Oleanane-Type Saponins from the Roots of Ligulariopsis shichuana and Their α-Glucosidase Inhibitory Activities
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. General Experimental Material
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Spectroscopic Data
3.5. Acid Hydrolysis of Saponins
3.6. α-Glucosidase Inhibitory Activity Assay
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mata, R.; Cristians, S.; Escandón-Rivera, S.; Juárez-Reyes, K.; Rivero-Cruz, I. Mexican antidiabetic herbs: Valuable sources of inhibitors of α-glucosidases. J. Nat. Prod. 2013, 76, 468–483. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jung, H.A.; Sohn, H.S.; Kim, J.W.; Choi, J.S. Potential of icariin metabolites from Epimedium Koreanum Nakai as antidiabetic therapeutic agents. Molecules 2017, 22, 986. [Google Scholar] [CrossRef] [PubMed]
- Grover, J.K.; Yadav, S.; Vats, V. Medicinal plants of India with anti-diabetic potential. J. Ethnopharmacol. 2002, 81, 81–100. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.S.; Gao, K.; Jia, Z.J. New eremophilenolides from Ligulariopsis shichuana. J. Nat. Prod. 2002, 65, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.S.; Gao, K.; Jia, Z.J. New sesquiterpenes from Ligulariopsis shichuana. J. Chin. Chem. Soc. 2004, 51, 417–422. [Google Scholar] [CrossRef]
- Wang, W.S.; Gao, K.; Wang, C.M.; Jia, Z.J. Cytotoxic triterpenes from Ligulariopsis shichuana. Int. J. Pharm. Sci. 2003, 58, 148–150. [Google Scholar] [CrossRef]
- Murakami, M.; Wei, M.X.; Ding, W.; Zhang, Q.D. Effects of Chinese herbs on salivary fluid secretion by isolated and perfused rat submandibular glands. World J. Gastroenterol. 2009, 15, 3908–3915. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.C.; Zhang, Q.W.; Liu, X.; Che, C.T.; Zhao, S.X. Oleanane saponins from Gymnema sylvestre. Phytochemistry 2000, 53, 893–899. [Google Scholar] [CrossRef]
- Yin, M.; Wang, X.; Wang, M.; Chen, Y.; Dong, Y.; Zhao, Y.; Feng, X. A new triterpenoid saponin and other saponins from Salicornia europaea. Chem. Nat. Compd. 2012, 48, 258–261. [Google Scholar] [CrossRef]
- Tapondjou, A.L.; Miyamoto, T.; Lacaille-Dubois, M.A. Glucuronide triterpene saponins from Bersama engleriana. Phytochemistry 2006, 67, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, T.; Kinoshita, K.; Koyama, K.; Takahashi, K.; Kondo, N.; Yuasa, H. A new triterpene from Rathbunia alamosensis. J. Nat. Prod. 1995, 58, 1913–1914. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, I.K.; Choi, S.U.; Lee, J.H.; Moon, E.; Kim, S.Y.; Lee, K.R. New triterpenoids from the tubers of Corydalis ternata: Structural elucidation and bioactivity evaluation. Planta Med. 2011, 77, 1555–1558. [Google Scholar] [CrossRef] [PubMed]
- Melek, F.R.; Miyase, T.; El-Gindi, O.D.; Abdel-Khalik, S.M.; Haggag, M.Y. Saponins from Fagonia mollis. Phytochemistry 1996, 42, 1405–1407. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Murakami, T.; Harada, E.; Murakami, N.; Yamahara, J.; Matsuda, H. Bioactive saponins and glycosides. VII. On the hypoglycemic principles from the root cortex of Aralia elata Seem.: Structure related hypoglycemic activity of oleanolic acid oligoglycoside. Chem. Pharm. Bull. 1996, 44, 1923–1927. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Li, Y.; Murakami, T.; Matsumura, N.; Yamahara, J.; Yoshikawa, M. Antidiabetic principles of natural medicines. III. Structure-related inhibitory activity and action mode of oleanolic acid glycosides on hypoglycemic activity. Chem. Pharm. Bull. 1998, 46, 1399–1403. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Matsuda, H. Antidiabetogenic activity of oleanolic acid glycosides from medicinal foodstuffs. Biofactors 2000, 13, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Omar, R.; Li, L.Y.; Yuan, T.; Seeram, N.P. α-Glucosidase inhibitory hydrolyzable tannins from Eugenia jambolana seeds. J. Nat. Prod. 2012, 75, 1505–1509. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |
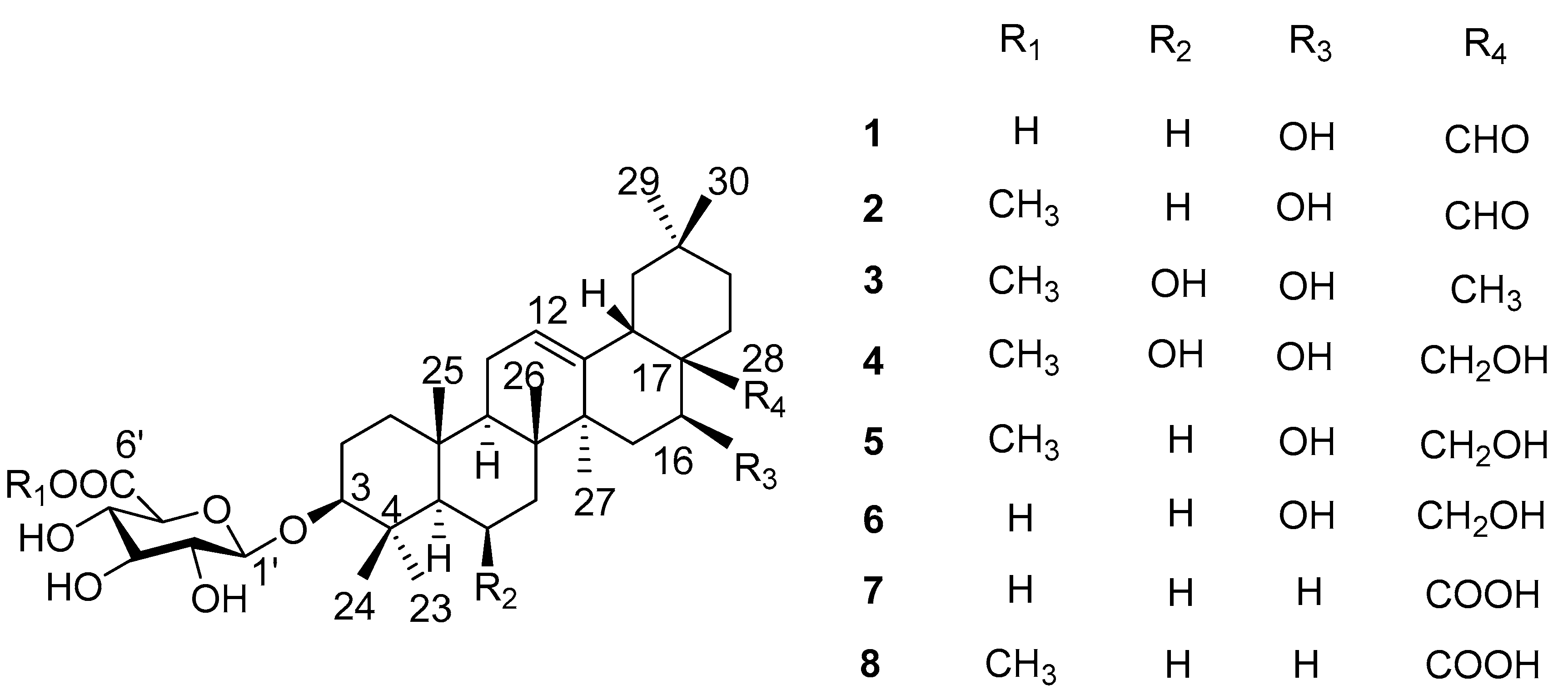
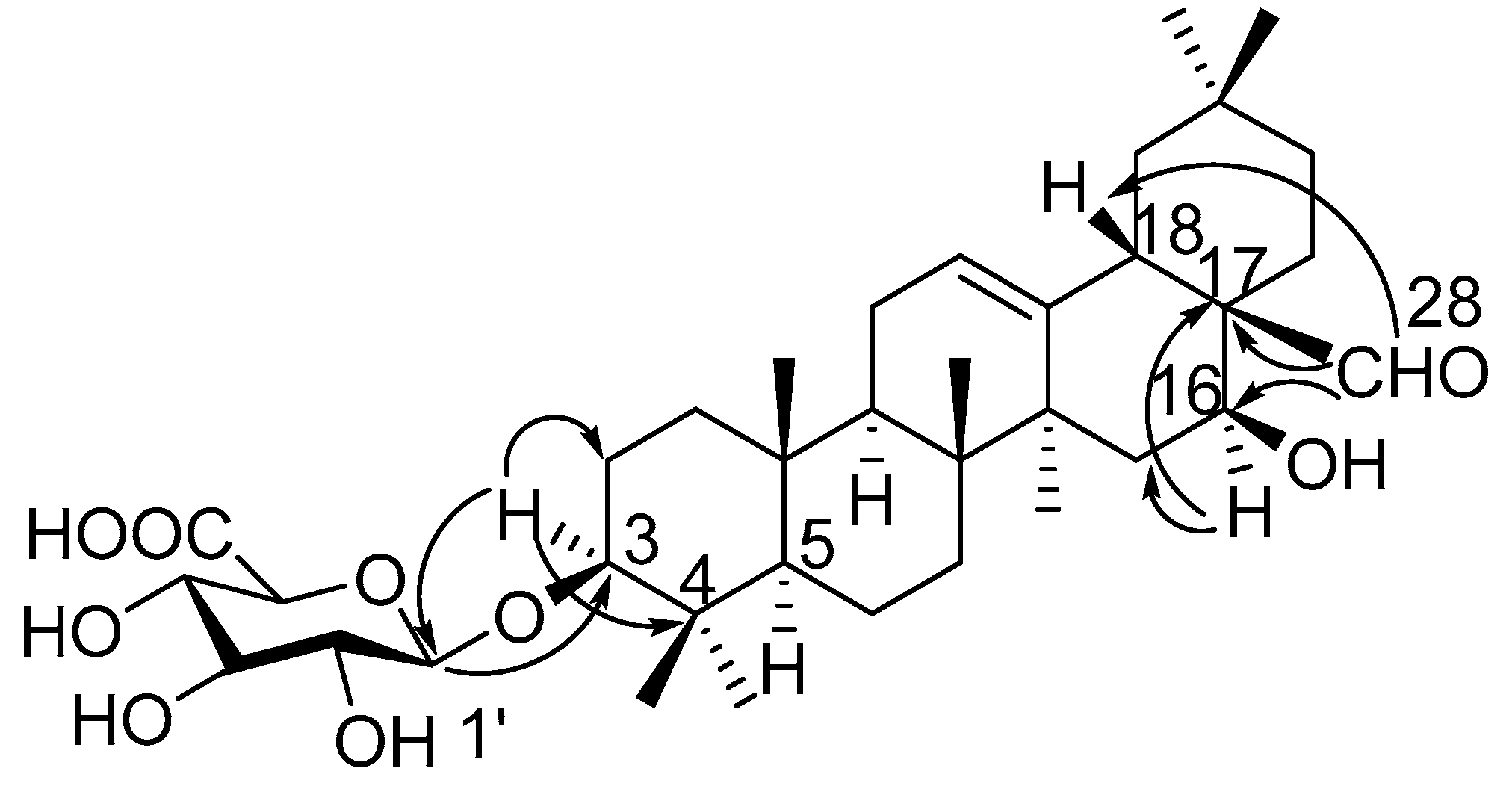

| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1a | 1.62, m | 1.60, m | 1.55, m | 1.55, m | 1.58, m |
| 1b | 1.00, m | 0.98, m | 0.95, m | 0.93, m | 0.95, m |
| 2a | 1.98, m | 1.96, m | 1.78, m | 1.80, m | 1.78, m |
| 2b | 1.72, m | 1.70, m | 1.70, m | 1.76, m | 1.68, m |
| 3 | 3.19, dd (11.7, 4.3) | 3.13, dd (11.8, 4.4) | 3.07, dd (9.9, 5.7) | 3.07, dd (9.9, 5.7) | 3.14, dd (11.2, 4.6) |
| 5 | 0.81, m | 0.77, m | 0.75, m | 0.75, m | 0.76, m |
| 6a | 1.59, m | 1.57, m | 4.51, br s | 4.51, br s | 1.57, m |
| 6b | 1.45, m | 1.43, m | 1.43, m | ||
| 7a | 1.43, m | 1.41, m | 1.72, m | 1.73, m | 1.55, m |
| 7b | 1.27, m | 1.25, m | 1.55, m | 1.58, m | 1.36, m |
| 9 | 1.58, m | 1.56, m | 1.58, m | 1.58, m | 1.54, m |
| 11a | 1.93, m | 1.92, m | 2.03, m | 2.02, m | 1.92, m |
| 11b | 1.92, m | 1.91, m | 1.91, m | 1.90, m | 1.85, m |
| 12 | 5.34, t-like (1.5) | 5.31, t-like (1.5) | 5.27, br s | 5.27, br s | 5.34, br s |
| 15a | 1.82, m | 1.79, m | 1.80, m | 1.40, m | 1.40, m |
| 15b | 1.53, m | 1.51, m | 1.25, m | 1.20, m | 1.21, m |
| 16 | 4.32, dd (11.9, 4.5) | 4.29, dd (12.0, 4.6) | 4.13, dd (11.4, 4.5) | 4.24, dd (11.7, 4.8) | 4.24, dd (12.0, 4.8) |
| 18 | 2.78, dd (14.0, 4.2) | 2.76, dd (14.0, 4.5) | 2.18, m | 2.21, m | 2.18, m |
| 19a | 1.70, m | 1.67, m | 1.73, m | 1.72, m | 1.71, m |
| 19b | 1.19, m | 1.16, m | 1.03, m | 1.06, m | 1.02, m |
| 21a | 1.58, m | 1.56, m | 1.90, m | 1.41, m | 1.41, m |
| 21b | 1.37, m | 1.34, m | 1.15, m | 1.21, m | 1.21, m |
| 22a | 2.09, m | 2.06, m | 1.39, m | 2.21, m | 2.10, m |
| 22b | 1.26, m | 1.23, m | 1.11, m | 1.41, m | 1.40, m |
| 23 | 1.08, s | 1.05, s | 1.13, s | 1.13, s | 1.06, s |
| 24 | 0.88, s | 0.85, s | 1.23, s | 1.23, s | 0.86, s |
| 25 | 0.98, s | 0.95, s | 1.33, s | 1.33, s | 0.97, s |
| 26 | 0.83, s | 0.81, s | 1.28, s | 1.30, s | 1.03, s |
| 27 | 1.25, s | 1.23, s | 1.19, s | 1.20, s | 1.24, s |
| 28a | 9.76, s | 9.73, s | 0.79, s | 3.80, d (9.7) | 3.81, d (9.7) |
| 28b | 3.26, m | 3.27, m | |||
| 29 | 0.96, s | 0.93, s | 0.89, s | 0.90, s | 0.90, s |
| 30 | 0.96, s | 0.94, s | 0.92, s | 0.93, s | 0.92, s |
| GluA-1′ | 4.38, d (8.2) | 4.37, d (7.8) | 4.37, d (7.7) | 4.37, d (7.8) | 4.40, d (7.5) |
| 2′ | 3.24, t (8.3) | 3.22, t (8.5) | 3.24, t (8.4) | 3.24, t (8.4) | 3.26, t (8.4) |
| 3′ | 3.39, t (9.2) | 3.34, t (9.1) | 3.35, t (9.1) | 3.35, t (9.1) | 3.37, t (9.0) |
| 4′ | 3.47, t (8.8) | 3.49, t (9.4) | 3.50, t (9.4) | 3.49, t (9.4) | 3.53, t (9.2) |
| 5′ | 3.62, d (10.0) | 3.81, d (9.8) | 3.80, d (9.8) | 3.80, d (9.7) | 3.83, br d (10.0) |
| 6′-OCH3 | 3.77, s | 3.76, s | 3.76, s | 3.79, s |
| Position | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| 1 | 38.4, CH2 | 38.3, CH2 | 42.1, CH2 | 42.1, CH2 | 39.9, CH2 |
| 2 | 25.5, CH2 | 25.6, CH2 | 27.3, CH2 | 27.3, CH2 | 27.0, CH2 |
| 3 | 89.4, CH | 89.6, CH | 91.3, CH | 91.3, CH | 91.1, CH |
| 4 | 38.8, C | 38.8, C | 41.2, C | 41.2, C | 41.6, C |
| 5 | 55.6, CH | 55.5, CH | 57.3, CH | 57.3, CH | 57.0, CH |
| 6 | 17.9, CH2 | 17.8, CH2 | 68.6, CH | 68.6, CH | 19.3, CH2 |
| 7 | 32.6, CH2 | 32.6, CH2 | 41.6, CH2 | 41.7, CH2 | 33.7, CH2 |
| 8 | 39.5, C | 39.4, C | 40.4, C | 40.4, C | 40.9, C |
| 9 | 46.8, CH | 46.7, CH | 48.7, CH | 48.6, CH | 48.3, CH |
| 10 | 36.4, C | 36.4, C | 37.4, C | 36.7, C | 36.7, C |
| 11 | 23.1, CH2 | 23.1, CH2 | 24.7, CH2 | 24.7, CH2 | 24.1, CH2 |
| 12 | 123.1, CH | 123.0, CH | 123.8, CH | 124.3, CH | 124.0, CH |
| 13 | 142.0, C | 141.9, C | 144.5, C | 143.6, C | 144.3, C |
| 14 | 43.4, C | 43.4, C | 45.4, C | 45.1, C | 44.7, C |
| 15 | 36.5, CH2 | 36.5, CH2 | 36.4, CH2 | 34.9, CH2 | 35.0, CH2 |
| 16 | 63.8, CH | 63.8, CH | 66.4, CH | 68.0, CH | 67.8, CH |
| 17 | 52.8, C | 52.7, C | 38.6, C | 41.5, C | 40.2, C |
| 18 | 41.8, CH | 41.7, CH | 50.8, CH | 45.1, CH | 45.1, CH |
| 19 | 45.7, CH2 | 45.8, CH2 | 48.1, CH2 | 47.9, CH2 | 47.9, CH2 |
| 20 | 30.0, C | 30.0, C | 31.9, C | 30.8, C | 31.7, C |
| 21 | 32.6, CH2 | 32.6, CH2 | 35.4, CH2 | 34.9, CH2 | 34.8, CH2 |
| 22 | 22.3, CH2 | 22.3, CH2 | 31.7, CH2 | 26.0, CH2 | 26.0, CH2 |
| 23 | 27.1, CH3 | 27.0, CH3 | 28.3, CH3 | 28.2, CH3 | 28.5, CH3 |
| 24 | 15.6, CH3 | 15.5, CH3 | 18.5, CH3 | 18.5, CH3 | 17.0, CH3 |
| 25 | 14.6, CH3 | 14.6, CH3 | 17.6, CH3 | 17.5, CH3 | 16.2, CH3 |
| 26 | 16.3, CH3 | 16.2, CH3 | 18.9, CH3 | 18.6, CH3 | 17.7, CH3 |
| 27 | 25.6, CH3 | 25.5, CH3 | 27.8, CH3 | 27.5, CH3 | 27.0, CH3 |
| 28 | 208.0, CH | 208.0, CH | 22.4, CH3 | 69.0, CH2 | 69.0, CH2 |
| 29 | 32.0, CH3 | 32.0, CH3 | 33.9, CH3 | 31.8, CH3 | 33.7, CH3 |
| 30 | 22.6, CH3 | 22.6, CH3 | 24.5, CH3 | 24.3, CH3 | 24.3, CH3 |
| GluA-1′ | 105.3, CH | 105.6, CH | 107.1, CH | 107.1, CH | 107.1, CH |
| 2′ | 75.3, CH | 73.9, CH | 75.4, CH | 75.4, CH | 75.4, CH |
| 3′ | 78.1, CH | 78.1, CH | 77.6, CH | 77.6, CH | 77.6, CH |
| 4′ | 74.1, CH | 71.8, CH | 73.3, CH | 73.3, CH | 73.3, CH |
| 5′ | 76.6, CH | 76.1, CH | 76.7, CH | 76.7, CH | 76.7, CH |
| 6′ | 175.5, C | 170.0, C | 171.5, C | 171.5, C | 171.5, C |
| 6′-OCH3 | 51.4, CH3 | 52.9, CH3 | 52.8, CH3 | 52.8, CH3 |
| Compounds | IC50 (μM) | Compounds | IC50 (μM) |
|---|---|---|---|
| 1 | 18.7 ± 1.7 | 5 | 154.3 ± 11.2 |
| 2 | 37.9 ± 3.6 | 6 | 42.6 ± 5.9 |
| 3 | 104.9 ± 4.3 | 7 | 57.6 ± 6.8 |
| 4 | 89.7 ± 5.1 | 8 | 133.7 ± 3.3 |
| Acarbose | 190.5 ± 3.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-B.; Liu, T.-T.; Wang, W.-S.; Feng, J.-C.; Tian, H.-M. Oleanane-Type Saponins from the Roots of Ligulariopsis shichuana and Their α-Glucosidase Inhibitory Activities. Molecules 2017, 22, 1981. https://doi.org/10.3390/molecules22111981
Wu H-B, Liu T-T, Wang W-S, Feng J-C, Tian H-M. Oleanane-Type Saponins from the Roots of Ligulariopsis shichuana and Their α-Glucosidase Inhibitory Activities. Molecules. 2017; 22(11):1981. https://doi.org/10.3390/molecules22111981
Chicago/Turabian StyleWu, Hai-Bo, Ting-Ting Liu, Wen-Shu Wang, Jin-Chao Feng, and Hong-Mei Tian. 2017. "Oleanane-Type Saponins from the Roots of Ligulariopsis shichuana and Their α-Glucosidase Inhibitory Activities" Molecules 22, no. 11: 1981. https://doi.org/10.3390/molecules22111981
APA StyleWu, H.-B., Liu, T.-T., Wang, W.-S., Feng, J.-C., & Tian, H.-M. (2017). Oleanane-Type Saponins from the Roots of Ligulariopsis shichuana and Their α-Glucosidase Inhibitory Activities. Molecules, 22(11), 1981. https://doi.org/10.3390/molecules22111981




