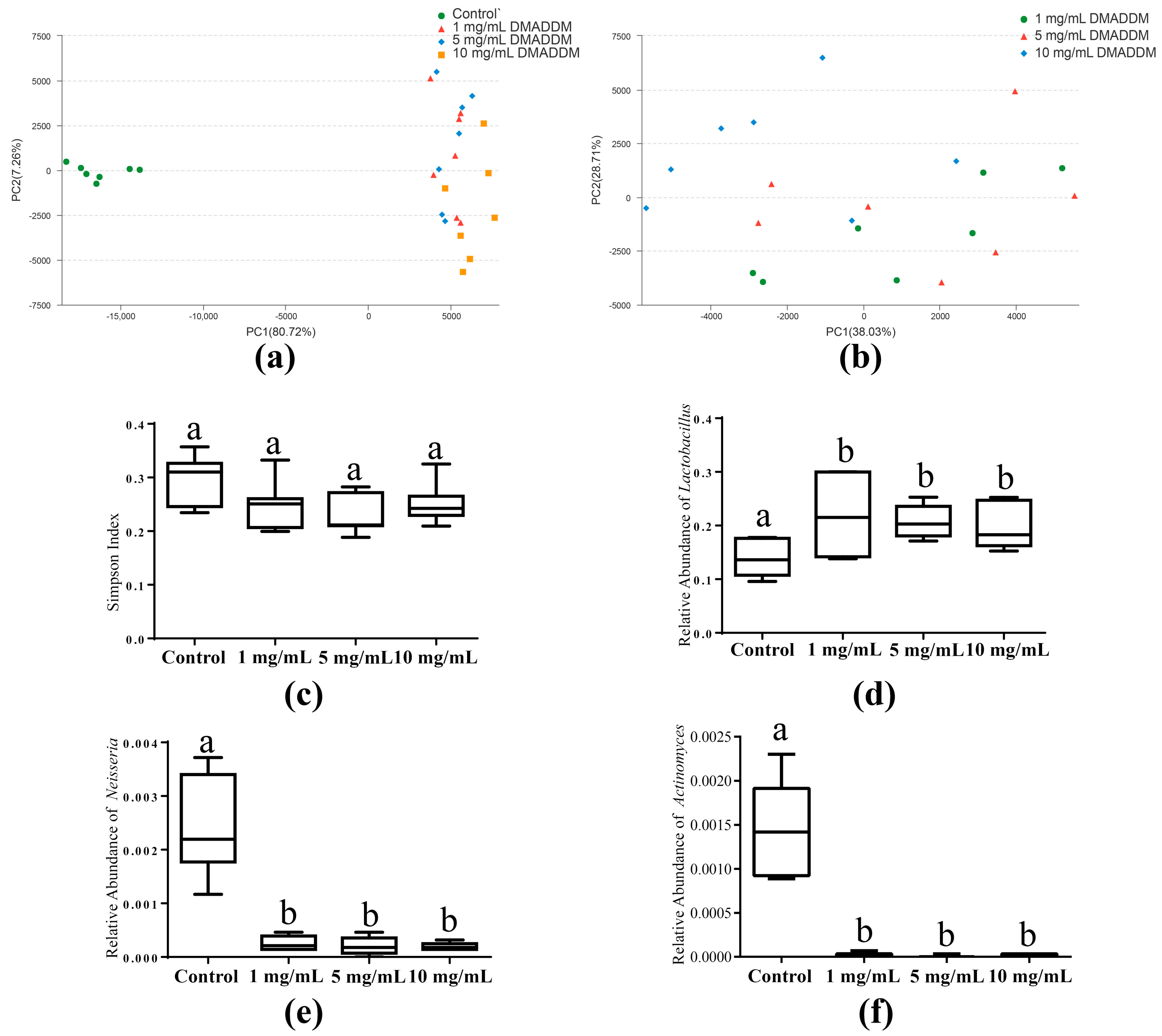
2.3. The Microbial Community of Saliva-Derived Biofilms
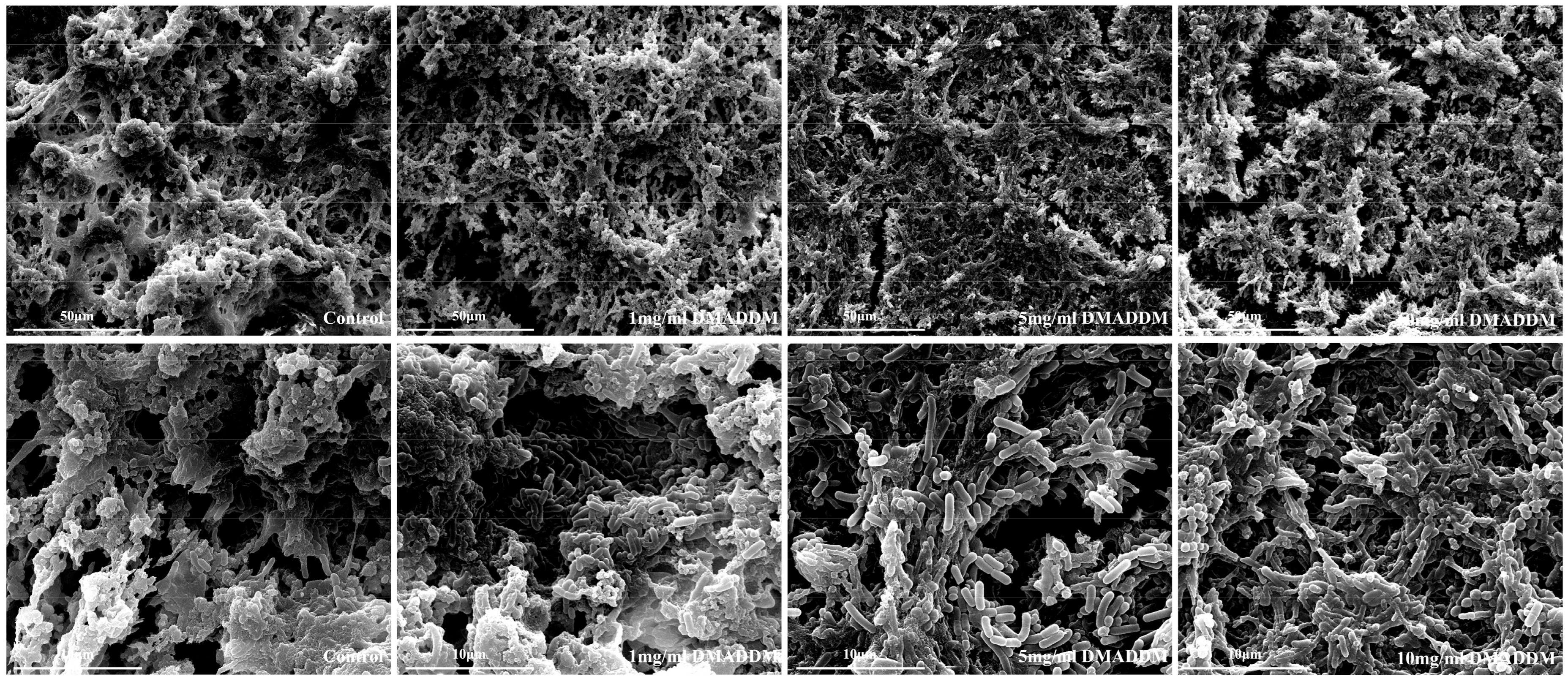
2.4. Scanning Electron Microscopy (SEM) Observation
3. Discussion
4. Materials and Methods
4.1. Coating the AH-Ti Substrates with DMADDM
4.2. Saliva Collection
4.3. Biofilm Development
4.4. Crystal Violet Assay and MTT Assay
4.5. Live/Dead Bacteria Staining
4.6. SEM Observation
4.7. 16S rRNA Gene Sequencing
4.8. Bioinformatics and Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DMADDM | Dimethylaminododecyl methacrylate |
Appendix A
References
- Pjetursson, B.E.; Asgeirsson, A.G.; Zwahlen, M.; Sailer, I. Improvements in implant dentistry over the last decade: Comparison of survival and complication rates in older and newer publications. Int. J. Oral Maxillofac. Implant. 2014, 29, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Simonis, P.; Dufour, T.; Tenenbaum, H. Long-term implant survival and success: A 10–16-year follow-up of non-submerged dental implants. Clin. Oral Implant. Res. 2010, 21, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Busscher, H.J.; Rinastiti, M.; Siswomihardjo, W.; van der Mei, H.C. Biofilm formation on dental restorative and implant materials. J. Dent. Res. 2010, 89, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Wang, H.L. Biofilm related to dental implants. Implant Dent. 2010, 19, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Lang, N.P.; Berglundh, T. Periimplant diseases: Where are we now?—Consensus of the seventh European workshop on periodontology. J. Clin. Periodontol. 2011, 38, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Lafaurie, G.I.; Sabogal, M.A.; Castillo, D.M.; Rincon, M.V.; Gomez, L.A.; Lesmes, Y.A.; Chambrone, L. Microbiome and microbial biofilm profiles of peri-implantitis: A systematic review. J. Periodontol. 2017, 88, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Xu, L.; Wang, Z.; Li, L.; Zhang, J.; Zhang, Q.; Chen, T.; Lin, J.; Chen, F. Subgingival microbiome in patients with healthy and ailing dental implants. Sci. Rep. 2015, 5, 10948. [Google Scholar] [CrossRef] [PubMed]
- Koyanagi, T.; Sakamoto, M.; Takeuchi, Y.; Ohkuma, M.; Izumi, Y. Analysis of microbiota associated with peri-implantitis using 16s rrna gene clone library. J. Oral Microbiol. 2010, 2. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S.; Mason, M.R.; Brooker, M.R.; O’Brien, K. Pyrosequencing reveals unique microbial signatures associated with healthy and failing dental implants. J. Clin. Periodontol. 2012, 39, 425–433. [Google Scholar] [CrossRef] [PubMed]
- De Melo, F.; do Nascimento, C.; Souza, D.O.; de Albuquerque, R.F. Identification of oral bacteria on titanium implant surfaces by 16s rdna sequencing. Clin. Oral Implant. Res. 2016, 28, 697–703. [Google Scholar] [CrossRef] [PubMed]
- De Avila, E.D.; Lima, B.P.; Sekiya, T.; Torii, Y.; Ogawa, T.; Shi, W.; Lux, R. Effect of uv-photofunctionalization on oral bacterial attachment and biofilm formation to titanium implant material. Biomaterials 2015, 67, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.C.; Fernandez, E.; Llama-Palacios, A.; Figuero, E.; Herrera, D.; Sanz, M. Response to antiseptic agents of periodontal pathogens in in vitro biofilms on titanium and zirconium surfaces. Dent. Mater. 2017, 33, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, M.C.; Llama-Palacios, A.; Fernandez, E.; Figuero, E.; Marin, M.J.; Leon, R.; Blanc, V.; Herrera, D.; Sanz, M. An in vitro biofilm model associated to dental implants: Structural and quantitative analysis of in vitro biofilm formation on different dental implant surfaces. Dent. Mater. 2014, 30, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Roehling, S.; Astasov-Frauenhoffer, M.; Hauser-Gerspach, I.; Braissant, O.; Woelfler, H.; Waltimo, T.; Kniha, H.; Gahlert, M. In vitro biofilm formation on titanium and zirconia implant surfaces. J. Periodontol. 2017, 88, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Jordan, R.P.; Marsh, L.; Ayre, W.N.; Jones, Q.; Parkes, M.; Austin, B.; Sloan, A.J.; Waddington, R.J. An assessment of early colonisation of implant-abutment metal surfaces by single species and co-cultured bacterial periodontal pathogens. J. Dent. 2016, 53, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Surmeneva, M.A.; Sharonova, A.A.; Chernousova, S.; Prymak, O.; Loza, K.; Tkachev, M.S.; Shulepov, I.A.; Epple, M.; Surmenev, R.A. Incorporation of silver nanoparticles into magnetron-sputtered calcium phosphate layers on titanium as an antibacterial coating. Colloids Surf. B Biointerfaces 2017, 156, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Man, H.C. Laser fabrication of ag-ha nanocomposites on ti6al4v implant for enhancing bioactivity and antibacterial capability. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 70, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H. Time-kill behaviour against eight bacterial species and cytotoxicity of antibacterial monomers. J. Dent. 2013, 41, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, K.; Zhou, X.; Xu, N.; Xu, H.H.; Weir, M.D.; Ge, Y.; Wang, S.; Li, M.; Li, Y.; et al. Antibacterial effect of dental adhesive containing dimethylaminododecyl methacrylate on the development of streptococcus mutans biofilm. Int. J. Mol. Sci. 2014, 15, 12791–12806. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Weir, M.D.; Zhang, K.; Arola, D.D.; Zhou, X.; Xu, H.H. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J. Dent. 2013, 41, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Cheng, L.; Wu, E.J.; Weir, M.D.; Bai, Y.; Xu, H.H. Effect of water-ageing on dentine bond strength and anti-biofilm activity of bonding agent containing new monomer dimethylaminododecyl methacrylate. J. Dent. 2013, 41, 504–513. [Google Scholar] [CrossRef] [PubMed]
- Rego, G.F.; Vidal, M.L.; Viana, G.M.; Cabral, L.M.; Schneider, L.F.J.; Portela, M.B.; Cavalcante, L.M. Antibiofilm properties of model composites containing quaternary ammonium methacrylates after surface texture modification. Dent. Mater. 2017, 33, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Li, B.; Zhou, X.; Ge, Y.; Wang, S.; Li, M.; Ren, B.; Wang, H.; Zhang, K.; Xu, H.H.K.; et al. Anti-caries effects of dental adhesives containing quaternary ammonium methacrylates with different chain lengths. Materials 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, S.; Zhou, X.; Xu, H.H.; Weir, M.D.; Ge, Y.; Li, M.; Wang, S.; Li, Y.; Xu, X.; et al. Effect of antibacterial dental adhesive on multispecies biofilms formation. J. Dent. Res. 2015, 94, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Ren, B.; Zhou, X.; Xu, H.H.K.; Wang, S.; Li, M.; Weir, M.D.; Feng, M.; Cheng, L. Novel dental adhesive with biofilm-regulating and remineralization capabilities. Materials 2017, 10. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, B.; He, J.; Cheng, L.; Lan, F.; Wu, Y. The response behavior of human gingival fibroblasts and inhibition bacteria adhesion on polydopamine modification of titanium implants for percutaneous application. Colloids Surf. B Biointerfaces 2017, submitted. [Google Scholar]
- Tian, Y.; He, X.; Torralba, M.; Yooseph, S.; Nelson, K.E.; Lux, R.; McLean, J.S.; Yu, G.; Shi, W. Using dgge profiling to develop a novel culture medium suitable for oral microbial communities. Mol. Oral Microbiol. 2010, 25, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Edlund, A.; Yang, Y.; Hall, A.P.; Guo, L.; Lux, R.; He, X.; Nelson, K.E.; Nealson, K.H.; Yooseph, S.; Shi, W.; et al. An in vitro biofilm model system maintaining a highly reproducible species and metabolic diversity approaching that of the human oral microbiome. Microbiome 2013, 1, 25. [Google Scholar] [CrossRef] [PubMed]
- Sela, M.N.; Badihi, L.; Rosen, G.; Steinberg, D.; Kohavi, D. Adsorption of human plasma proteins to modified titanium surfaces. Clin. Oral Implant. Res. 2007, 18, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Badihi Hauslich, L.; Sela, M.N.; Steinberg, D.; Rosen, G.; Kohavi, D. The adhesion of oral bacteria to modified titanium surfaces: Role of plasma proteins and electrostatic forces. Clin. Oral Implant. Res. 2013, 24, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, I.M.; Ricomini Filho, A.P.; Lucena-Ferreira, S.C.; da Silva, W.J.; Paes Leme, A.F.; Senna, P.M.; Del Bel Cury, A.A. Salivary pellicle composition and multispecies biofilm developed on titanium nitrided by cold plasma. Arch. Oral Biol. 2014, 59, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Rettedal, E.A.; Clay, S.; Brozel, V.S. Gc-clamp primer batches yield 16s rrna gene amplicon pools with variable gc clamps, affecting denaturing gradient gel electrophoresis profiles. FEMS Microbiol. Lett. 2010, 312, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, H.; Tomioka, N.; Nakahara, T.; Uchiyama, H. A single band does not always represent single bacterial strains in denaturing gradient gel electrophoresis analysis. Biotechnol. Lett. 2001, 23, 1205–1208. [Google Scholar] [CrossRef]
- Li, B.; Zhou, X.; Zhou, X.; Wu, P.; Li, M.; Feng, M.; Peng, X.; Ren, B.; Cheng, L. Effects of different substrates/growth media on microbial community of saliva-derived biofilm. FEMS Microbiol. Lett. 2017, 364. [Google Scholar] [CrossRef] [PubMed]
- Ledder, R.G.; Sreenivasan, P.K.; DeVizio, W.; McBain, A.J. Evaluation of the specificity and effectiveness of selected oral hygiene actives in salivary biofilm microcosms. J. Med. Microbiol. 2010, 59, 1462–1468. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Ma, S.; Chen, J.H.; Xu, H.H. Therapeutic polymers for dental adhesives: Loading resins with bio-active components. Dent. Mater. 2014, 30, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Yudovin-Farber, I.; Bahir, R.; Domb, A.J.; Weiss, E.I. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against streptococcus mutans. Biomaterials 2006, 27, 3995–4002. [Google Scholar] [CrossRef] [PubMed]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Liu, Y.; Kim, D.; Li, Y.; Hwang, G.; Naha, P.C.; Cormode, D.P.; Koo, H. Nanocatalysts promote streptococcus mutans biofilm matrix degradation and enhance bacterial killing to suppress dental caries in vivo. Biomaterials 2016, 101, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Vogels, R.; Peeters, W.; van Steenberghe, D.; Naert, I.; Haffajee, A. Dynamics of initial subgingival colonization of ‘pristine’ peri-implant pockets. Clin. Oral Implant. Res. 2006, 17, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Hernandez, M.; Olivares-Navarrete, R.; Almaguer-Flores, A. Influence of the periodontal status on the initial-biofilm formation on titanium surfaces. Clin. Implant Dent. Relat. Res. 2016, 18, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Vieira Colombo, A.P.; Magalhaes, C.B.; Hartenbach, F.A.; Martins do Souto, R.; Maciel da Silva-Boghossian, C. Periodontal-disease-associated biofilm: A reservoir for pathogens of medical importance. Microb. Pathog. 2016, 94, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Masaki, C.; Tsuka, S.; Mukaibo, T.; Kondo, Y.; Hosokawa, R. The effects of lactobacillus reuteri probiotics combined with azithromycin on peri-implantitis: A randomized placebo-controlled study. J. Prosthodont. Res. 2017. [Google Scholar] [CrossRef] [PubMed]
- Flichy-Fernandez, A.J.; Ata-Ali, J.; Alegre-Domingo, T.; Candel-Marti, E.; Ata-Ali, F.; Palacio, J.R.; Penarrocha-Diago, M. The effect of orally administered probiotic lactobacillus reuteri-containing tablets in peri-implant mucositis: A double-blind randomized controlled trial. J. Periodontal Res. 2015, 50, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Szkaradkiewicz, A.K.; Stopa, J.; Karpinski, T.M. Effect of oral administration involving a probiotic strain of lactobacillus reuteri on pro-inflammatory cytokine response in patients with chronic periodontitis. Arch. Immunol. Ther. Exp. 2014, 62, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Sajedinejad, N.; Paknejad, M.; Houshmand, B.; Sharafi, H.; Jelodar, R.; Shahbani Zahiri, H.; Noghabi, K.A. Lactobacillus salivarius NK02: A potent probiotic for clinical application in mouthwash. Probiotics Antimicrob. Proteins 2017. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, T.; Hajishengallis, G. Topical treatment with probiotic lactobacillus brevis CD2 inhibits experimental periodontal inflammation and bone loss. J. Periodontal Res. 2014, 49, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Nishiguchi, S.; Fujibayashi, S.; Kim, H.M.; Kokubo, T.; Nakamura, T. Biology of alkali- and heat-treated titanium implants. J. Biomed. Mater. Res. A 2003, 67, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H. Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dent. Mater. 2014, 30, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Weir, M.D.; Zhang, K.; Deng, D.; Cheng, L.; Xu, H.H. Synthesis of new antibacterial quaternary ammonium monomer for incorporation into cap nanocomposite. Dent. Mater. 2013, 29, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Zhang, K.; Melo, M.A.; Weir, M.D.; Zhou, X.; Xu, H.H. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. J. Dent. Res. 2012, 91, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Exterkate, R.A.; ten Cate, J.M. Factors associated with alkali production from arginine in dental biofilms. J. Dent. Res. 2012, 91, 1130–1134. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.; Gu, X.; Yin, T.; Wen, H.; Gao, X.; Zheng, X. Study of enteropathogenic bacteria in children with acute diarrhoea aged from 7 to 10 years in xuzhou, china. Microb. Pathog. 2016, 91, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.H.; Li, M.; Li, C.Q.; Kou, G.J.; Zuo, X.L.; Li, Y.Q. Human colorectal mucosal microbiota correlates with its host niche physiology revealed by endomicroscopy. Sci. Rep. 2016, 6, 21952. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Lin, X.; Zhao, F.; Shi, X.; Li, H.; Li, Y.; Zhu, W.; Xu, X.; Li, C.; Zhou, G. Meat, dairy and plant proteins alter bacterial composition of rat gut bacteria. Sci. Rep. 2015, 5, 15220. [Google Scholar] [CrossRef] [PubMed]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Pena, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. Qiime allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.R.; Chai, B.; Farris, R.J.; Wang, Q.; Kulam, S.A.; McGarrell, D.M.; Garrity, G.M.; Tiedje, J.M. The ribosomal database project (rdp-ii): Sequences and tools for high-throughput rrna analysis. Nucleic Acids Res. 2005, 33, D294–D296. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Lee, S.M.; Jeng, S.L. Estimating population size for capture-recapture data when capture probabilities vary by time and individual animal. Biometrics 1992, 48, 201–216. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Ge, Y.; Wu, Y.; Chen, J.; Xu, H.H.K.; Yang, M.; Li, M.; Ren, B.; Feng, M.; Weir, M.D.; et al. Anti-Bacterial and Microecosystem-Regulating Effects of Dental Implant Coated with Dimethylaminododecyl Methacrylate. Molecules 2017, 22, 2013. https://doi.org/10.3390/molecules22112013
Li B, Ge Y, Wu Y, Chen J, Xu HHK, Yang M, Li M, Ren B, Feng M, Weir MD, et al. Anti-Bacterial and Microecosystem-Regulating Effects of Dental Implant Coated with Dimethylaminododecyl Methacrylate. Molecules. 2017; 22(11):2013. https://doi.org/10.3390/molecules22112013
Chicago/Turabian StyleLi, Bolei, Yang Ge, Yao Wu, Jing Chen, Hockin H. K. Xu, Minggang Yang, Mingyun Li, Biao Ren, Mingye Feng, Michael D. Weir, and et al. 2017. "Anti-Bacterial and Microecosystem-Regulating Effects of Dental Implant Coated with Dimethylaminododecyl Methacrylate" Molecules 22, no. 11: 2013. https://doi.org/10.3390/molecules22112013
APA StyleLi, B., Ge, Y., Wu, Y., Chen, J., Xu, H. H. K., Yang, M., Li, M., Ren, B., Feng, M., Weir, M. D., Peng, X., Cheng, L., & Zhou, X. (2017). Anti-Bacterial and Microecosystem-Regulating Effects of Dental Implant Coated with Dimethylaminododecyl Methacrylate. Molecules, 22(11), 2013. https://doi.org/10.3390/molecules22112013





