Abstract
Substituted seven-membered N-heterocycles are prevalent bioactive epitopes and useful synthons for preparing enzyme inhibitors or molecular recognition systems. To fully exploit the chemical properties of this flexible N-heterocycle scaffold, efficient methods for its diverse functionalization are required. Here we utilize the late-stage oxidation of tetrahydroazepines as an approach to access densely functionalized oxo-azepines in a total of 8 steps and ~30% overall yield from commercially available starting materials. Hydroboration of tetrahydroazepines proceeded with diastereoselectivity in a substrate-dependent manner to yield regioisomeric azepanols before their oxidation to the corresponding oxo-azepines. Regioselectivity of the hydroboration step may be improved moderately by a rhodium catalyst, albeit with loss of conversion to a competing hydrogenation pathway. Overall our method allows efficient access to azepanols and oxo-azepines as versatile epitopes and synthons with a high degree of diastereoselectivity and moderate regioselectivity.
1. Introduction
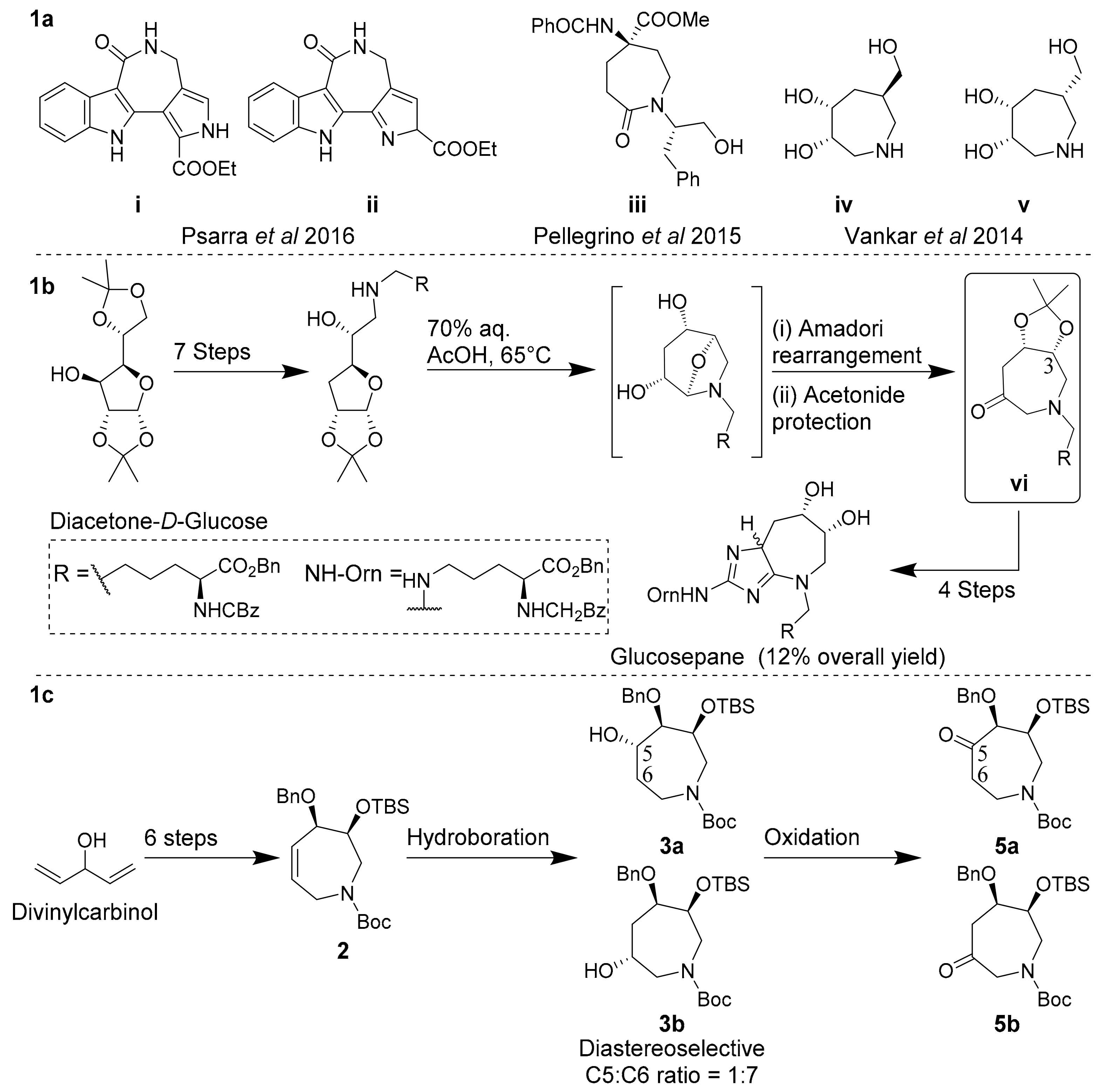
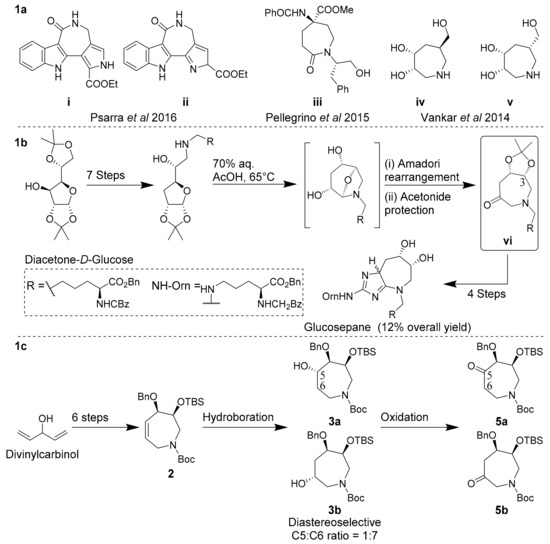
The seven-membered, N-heterocyclic azepane scaffold is a biologically active epitope and useful building block in the construction of novel glycosidase inhibitors [1,2,3,4,5], anticancers [6,7,8,9,10], antidiabetics [11,12,13,14,15], antivirals [16,17] and DNA minor groove-binding agents [18,19,20]. In particular, azepanols and oxo-azepines have received much recent attention as useful epitopes in both materials and medicinal chemistry due to the unique flexibility of the seven-membered ring and resultant spatial distribution of oxygen substituents. For example (Scheme 1a), 2-oxo-azepine i reported by Psarra et al. in 2016 exhibited a selective 78% inhibition of TAK1 kinase activity versus other kinase targets, while its constitutional isomer ii exhibited comparatively modest and non-selective inhibition of TAK1 activity (58%) [21]. Oxo-azepine i may now serve as a scaffold for a detailed structure-activity relationship (SAR) study towards optimized TAK1 inhibition. Pellegrino et al. enantioselectively synthesized and incorporated oxo-azepine iii into a tetrapeptide to induce a 310 helical turn into the molecule, as confirmed by NMR and molecular dynamics [22]. This 310 helical conformation was found to be stabilized by key intramolecular hydrogen bonds between the 2-oxo-azepine ketone oxygen and amino acid residues [22,23]. Vankar et al. in 2014 reported syn-dihydroxyazepane iv as a potent and selective inhibitor of jack bean α-mannosidase, while its epimer v was two orders of magnitude less active towards this enzyme [2]. In a recent landmark example, Spiegel et al. prepared glucosepane enantioselectively from oxo-azepine vi, yielding a guanidine-containing oxo-azepine (Scheme 1b) [24]. Collectively, these examples highlight the importance of stereochemically well-defined and highly functionalized azepanols and oxo-azepines as biologically active epitopes and synthons. These examples also highlight the need for efficient synthetic methods to access these molecules with regio- and diastereocontrol.
Scheme 1.
(a) Representative oxo-azepines as glycosidase inhibitors; (b) Concise, enantioselective synthesis of glucosepane by Spiegel et al. [24]; (c) Current work.
Azepanols and oxo-azepines are typically constructed via the ring-expansion or cyclization of appropriately derivatized sugars or furans with pre-established substitution patterns and stereochemistry. Methods of ring-expansion and cyclization have most-commonly included reductive amination [19,20,25,26,27,28,29] or epoxide ring opening with suitable amines [30,31], ring-closing metathesis (RCM) [32,33,34], alkene–nitrone cycloaddition [35] and tandem Staudinger aza–Wittig reactions [36]. The Spiegel synthesis of glucosepane utilized commercially available diacetone-d-glucose ($3.20/g) as a precursor to oxo-azepine vi via a key Amadori rearrangement (Scheme 1b) [24]. Oxo-azepine vi was then further elaborated via the C3-ketone with a sigmatropic rearrangement and cyclization sequence to yield glucosepane in a total of 13 steps and 12% overall yield from diacetone-d-glucose [24]. As part of our work on densely functionalized and conformationally diverse azepanes, we have sought to develop a more-concise route to access oxo-azepines via a late-stage oxidation of tetrahydroazepines [37,38]. Here we report the diastereoselective synthesis of useful oxo-azepine synthons that can be incorporated into complex oxo-azepines such as the glucosepanes (Scheme 1c).
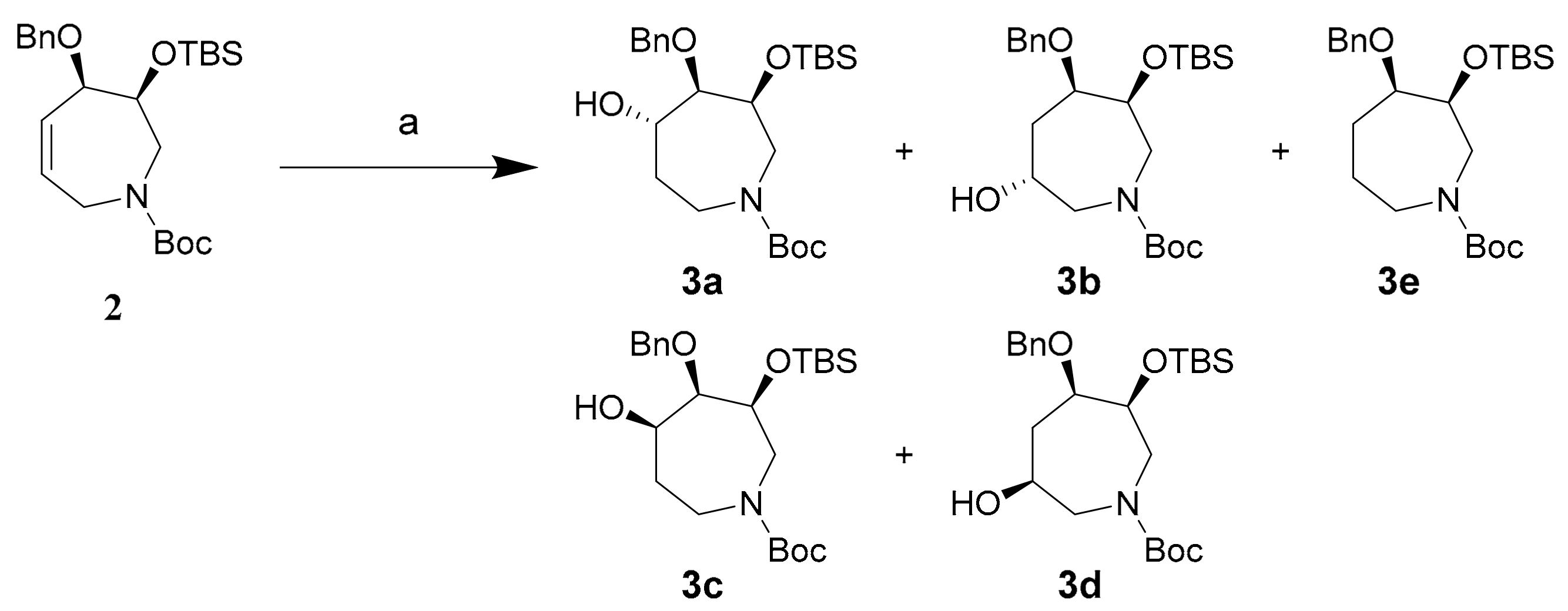
Our previous work showed that hydroboration of the (3S)-azido-(4R)-tetrahydroazepine proceeded in low regio- and diastereoselectivity [37,38,39]. Herein, we investigate regio- and diastereoselectivity in the hydroboration of a related tetrahydroazepine 2 (Scheme 1c). Standard and catalytic hydroboration reactions yielded a mixture of the expected (5S) and (6R) alcohols 3a and 3b, respectively, with complete diastereofacial selectivity and moderate regioselectivity. These azepanols were then converted to the corresponding oxo-azepines 5a and 5b by oxidation. Overall, the current work with tetrahydroazepine 2 constitutes an efficient and shorter route (8 steps from commercially available simple starting material) to azepanols and oxo-azepines that can be used as synthons for complex oxo-azepines such as the glucosepanes (13 steps, 12% overall yield).
2. Results
2.1. Synthesis of Silyl Ether 2
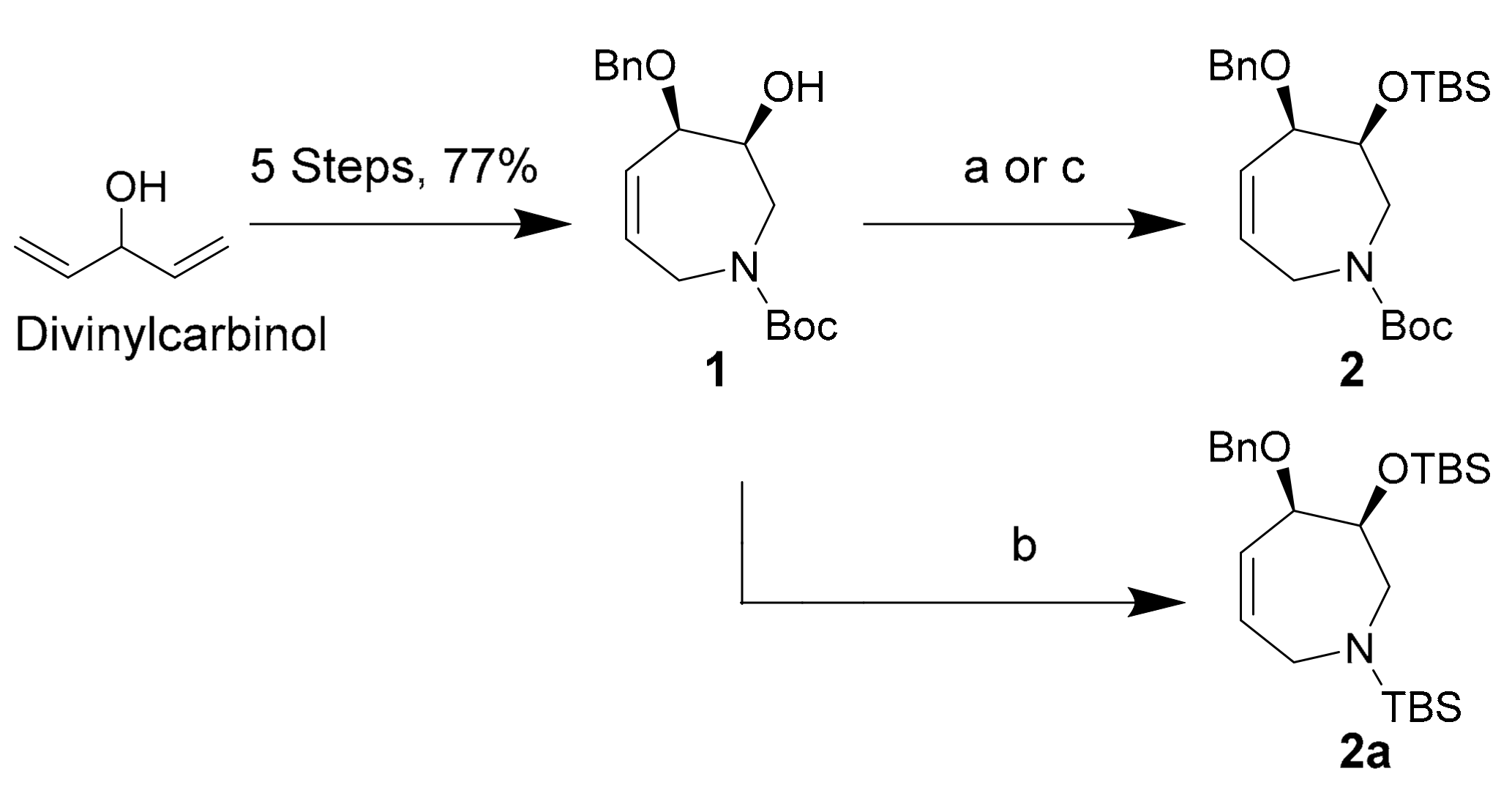
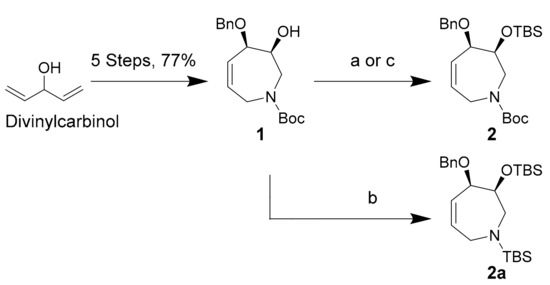
Tetrahydroazepine starting material 1 was synthesized from commercially available divinylcarbinol using a concise, 5-step protocol described by Fürstner et al. in 77% overall yield [32]. Silyl ether 2 was then produced in 90% yield using Corey’s TBSOTf conditions over 2 h at −80 °C (Scheme 2, conditions (a)) [40]. The same reaction conducted at −20 °C led to N-Boc deprotection of 1 and to the formation of O,N-TBS protected species 2a according to preliminary NMR spectroscopic data (Scheme 2, conditions (b)). Compound 2a appears to degrade by N-TBS hydrolysis over several days at −20 °C. The use of Corey’s conditions with TBSCl and imidazole in DMF resulted in long reaction times and poor yields for our sterically congested substrate 1 (Scheme 2, conditions (c)), with only 30% conversion to 2 after 48 h at 35 °C [41].
Scheme 2.
Silyl-protection conditions for secondary alcohol 1. Conditions (a) TBSOTf (1.0 equiv), 2,6-lutidine (1.2 equiv), −80 °C, DCM, 2 h, 90%; (b) TBSOTf (1.0 equiv), 2,6-lutidine (1.2 equiv), −20 °C, DCM, 2 h, 50% (of 2a); (c) TBSCl (3.0 equiv), imidazole (3.5 equiv), 35 °C, DMF, 24 h, 30%.
2.2. Hydroboration of (3S)-OTBS Tetrahydroazepine 2
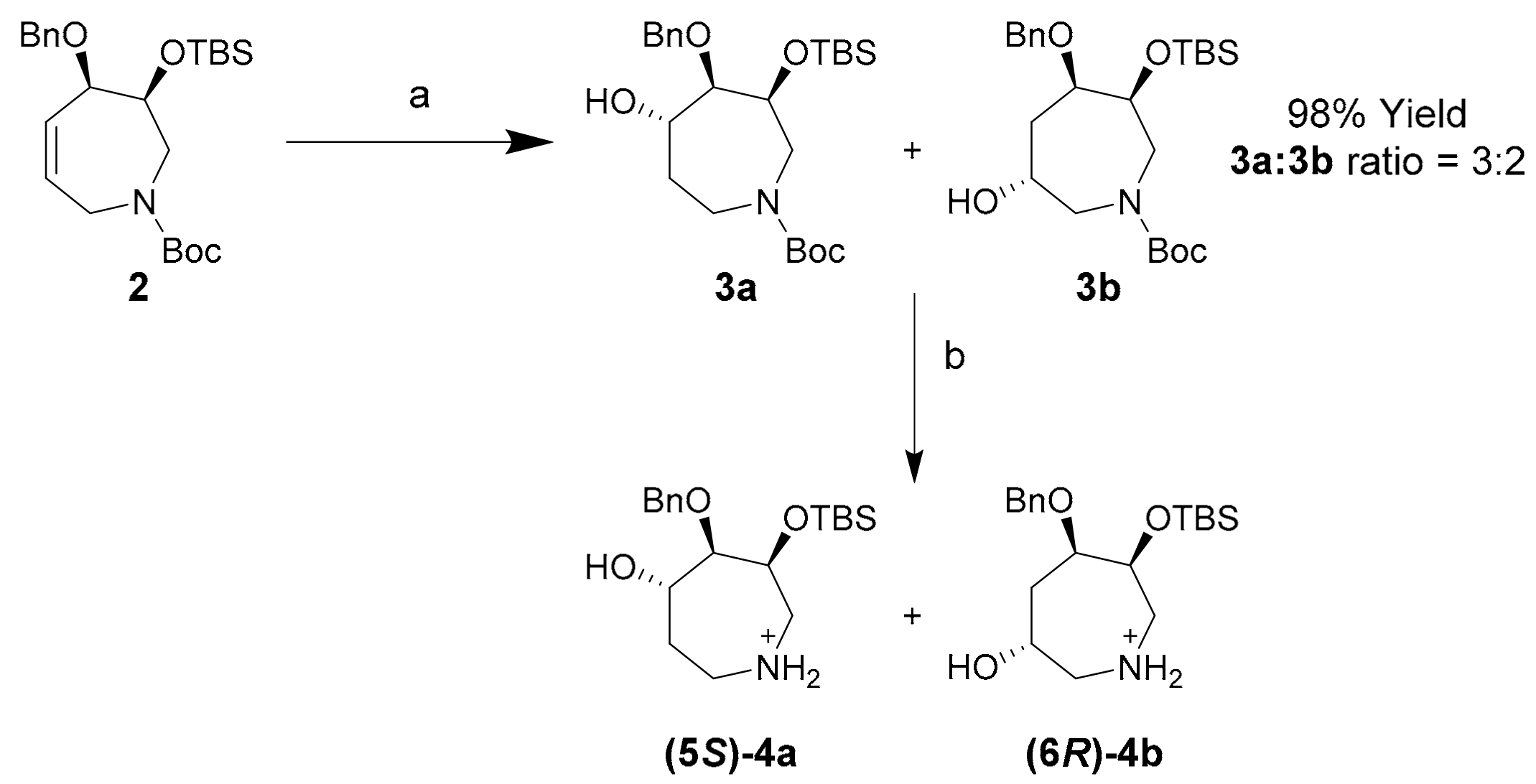
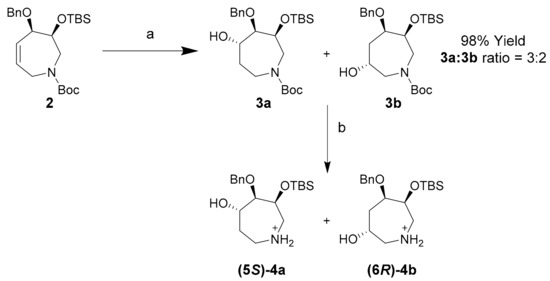
Hydroboration of tetrahydroazepine 2 proceeded smoothly to yield a 3:2 mixture of regioisomers 3a and 3b with excellent diastereofacial selectivity and high yield utilizing borane dimethylsulfide (BH3∙SMe2) (Scheme 3). These compounds were isolated by semi-preparative reverse-phase HPLC using acetonitrile:water:trifluoroacetic acid (TFA) (from 50:50:0.1 to 80:20:0.1 over 45 min; flow rate: 4.18 mL/min) as the mobile phase. Separated regioisomers 3a and 3b were de-protected to yield azepanols (5S)-4a and (6R)-4b for characterization by 2D-NMR in the absence of N-Boc rotamers.
Scheme 3.
Synthesis of azepanols 3a and 3b and their deprotected derivatives (5S)-4a and (6R)-4b. Conditions (a) (i) BH3∙SMe2, THF, r.t., 1 h; (ii) NaOH, H2O2, EtOH, 80 °C, 1 h, 92%; (b) TFA, r.t., 5 min, 99%.
2.3. Structural Assignment of (5S)-4a and (6R)-4b
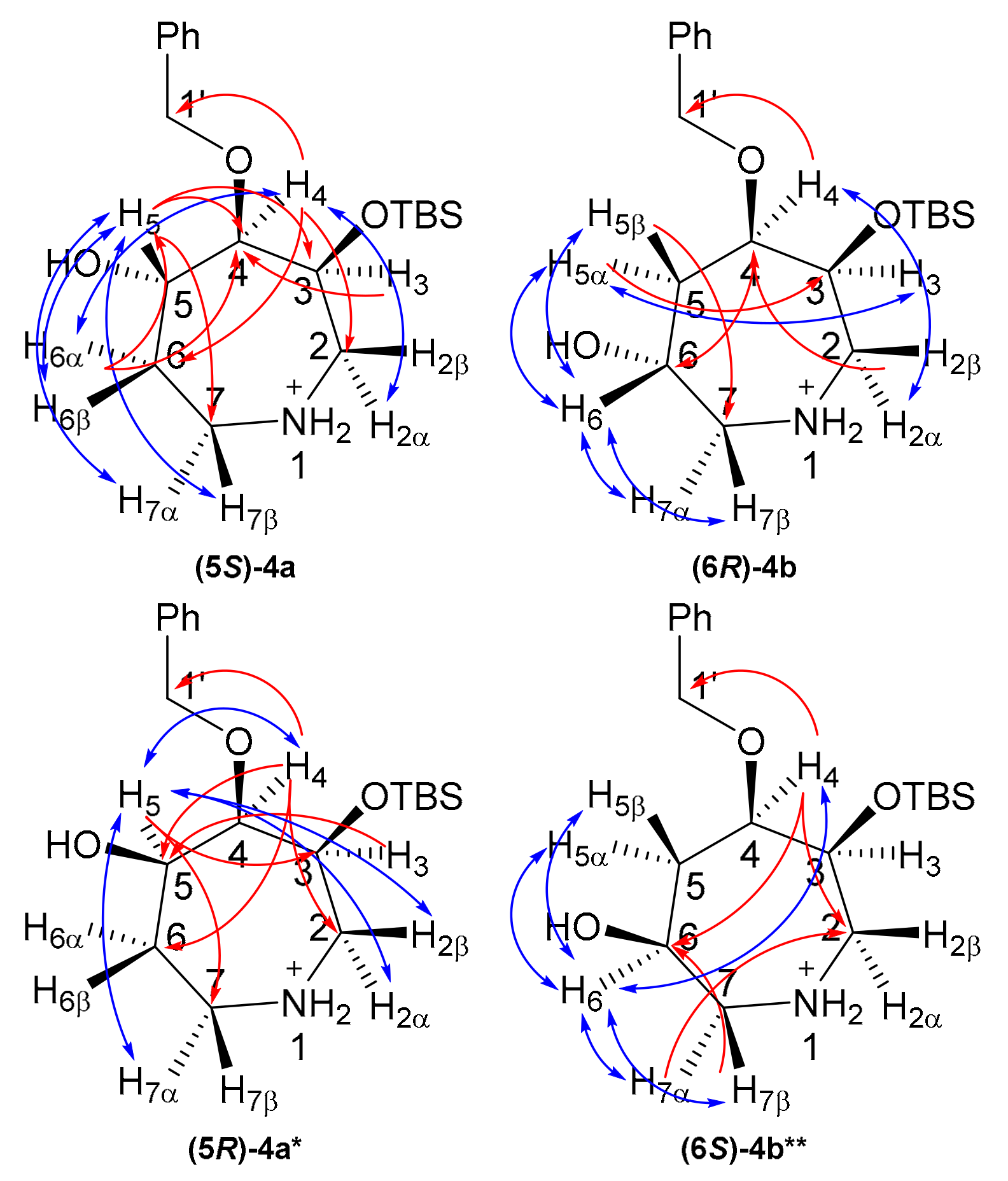
Key 2D-NMR correlations (Figure 1) were used to completely assign the 1H and 13C resonances (see Supplementary Materials). Briefly for (5S)-4a, an HMBC correlation between C1′ (δC 74.0 ppm) and H4 (δH 3.55 ppm), in combination with an HSQC correlation between H4 and C4 (δC 85.6 ppm) was used to identify C4/H4. COSY correlations and HSQCs were then used to assign the rest of the azepine ring (Table S1). NOESY correlations between H4/H6α and H4/H2α allowed stereochemical assignment of H6 and H2 protons. The presence of a strong nOe correlation between H5 and H6β and the absence of nOe correlations between H5 and H3 or H2α suggested 5S stereochemistry at the site of hydroxylation. To further substantiate this hypothesis, compound (5S)-4a was synthesized by oxidation of (5S)-4a to the 5-ketone 5a, followed by reduction to a mixture of (5S)-4a and (5S)-4a. A (5S)-4a:(5R)-4a ratio of 4:1 was observed for this reduction, likely due to the steric bulk of the re face of the azepine ring leading to the observed diastereofacial selectivity in the hydroboration reaction. For (5R)-4a, nOe correlations from H5 to H4 and H7α and an absence of nOe correlations between H5 and H7β (Table S2) suggested 5R stereochemistry for the minor component, but the NMR data was far from convincing due to overlapping peaks, especially for the C2 and C7 protons. DFT calculations (next section) were used to substantiate the stereochemical assignments.
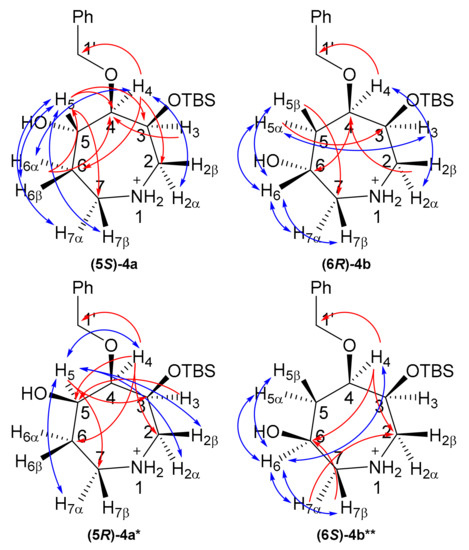
Figure 1.
2D-NMR correlations for azepanols (5S)-4a and (6R)-4b. Red, HMBC; blue, NOESY. * Characterized as a mixture with (5S)-4a; ** Characterized as a mixture with (6R)-4b.
Compound (6R)-4b was characterized by the same approach. The combination of an HMBC between C1′ (δC 72.5 ppm) and H4 (δH 3.84 ppm), and an HSQC between H4 and C4 (δC 77.5 ppm) was used to identify C4/H4. COSY correlations and HSQCs were used to identify the remainder of the azepine ring and site of hydroxylation (C6; 63.7 ppm, Table S3). The 6R stereochemistry of (6R)-4b was supported by strong nOe correlations from H6 to H5α, H5β, H7α and H7β. This suggested that H6 is synclinal to H5α, H5β, H7α and H7β, but this could not be used to confirm the stereochemistry. Similarly, the absence of nOe correlations from H6 to H2α, H3 and H4 was weak evidence of 6R stereochemistry. To help with the assignment, a mixture of (6R)-4b and (6S)-4b was synthesized using the same oxidation/reduction approach as for 4a (Table S4). In compound (6S)-4b, H6 showed stronger nOe correlations to H5α and H7α than to H5β and H7β, consistent with a β-OH and thus 6S stereochemistry for this compound and in support of 6R stereochemistry for 4b.
2.4. DFT Analysis of (5S)-4a and (5R)-4a
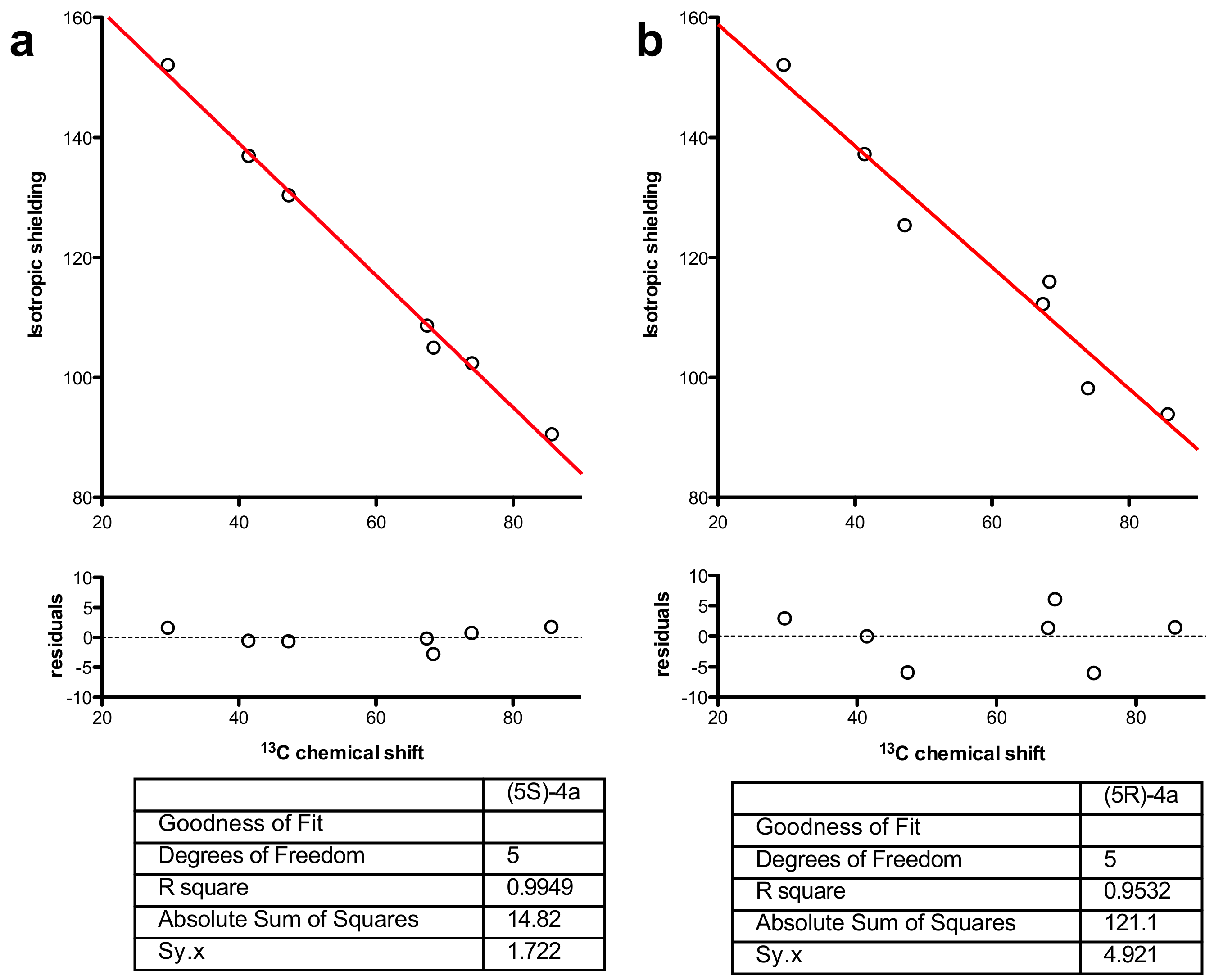
Models of (5S)-4a and (5R)-4a were built in MOE (Molecular Operating Environment) [42], and a conformational search using low-mode molecular dynamics was made from each structure and the remaining azepine geometry using the MMFF94x force-field for minimization. The TBS and Ph groups were then stripped from the structures and the remaining azepines’ geometry optimized using Turbomole 7.1 (DFT//BP86/SV(P) then DFT//B3-LYP/def2-TZVPP) [43]. The NMR shielding constants were then calculated at the same level of theory (DFT//B3-LYP/def2-TZVPP) and plotted against the actual 13C- and 1H-NMR data of (5S)-4a and (5R)-4a (see Supplementary Materials). Non-linear least-squares fitting to a straight line (GraphPad Prism 5.0) resulted in a goodness of fit (R; Figure 2).
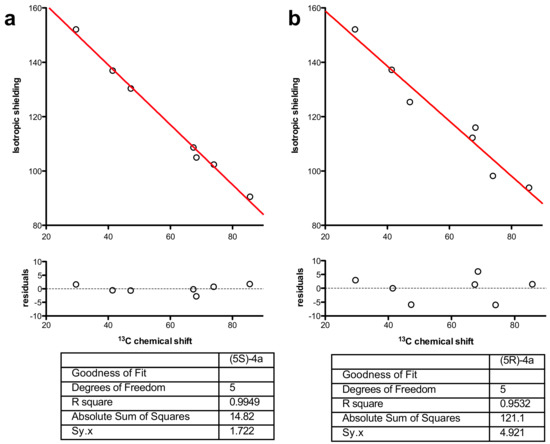
Figure 2.
Non-linear regression fitted to a straight line of chemical shifts against calculated isotropic shielding (DFT//B3-LYP/TZVPP) for (a) (5S)-4a and (b) (5R)-4a with residuals and goodness-of-fit values.
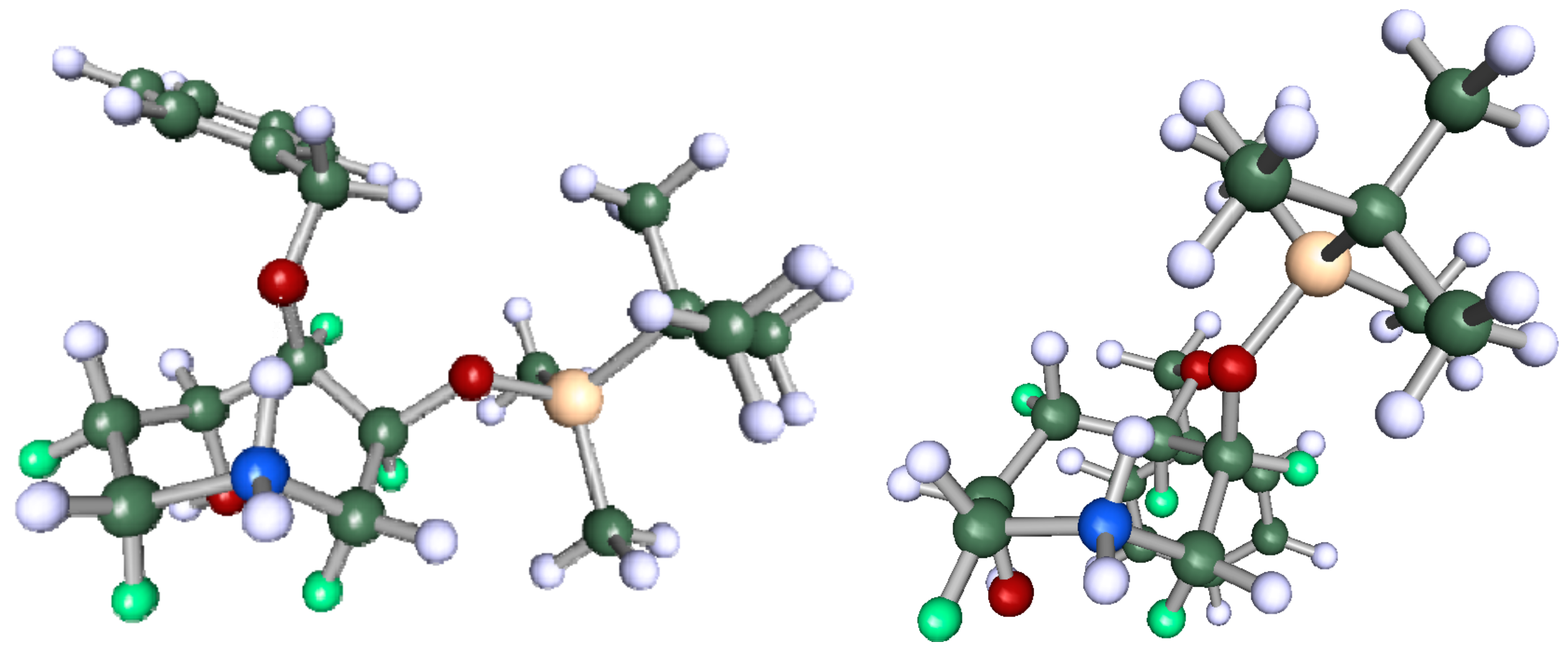
Non-linear regression showed a better fit for 13C-NMR chemical shifts for (5S)-4a (Figure 2a) than for (5R)-4a, whereas the lowest-energy structure gave a poorer fit (R2 = 0.9532 vs. 0.9949) with several 13C resonances (notably C5 and the benzylic carbon) greater than 5 ppm from the line of best fit (Figure 2b). In light of this regression analysis and experimental spectral data for 4a, we conclude that the molecule has, on the weight of evidence, 5S stereochemistry. Finally, it is noteworthy that the DFT calculations predict a conformation (Figure 3) where H5 would not show any diagnostic nOes to H3 and H2α. Similarly, the minimum-energy structure for (6R)-4b (Figure 3, right) showed that H5 is synclinal to H5α, H5β, H7α and H7β, and that H5 would indeed not be expected to show nOe correlations to H2α, H3 or H4 and nOes of roughly equal intensity to H5α, H5β, H7α and H7β, as is observed (see Supplementary Materials).
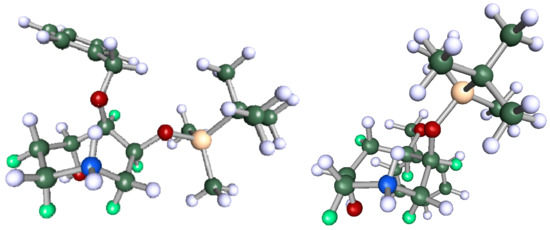
Figure 3.
Global lowest-energy structure of (5S)-4a (left) and (6R)-4b (right), α-protons coloured green.
2.5. Reagent and Catalyst Screen for the Hydroboration of 2
The stereochemical and regiochemical outcomes of hydroborations of substituted alkenes is related to the steric and stereoelectronic environment of both the reagent and substrate. In this case, the hydroboration of (3R)-OTBS tetrahydroazepine 2 using BH3∙SMe2 proceeded with excellent diastereofacial selectivity but low regioselectivity to provide 3a and 3b. In contrast, hydroboration of a (3S)-azidotetrahydroazepine in our earlier work proceeded with low diastereo- and regioselectivity, yielding all four possible C5/C6 azepanols (product ratio 5S:5R:6S:6R = 1:36:32:28) [39]. It is likely that the bulky (3R)-OTBS group, along with the other substituents of tetrahydroazepine 2, present significant steric bias to the cyclic alkene that was absent in the (3S)-azido substrate. We were thus interested in screening reagents and catalysts to explore potential regioselectivity in the hydroboration of substrate 2 (Scheme 4).
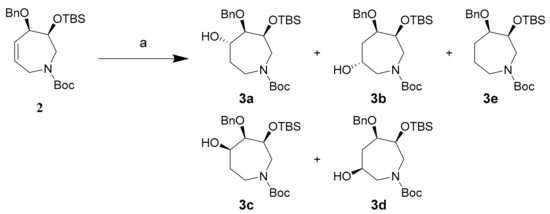
Scheme 4.
Reagent and catalyst screen for the hydroboration of 2. Conditions (a) (i) BHR2, see Table 2, THF; (ii) NaOH, H2O2, EtOH, 80 °C, 1 h, 99%.
Hydroboration of 2 with a variety of common borylating reagents (Table 1) proceeded with poor or no yield of the azepanols (Table 1, Entries 2–7) except for BH3∙SMe2 (Table 1, Entry 1). The use of more-stable borylating reagents, including catechol borane, pinacol borane and 9-BBN, yielded no azepanol product upon oxidation, even when borylation was conducted in refluxing THF. In the case of catechol borane, the addition of Wilkinson’s catalyst (Table 1, Entry 5) resulted exclusively in the formation of hydrogenation product 3e.

Table 1.
Screening of different hydroboration reagents.
Transition-metal catalysts for hydroborations have been shown to exhibit remarkable chemo- [44], regio- [45] and stereoselectivities [44,46,47,48,49] for a variety of acyclic and cyclic substrates [44,50,51,52,53]. We screened a variety of catalysts for the hydroboration of tetrahydroazepine 2 (Table 2). Use of iridium and rhodium catalysts led to the formation of hydrogenation product 3e as the major product (Table 2, Entries 1–3). This suggests the addition of our densely functionalized substrate, 2, to the metal centre is slow, as expected for unreactive olefins [54,55]. Loading of 2 is likely out-competed by diboration of the metal centre with accompanying hydrogen release that leads to formation of 3e in the presence of the metal catalyst [54,55].

Table 2.
Catalyst screen for the hydroboration of 2.
Due to the difficulty in isolating hydrogenation product 3e from starting material 2 by semi-preparative LC, 3e was formed directly by hydrogenation of compound 2. The 1H-NMR spectrum of compound 3e was then compared with crude reaction mixtures (Table 2, Entries 1–2) to verify its formation under catalytic hydroboration conditions (for chromatograms, see Supplementary Materials). Compound 3e was deprotected to yield 4c and characterized by 2D-NMR in the absence of N-Boc rotamers (Table S5).
In the case of PinBH with Rh(COD)(DPPB)BF4, modest conversion to the desired azepanol products was observed with a considerable bias towards the 6R regioisomer 3b while preserving the diastereoselectivity (Table 2, Entry 2). Improved regioselectivity may be accounted for by borylation at the most sterically accessible position, as is observed with other OTBS-protected substrates. This steric effect appears to outweigh any potential directed borylation via the allylic OBn group to the syn face of the substrate. Addition of the Lewis acid additive tris(pentafluorophenyl)borane (FAB), as reported by Lata and Crudden [56], increased the formation of 3e without improving the regioselectivity between 3a and 3b (Table 2, Entry 3). The use of a copper (I) metal centre with TangPhos (Table 2, Entry 4) and bulky (R)-DTBM SegPhos (Table 2, Entry 5) ligands resulted in no formation of any product [57,58].
In contrast to neutral rhodium catalysts for hydroboration, rhodium (I) catalysts exhibit marked solvent-dependent variations in yield, stereo- and regioselectivity for a variety of substrates [52,54,55]. In an attempt to optimize the hydroboration of 2 with PinBH/5 mol % Rh(COD)(DPPB)BF4, the most-active catalyst for the substrate, a solvent screen was conducted (Table 3). In the relatively high-polarity solvent DCE at 60 °C, hydroboration proceeded with good conversion and suppression of the hydrogenation product 3e (Table 3, Entry 1) compared to the same reaction at ambient temperature (Table 2, Entry 2). This reaction also proceeded with comparable diastereoselectivity and an increase in regioselectivity (Table 3, Entry 1, 3a:3b = 1:8) in favour of 3b as the kinetic product compared to the same reaction at ambient temperature (Table 2, Entry 2, 3a:3b = 1:7).

Table 3.
Solvent screen for the hydroboration of 2 using PinBH and 5–10 mol % Rh(COD)(DPPB)BF4.
In THF and toluene, the reaction proceeded with the highest regioselectivity (3a:3b = 1:8, Table 3, Entry 5) in favour of 3b with similarly high conversion upon heating (Table 3, Entries 4 and 6). In these solvents, THF exhibited the least formation of hydrogenation product 3e, while toluene exhibited the most. Considering that DCE and toluene, the most- and least-polar of the solvents screened, respectively, had comparable amounts of hydrogenation product 3e, the formation of 3e does not appear to be dependent on solvent polarity. It may be possible, then, that the ability of THF to coordinate the borane and metal centre precludes diboration of the metal centre and therefore mildly suppresses formation of 3e. In addition, reduction of the catalyst loading from 5–10 mol % to 1 mol % in DCE (Table 3, Entry 2) increased the proportion of 3e formed, suggesting borane addition to the metal centre effectively out-competes alkene addition. In toluene (Table 3, Entry 7), reduction of the catalyst loading in the same manner yielded an 18% decrease in the amount of 3e formed, although conversion was still poor over 18 h. In summary, hydrogenation is competitive with hydroboration of our very sterically congested azepane substrate 2. As a compromise between conversion, regioselectivity and suppression of 3e formation, the optimal conditions for the catalytic hydroboration of 2 with PinBH/5 mol % Rh(COD)(DPPB)BF4 is THF at 60 °C (Table 3, Entry 4). These conditions facilitate nearly complete conversion of substrate 2 to azepanol regioisomers 3a and 3b in a ratio of 1:7, with minimal formation of hydrogenation product 3e.
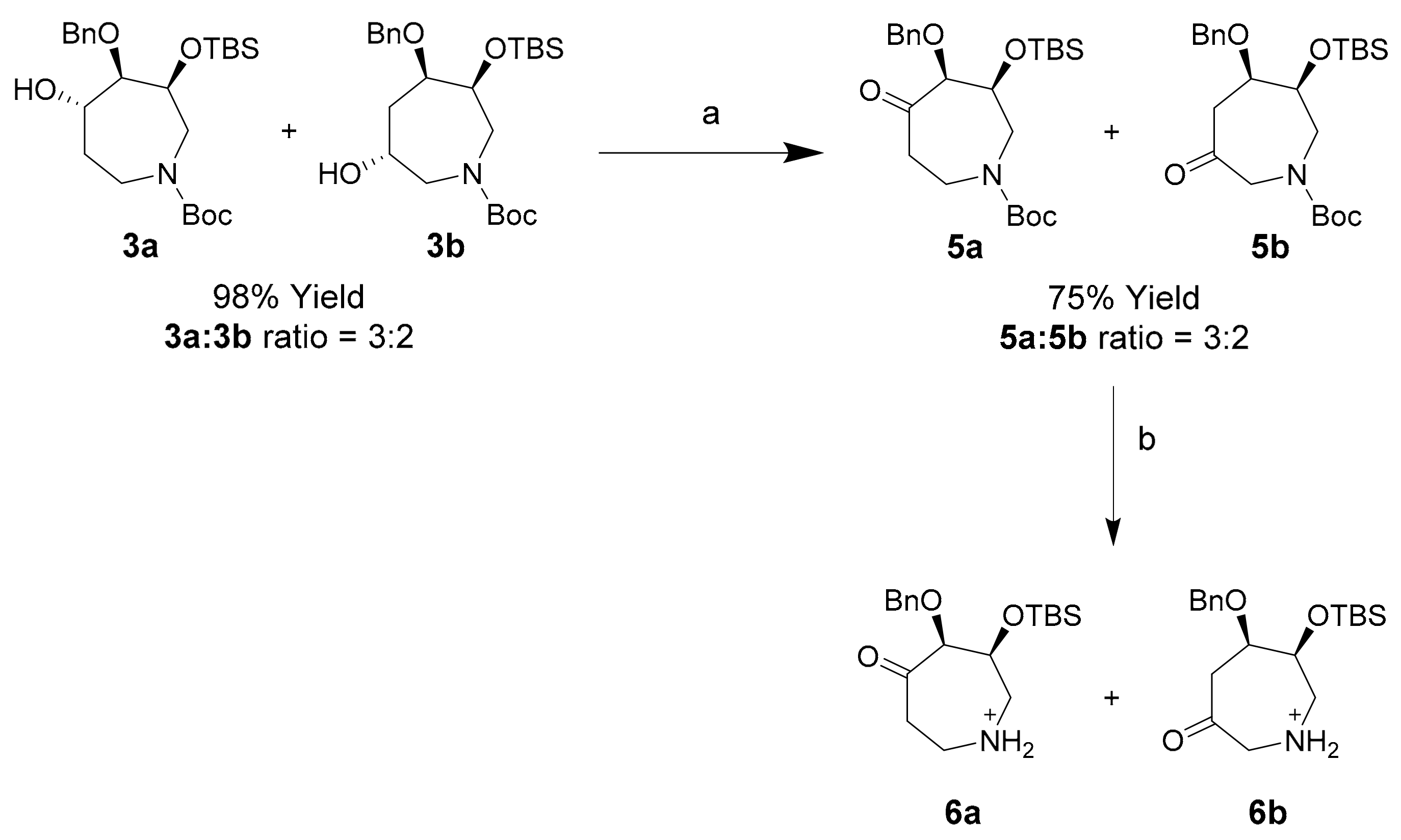
2.6. Synthesis and Structural Assignment of (3R)-OTBS-5/6-Oxo-Azepines 5a and 5b
Oxidation of crude 3a/3b azepanol mix using pyridinium chlorochromate proceeded to completion in 1 h, however in modest yield of oxo-azepines 5a and 5b (Scheme 5). Hydration of these products during work-up resulted in loss during column chromatography on Celite and silica gel. This problem was circumvented using dried 4Å molecular sieves (MS) for work-up, improving the combined isolated yield of 5a and 5b to 75%. N-Boc deprotected derivatives 6a and 6b were characterized by 2D-NMR spectroscopy.
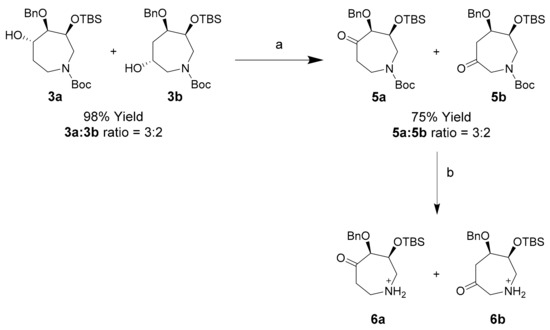
Scheme 5.
Synthesis of oxo-azepines 5a and 5b and their N-Boc deprotected counterparts 6a and 6b. Conditions (a) PCC, DCM, 25 °C, 45 min, 75%; (b) TFA, r.t., 5 min, 99%.
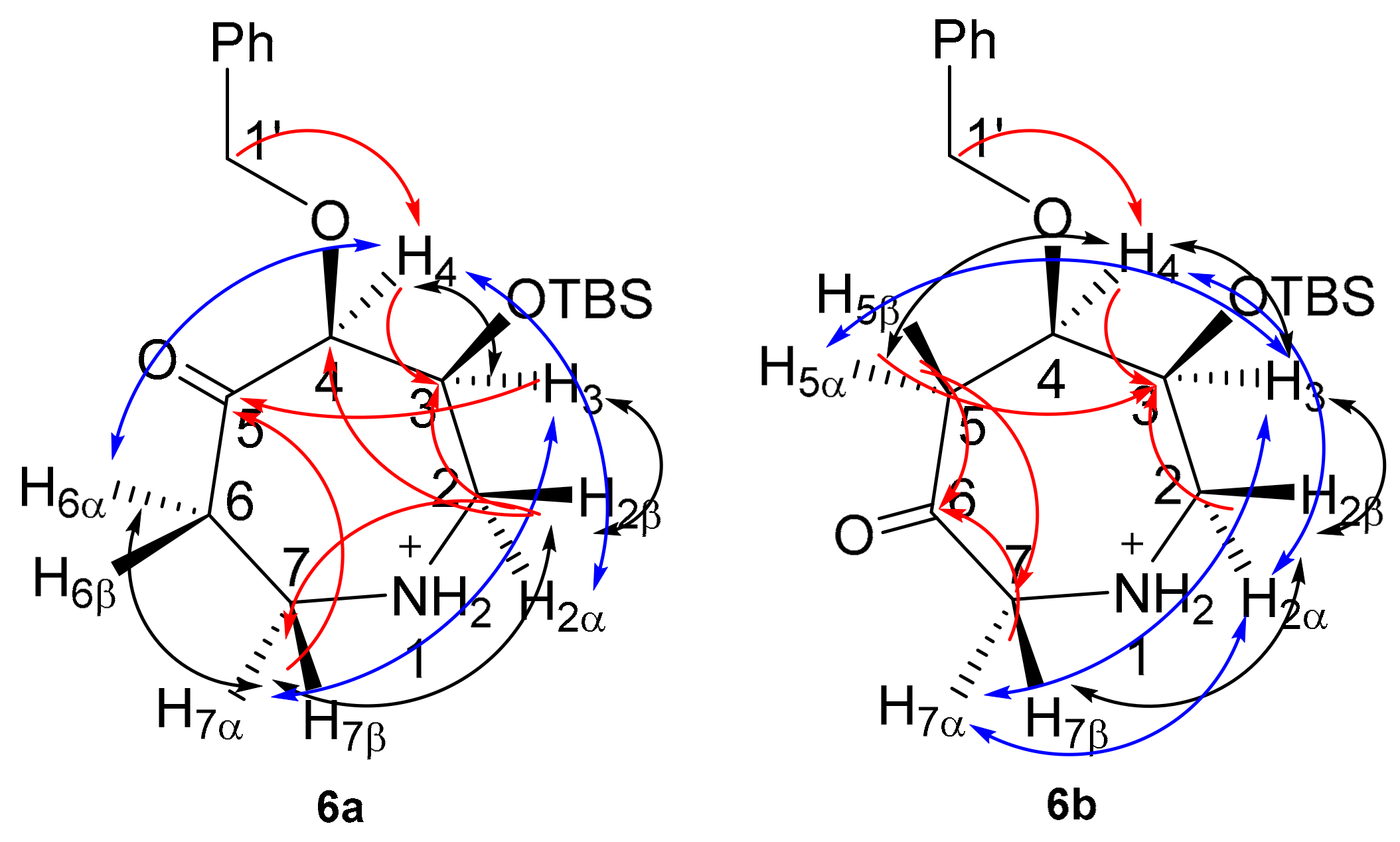
In comparison to azepanols (5S)-4a and (6R)-4b, structural assignment of ketones 6a (Table S6) and 6b (Table S7) is considerably less ambiguous in the absence of the monoalcohol stereocentre. These ketones, with their characteristic carbonyl 13C chemical shift, can be assigned directly from HMBC correlations and indirectly by a combination of COSY and HSQC correlations. In addition, the stereoelectronic bias exerted by the ketone reduces the conformational freedom of the ring, enhancing observed COSY and NOESY correlations (Figure 4).
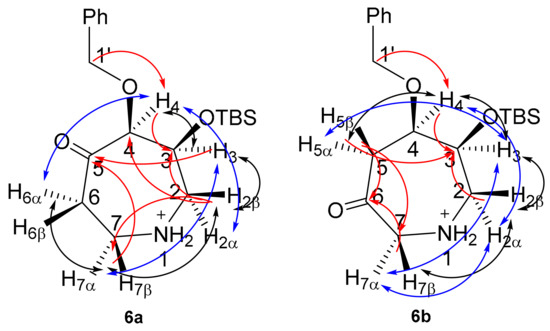
Figure 4.
2D-NMR correlations for monohydroxyl azepanes 6a and 6b. Red, HMBC; black, COSY; blue, NOESY.
For 6a, an HMBC between H1’ (δH 4.47, 4.85 ppm) and C4 (δC 87.2 ppm), combined with an HSQC between C4 and H4 (δH 4.32 ppm), was used to identify C4 (Table S6). In the same manner, COSY and HSQC correlations were used to assign the rest of the azepine ring (Table S6). Strong HMBC correlations from C5 (δC 202.1 ppm) to H3, H7α and H7β were used to identify the ketone at C5.
For 6b, an HMBC between H1’ (δH 4.67 ppm) and C4 (δC 75.5 ppm), combined with an HSQC between C4 and H4 (δH 3.84 ppm), was used to identify C4. COSY correlations and HSQC were then used to assign the rest of the azepine ring (Table S7). A strong 3JCH correlation from H4 to C6 (δC 200.5 ppm) and 2JCH correlations from H5α, H5β, H7α and H7β to C6 were used to identify the ketone at C6. Stereospecific proton assignments were based on nOe correlations (Tables S6 and S7).
3. Materials and Methods
3.1. General Methods
All reagents were purchased from Sigma-Aldrich and used without further purification. Dichloromethane (DCM) and tetrahydrofuran (THF) were distilled from calcium hydride. All reactions were conducted in flame-dried glassware and under nitrogen atmosphere unless stated otherwise. All reactions were magnetically stirred and monitored by thin-layer chromatography (TLC) using Merck silica gel 60 F254 pre-coated plates (0.25 mm). Flash column chromatography was performed using silica gel (0.032–0.063 mm particle size) from Fisher Scientific. All 1H-, 13C- and 2D-NMR experiments were performed on either a Bruker Avance DPX 400 MHz, Bruker Avance III HD NanoBay 400 MHz or DRX600 NMR spectrometer equipped with a TXI (5 mm) cryoprobe. Chemical shifts were reported in ppm using residual CDCl3 (δH; 7.26 ppm, δC; 77.36 ppm), or (CD3)2CO (δH; 2.05 ppm, δC; 29.84 ppm), or CD3OD (δH; 3.31 ppm, δC; 49.00 ppm) as an internal reference. Polarimetry data was collected on a JASCO polarimeter (model no. P-1010). LC–MS was conducted using an Agilent 6130 single-quadrupole LC–MS system. High-resolution mass-spectrometry (HRMS) was conducted using a Thermo-Fisher QExactive-Plus Orbitrap instrument. Infrared spectroscopy was conducted using a Thermo-Fisher Nicolet iS5 FT–IR spectrometer.
3.2. Synthetic Procedures
3.2.1. tert-Butyl(3S,4R)-4-(benzyloxy)-3-((tert-butyldimethylsilyl)oxy)-2,3,4,7-tetrahydro-1H-azepine-1-carboxylate (2)
To a stirred solution of tetrahydroazepanol 1 (0.571 g, 1.8 mmol) in dry DCM (8.9 mL) at −40 °C was added successively 2,6-lutidine (0.497 mL, 4.3 mmol) and tert-butyldimethylsilyl trifluoromethanesulfonate (0.822 mL, 2.1 mmol). The reaction proceeded at the same temperature for 40 min until completion according to TLC before being quenched by the addition of H2O. The organic layer was extracted twice with DCM, dried with MgSO4 and concentrated in vacuo. Crude residue was purified by flash chromatography (petroleum ether/ethyl acetate 9:1) to yield 2 as a clear, light yellow oil (90%). –43 (c 0.19, CHCl3); FTIR (ATR): 2953, 2929, 1697, 1411, 1245, 1169, 1137, 834, 776 cm−1. 1H-NMR (400 MHz, CDCl3): δ 7.37–7.23 (m, 5H), 5.93–5.62 (m, 2H), 4.67–4.54 (m, 2H), 4.28–4.24 (m, 1H), 4.15–4.06 (m, 1H), 3.99–3.81 (m, 2H), 3.73–3.62 (m, 1H), 3.33–3.23 (m, 1H), 1.41 (s, 9H), 0.89 (s, 5H), 0.87 (s, 4H), 0.07–0.06 (m, 6H). 13C-NMR (150 MHz, CDCl3): δ 138.94, 130.93, 129.43, 128.61, 127.88, 127.76, 78.22, 72.26, 71.60, 51.46, 46.29, 28.76, 26.17, 18.52, −4.2418, −4.50. HRMS–ESI: [M + H]+ calcd. for C24H40NO4Si: 434.2727; found 434.2718.
3.2.2. tert-Butyl(3S,4R,5S)-4-(benzyloxy)-3-((tert-butyldimethylsilyl)oxy)-5-hydroxyazepane-1-carboxylate (3a); tert-Butyl(3S,4R,6S)-4-(benzyloxy)-3-((tert-butyldimethylsilyl)oxy)-5-hydroxyazepane-1-carboxylate (3b)
To a solution of silyl ether 2 (142 mg, 0.33 mmol) in THF (316 µL) at 25 °C and under N2 atmosphere was added BH3∙SMe2 (2.0 M in THF, 180 µL, 0.36 mmol) dropwise. The reaction mixture was left to stir at the same temperature for 1 h before sequential addition of ethanol (1.92 mL), 6 M sodium hydroxide (1.44 mL) and 30% H2O2 (0.67 mL), followed by heating to 60 °C for 1 h. The reaction was cooled and quenched by the addition of saturated NH4Cl, after which the layers separated. The aqueous layer was then extracted with ethyl acetate (3 × 5 mL), dried with MgSO4 and rotary evaporated to yield crude alcohols 3a and 3b (136 mg, 92% yield) as a pale yellow oil. Crude product was used for PCC oxidation without purification. In addition, preparative HPLC (mobile phase—acetonitrile:water:TFA from 50:50:0.1 to 80:20:0.1 over 45 min; flow rate–4.18 mL/min) was used to isolate regioisomeric alcohols 3a and 3b as colourless oils with an overall yield of 92% and 3a:3b ratio of 3:2.
(3a) +19 (c 0.02, CHCl3). FTIR (ATR): 2953, 2929, 1671, 1200, 1132, 835, 721 cm−1. 1H-NMR (400 MHz, CDCl3): δ 7.40–7.26 (m, 5H), 4.78–4.70 (m, 1H), 4.55–4.40 (m, 1H), 4.40–4.26 (m, 1H), 4.06–3.96 (m, 1H), 3.80–3.40 (m, 3H), 3.34–3.15 (m, 2H), 2.58 (s, broad, 1H), 2.15–2.00 (m, 1H), 1.70–1.53 (m, 1H), 1.45 (s, 9H), 0.95–0.85 (m, 9H), 0.10–0.05 (m, 6H). 13C-NMR (150 MHz, CDCl3): δ 155.73, 138.05, 128.89, 128.26, 128.19, 86.66, 79.92, 72.86, 69.62, 68.92, 49.46, 41.96, 33.00, 28.79, 26.11, 18.33, −4.29, −4.65. HRMS–ESI: [M + H]+ calcd. for C24H42NO5Si: 452.2832; found 452.2825.
(3b) –25 (c 1, CHCl3). FTIR (ATR): 2951, 2929, 1669, 1413, 1250, 1165, 1074, 836, 776 cm−1. 1H-NMR (400 MHz, CDCl3): δ 7.40–7.25 (m, 5H), 4.71 (d, J = 12.12 Hz, 1H), 4.62 (d, J = 12.08 Hz, 1H), 4.10–3.95 (m, 2H), 3.95–3.73 (m, 4H), 3.47–3.25 (m, 1H), 3.10–2.90 (m, 1H), 2.50–2.30 (m, 1H), 1.62 (s, broad, 1H), 1.47 (s, 9H), 0.91 (s, 9H), 0.08 (s, 6H). 13C-NMR (150 MHz, CDCl3): δ 158.32, 139.28, 128.64, 127.77, 127.67, 80.95, 78.03, 74.72, 73.07, 69.79, 54.87, 51.24, 35.16, 28.74, 26.15, 18.40, −4.36, −4.45. HRMS–ESI: [M + H]+ calcd. for C24H42NO5Si: 452.2832; found 452.2825.
3.2.3. (3R,4R,5S)-4-(Benzyloxy)-3-((tert-butyldimethylsilyl)oxy)-5-hydroxyazepane ((5S)-4a)
Alcohol 3a (5.0 mg, 11 µmol) was dissolved in trifluoroacetic acid (TFA, 500 µL) at 25 °C and stirred for 5 min before evaporation of the TFA under N2 flow. Traces of TFA were then removed under high-vacuum (0.01 torr, 25 °C) for 3 h, yielding alcohol (5S)-4a as a light yellow oil (3.9 mg, quant.). +8 (c 0.12, CHCl3). FTIR (ATR): 2953, 2929, 1671, 1200, 1132, 835, 778 cm−1. 1H-NMR (400 MHz, CDCl3): δ 9.95 (s, broad, 1H), 8.45 (s, broad, 1H), 7.29–7.40 (m, 5H), 4.80 (d, J = 11.7 Hz, 1H), 4.57 (d, J = 11.6 Hz, 1H), 4.42 (dd, J = 7.1, 2.8 Hz, 1H), 4.01 (ddd, J = 6.8, 6.8, 4.4 Hz, 1H), 3.58 (dd, J = 6.8, 1.3 Hz, 1H), 3.32 (m, 1H), 3.29 (m, 2H), 3.28 (s, broad, 1H), 3.16 (m, 1H), 2.26 (dddd, J = 15.9, 8.1, 4.3, 3.6 Hz, 1H), 1.92 (dddd, J = 15.8, 9.0, 6.5, 2.6 Hz, 1H), 0.89 (s, 9H), 0.11 (s, 3H), 0.09 (s, 3H). 13C-NMR (150 MHz, CDCl3): δ 137.78, 128.98, 128.50, 128.23, 85.62, 73.97, 68.38, 67.41, 47.24, 41.39, 29.60, 25.99, 18.24, −4.50, −4.84. HRMS–ESI: [M]+ calcd. for C19H34NO3Si: 352.2308; found 352.2300.
3.2.4. (3R,4R,6S)-4-(Benzyloxy)-3-((tert-butyldimethylsilyl)oxy)-5-hydroxyazepane ((6R)-4b)
Alcohol 3b (5.0 mg, 11 µmol) was N-Boc deprotected as above for 3a, yielding alcohol (6R)-4b as a pale yellow oil (3.9 mg, quant.). +8 (c 0.07, CHCl3); FTIR (ATR): 2953, 2929, 1672, 1199, 1132, 835, 778 cm−1. 1H-NMR (400 MHz, CDCl3): δ 9.50 (s, broad, 1H), 8.42 (s, broad, 1H), 7.37–7.25 (m, 5H), 4.53 (d, J = 11.8 Hz, 1H), 4.48 (d, J = 11.8 Hz, 1H), 4.26 (m, 1H), 4.21 (m, 1H), 3.84 (dd, J = 9.5, 2.3 Hz, 1H), 3.41 (m, 2H), 3.33 (dd, J = 13.7, 3.3 Hz, 1H), 3.17 (dd, J = 13.3, 5.5 Hz, 1H), 2.26 (dq, J = 14.4, 4.8 Hz, 1H), 2.01 (dt, J = 14.5, 3.8 Hz, 1H), 0.89 (s, 9H), 0.08–0.06 (m, 6H). 13C-NMR (150 MHz, CDCl3): δ 138.37, 128.75, 128.06, 127.85, 77.48, 72.50, 71.48, 63.69, 50.63, 48.73, 34.63, 26.01, 18.26, −4.37, −4.94. HRMS–ESI: [M + H]+ calcd. for C19H34NO3Si: 352.2308; found 352.2300.
3.2.5. tert-Butyl (3S,4S)-4-(benzyloxy)-3-((tert-butyldimethylsilyl)oxy)-5-oxo-azepine-1-carboxylate (5a); tert-Butyl (3S,4S)-4-(benzyloxy)-3-((tert-butyldimethylsilyl)oxy)-6-oxo-azepine-1-carboxylate (5b)
Pyridinium chlorochromate (PCC, 130 mg, 0.60 mmol) was added to a well-stirred suspension of alcohol diastereomers 3a and 3b (91 mg, 0.20 mmol) and 4 Å MS (137 mg) in dry DCM (10 mL) under N2 atmosphere at 25 °C. The reaction mixture was then stirred for 1 h followed by passage through a plug of dry 4 Å MS and flash column chromatography (petroleum ether/ethyl acetate 9:1) to yield pure fractions of 5a (28.5 mg) and 5b (40.2 mg) in 75% overall yield.
(5a) +11 (c 1, CHCl3); FTIR (ATR): 2955, 2929, 1696, 1406, 1159, 1093, 834, 776 cm−1. 1H-NMR (400 MHz, CDCl3): δ 7.40–7.25 (m, 5H), 4.73–4.66 (m, 2H), 4.15–4.05 (m, 1H), 3.95–3.60 (m, 4H), 3.45–3.35 (m, 1H), 2.85–2.55 (m, 2H), 1.43 (s, 9H), 0.91–0.87 (m, 9H), 0.14–0.06 (m, 6H). 13C-NMR (150 MHz, CDCl3): δ 209.71, 154.90, 137.92, 128.83, 128.82, 128.00, 90.98, 80.38, 74.23, 72.36, 50.51, 42.65, 41.84, 28.61, 26.06, 18.35, −4.56, −4.66. HRMS–ESI: [M+H]+ calcd. for C24H40NO5Si: 450.2676; found 450.2669.
(5b) +32 (c 1, CHCl3); FTIR (ATR): 2954, 2929, 1697, 1409, 1155, 1109, 1079, 835, 775 cm−1. 1H-NMR (400 MHz, CDCl3): δ 7.40–7.20 (m, 5H), 4.80–4.60 (m, 2H), 4.60–4.20 (m, 1H), 4.10–3.70 (m, 3H), 3.60–3.10 (m, 2H), 2.81 (dd, J = 14.36, 8.16 Hz, 1H), 2.6 (dd, J = 21.09, 13.93 Hz, 1H), 1.50 (s, 5H), 1.43 (s, 4H), 0.93 (s, 9H), 0.20–0.08 (m, 6H). 13C-NMR (150 MHz, CDCl3): δ 208.93, 155.58, 138.42, 128.66, 127.95, 127.64, 81.28, 76.79, 74.55, 73.06, 59.13, 49.84, 43.74, 28.85, 26.12, 18.35, −4.34, −4.58. HRMS–ESI: [M + H]+ calcd. for C24H40NO5Si: 450.2676; found 450.2668.
3.2.6. (3S,4S)-4-(Benzyloxy)-3-((tert-butyldimethylsilyl)oxy)-5-oxoazepan-1-ium (6a)
Ketone 6a (5.0 mg, 11 µmol) was produced as above for (5S)-4a and was isolated as a pale light yellow powder (3.9 mg, 95%). +10 (c 1, CHCl3); FTIR (ATR): 2953, 2930, 1672, 1171, 1132, 835, 781 cm−1. 1H-NMR (400 MHz, CDCl3): δ 7.38–7.26 (m, 5H), 4.84 (d, J = 11.68 Hz, 1H), 4.47 (d, J = 11.68 Hz, 1H), 4.32 (s, broad, 1H), 4.30 (m, 1H), 3.74–3.60 (m, 1 H), 3.50 (dd, J = 13.62, 5.98 Hz, 1H), 3.45–3.20 (m, 2H), 2.79 (t, J = 4.46, 2H), 0.85 (s, 9H), 0.09 (s, 3H), 0.08 (s, 3H). 13C-NMR (150 MHz, CDCl3): δ 202.10, 137.19, 128.93, 128.28, 128.09, 87.18, 69.93, 51.33, 41.99, 38.29, 25.93, 25.84, 18.24, −4.63, −4.95. HRMS–ESI: [M + H]+ calcd. for C19H32NO3Si: 350.2152; found 350.2142.
3.2.7. (3S,4S)-4-(Benzyloxy)-3-((tert-butyldimethylsilyl)oxy)-6-oxoazepan-1-ium (6b)
Ketone 6b (5.0 mg, 11 µmol) was produced as above for (5S)-4a and was isolated as a pale light yellow oil (3.9 mg, 95%). +25 (c 1, CHCl3); FTIR (ATR): 2955, 2929, 1676, 1655, 1175, 1136, 836, 778 cm−1. 1H-NMR (400 MHz, CDCl3): δ 7.37–7.26 (m, 5H), 4.67 (s, broad, 2H), 4.32 (m, 1H), 3.84 (m, 1H), 3.68 (d, J = 17.92 Hz, 2H), 3.43 (dd, J = 13.20, 9.53 Hz, 1H), 3.33 (dd, J = 13.20, 3.99 Hz, 1H), 3.13 (dd, J = 15.26, 8.22 Hz, 1H), 3.03 (dd, J = 15.68, 0.60 Hz, 1H), 0.90 (s, 9H), 0.10 (s, 3H), 0.09 (s, 3H). 13C-NMR (150 MHz, CDCl3): δ 200.46, 137.45, 128.88, 128.42, 128.11, 75.48, 73.21, 71.68, 56.42, 49.13, 43.51, 25.92, 18.25, −4.64, −4.96. HRMS–ESI: [M + H]+ calcd. for C19H32NO3Si: 350.2152; found 350.2142.
3.2.8. tert-Butyl (3S,4R)-4-(benzyloxy)-3-((tert-butyldimethylsilyl)oxy)azepane-1-carboxylate (3e)
To a solution of tetrahydroazepine 2 (75 mg, 170 µmol) in methanol (350 µL) was added 5% w/w Pd/C (2 mg, 1 µmol, 0.5 mol %). The suspension was then stirred under H2 (1 atm) for 16 h until completion as confirmed by TLC. Catalyst was removed by passage through a plug of Celite with methanol rinsing. Solvent was then removed in vacuo to yield azepane 9 as a colourless oil (36 mg, 48%). −200 (c 0.14, CHCl3); FTIR (ATR): 2929, 2856, 1691, 1165, 1082, 834, 774 cm−1. 1H-NMR (400 MHz, CDCl3): 7.40–7.23 (m, 5H), 4.70–4.55 (m, 2H), 3.93 (m, 0.5H), 3.87–3.54 (m, 3.5H), 3.20–3.00 (m, 2H), 2.05–1.94 (m, 1H), 1.85–1.69 (m, 1.5H), 1.68–1.57 (m, 1H), 1.56–1.38 (m, 9.5H), 0.95–0.88 (m, 9H), 0.13–0.05 (m, 6H). δ 13C-NMR (150 MHz, CDCl3): δ 155.57, 139.54, 128.57, 127.65, 127.58, 80.39, 74.51, 72.30, 72.19, 50.06, 46.76, 28.89, 26.20, 26.20, 23.43, 18.44, −4.26, −4.38. HRMS–ESI: [M + H]+ calcd. for C24H42NO4Si: 436.2883; found 436.2875.
3.2.9. (3S,4R)-4-(Benzyloxy)-3-((tert-butyldimethylsilyl)oxy)azepan-1-ium (4c)
Azepane 3e (5.0 mg, 11 µmol) was N-Boc deprotected as above for 3a, yielding 4c as a colourless oil (3.9 mg, quant.). −84 (c 0.03, CHCl3); FTIR (ATR): 2930, 2857, 1674, 1199, 1131, 834, 778 cm−1. 1H-NMR (400 MHz, CDCl3): δ 10.12 (s, broad, 1H), 8.07 (s, broad, 1H), 7.40–7.26 (m, 5H), 4.67–4.51 (m, 2H), 4.22 (m, 1H), 3.66–3.52 (m, 1H), 3.32 (m, 2H), 3.16 (m, 2H), 1.98 (m, 2H), 1.80 (m, 2H), 0.89 (s, 9H), 0.086 (m, 6H). 13C-NMR (150 MHz, CDCl3): δ 138.32, 128.79, 128.14, 127.94, 81.05, 72.42, 71.06, 47.28, 45.94, 26.71, 26.03, 20.97, 18.32, −4.41, −4.84. HRMS–ESI: [M + H]+ calcd. for C19H34NO2Si: 336.2359; found 336.2348.
4. Conclusions
Overall, we have shown here that the late-stage oxidation of tetrahydroazepine 2 constitutes an efficient way to access novel oxo-azepines. Hydroboration of 2 with BH3∙SMe2 yielded azepanols 3a and 3b in a 3:2 product ratio and, following oxidation, afforded regioisomeric ketones 5a and 5b. Separation of these ketones was readily achieved to provide 5a and 5b in 29% and 19% overall yield, respectively. Regioselectivity can be improved by using additives such as Rh(COD)(DPPB)BF4, albeit with competitive hydrogenation (Table 3, Entry 4), to access ketones 5a and 5b in a 1:7 product ratio, over 8 steps and in 30% overall yield for 5b. Azepanols 3a/b and oxo-azepines 5a/b are versatile synthons and, following complete deprotection, useful epitopes in the construction of novel azepane-based biological agents. As a point of comparison, the work of Spiegel et al. highlighted the synthetic utility of their C6 ketone vi in the concise synthesis of glucosepane via a sigmatropic rearrangement and cyclization sequence in a total of 13 steps and 12% overall yield from diacetone-d-glucose (Scheme 1b). Ketones 5a and 5b structurally resemble compound vi as trisubstituted oxo-azepines bearing a protected vicinal dihydroxyl motif with syn stereochemistry at C3 and C4. The availability of a more-efficient route may assist in future biological investigations focused on glucosepanes.
Supplementary Materials
Supplementary materials are available online. 2D-NMR spectral data for compounds 4a/b, 6a/b and 4c; LC–MS data for key metal-catalyzed hydroboration reactions.
Acknowledgments
This research was supported by an APA scholarship to H.S. from Macquarie University. We would like to thank Erika Davies for assistance with 2D-NMR spectroscopy.
Author Contributions
H.S. and F.L. conceived and designed the experiments; H.S. performed the experiments and H.S., P.K. and F.L. analyzed the data. P.K. performed the DFT study and analyzed the data. H.S., P.K. and F.L. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bouquet, J.; Bleriot, Y.; Desire, J.; King, D.T.; Vocadlo, D.J.; Vadlamani, G.; Benzie, G.R.; Mark, B.L.; Iorga, B.; Ide, D.; et al. Selective Trihydroxylated Azepane Inhibitors of NagZ, a Glycosidase Involved in Pseudomonas aeruginosa Resistance to β-Lactam Antibiotics. Org. Biomol. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Basak, R.K.; Vankar, Y.D. Synthesis and Comparative Study of Homoisofagomines and Analogues as Glycosidase Inhibitors. Eur. J. Org. Chem. 2014, 2014, 844–859. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Favre, S.; Vogel, P.; Sollogoub, M.; Bleriot, Y. Synthesis of Branched Seven-Membered 1-N-Imino-Sugars and Their Evaluation as Glycosidase Inhibitors. Carbohydr. Res. 2012, 356, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Kalamkar, N.B.; Kasture, V.M.; Chavan, S.T.; Sabharwal, S.G.; Dhavale, D.D. Concise and Practical Route to Tri- and Tetra-Hydroxy Seven-Membered Imino-Cyclitols as Glycosidase Inhibitors from D-(+)-Glucurono-γ-Lactone. Tetrahedron 2010, 66, 8522–8526. [Google Scholar] [CrossRef]
- Shih, T.-L.; Yang, R.-Y.; Li, S.-T.; Chiang, C.-F.; Lin, C.-H. Expeditious Synthesis of Tri- and Tetrahydroxyazepanes from D-(-)-Quinic Acid as Potent Glycosidase Inhibitors. J. Org. Chem. 2007, 72, 4258–4261. [Google Scholar] [CrossRef] [PubMed]
- Parker, L.J.; Watanabe, H.; Tsuganezawa, K.; Tomabechi, Y.; Handa, N.; Shirouzu, M.; Yuki, H.; Honma, T.; Ogawa, N.; Nagano, T. Flexibility of the P-Loop of Pim-1 Kinase: Observation of a Novel Conformation Induced by Interaction With an Inhibitor. Acta Crystallogr. Sec. F Struct. Biol. Cryst. Commun. 2012, 68, 860–866. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, D.S.; Marquis, R.W.; Xie, R.; Nidamarthy, S.D.; Oh, H.-J.; Jeong, J.U.; Erhard, K.F.; Ward, K.W.; Roethke, T.J.; Smith, B.R. Structure Activity Relationships of 5-, 6-, and 7-Methyl-Substituted Azepan-3-one Cathepsin K Inhibitors. J. Med. Chem. 2006, 49, 1597–1612. [Google Scholar] [CrossRef] [PubMed]
- Breitenlechner, C.B.; Wegge, T.; Berillon, L.; Graul, K.; Marzenell, K.; Friebe, W.-G.; Thomas, U.; Schumacher, R.; Huber, R.; Engh, R.A. Structure-Based Optimization of Novel Azepane Derivatives as PKB Inhibitors. J. Med. Chem. 2004, 47, 1375–1390. [Google Scholar] [CrossRef] [PubMed]
- Cipolla, L.; Araújo, A.C.; Airoldi, C.; Bini, D. Pyrrolo [2,1-c][1,4] Benzodiazepine as a Scaffold for the Design and Synthesis of Anti-Tumour Drugs. Anti Cancer Agents Med. Chem. Former. Curr. Med. Chem. Anti Cancer Agents 2009, 9, 1–31. [Google Scholar] [CrossRef]
- Rajanarendar, E.; Reddy, M.N.; Krishna, S.R.; Reddy, K.G.; Reddy, Y.; Rajam, M. Design, Synthesis, in vitro Antimicrobial and Anticancer Activity of Novel Methylenebis-isoxazolo[4,5-b]azepines Derivatives. Eur. J. Med. Chem. 2012, 50, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Smid, P.; Mlinaric, M.; Koehler, K.F.; Nunez Garcia, S.; Wegener, E.; Lange, J.H.M. Spiro Azepane-Oxazolidinones as Kv1.3 Potassium Channel Blockers. US8575148B2, 18 October 2010. [Google Scholar]
- Uchikawa, O.; Sakai, N.; Terao, Y.; Suzuki, H. Preparation of Fused Heterocyclic Compounds as Apoptosis Signal Regulating Kinase 1 (ASK1) inhibitors. WO2008016131A1, 7 February 2008. [Google Scholar]
- Anderson, K.W.; Fotouhi, N.; Gillespie, P.; Goodnow, R.A., Jr.; Guertin, K.R.; Haynes, N.-E.; Myers, M.P.; Pietranico-Cole, S.L.; Qi, L.; Rossman, P.L.; et al. Preparation of Pyrazole-4-Carboxamide Derivatives as 11-β-Hydroxysteroid Dehydrogenase Form I (11-β-HSD1) Inhibitors. WO2007107470A2, 27 September 2007. [Google Scholar]
- Brummerhop, H.; Frick, W.; Glombik, H.; Plettenburg, O.; Bickel, M.; Heuer, H.; Theis, S. Synthesis of Fluoro-Glycoside Derivatives of Pyrazoles for Use in Treatment of Diabetes or For Lowering Blood Sugar Levels. WO2005121161A1, 22 December 2005. [Google Scholar]
- Ammenn, J.; Gillig, J.R.; Heinz, L.J.; Hipskind, P.A.; Kinnick, M.D.; Lai, Y.-S.; Morin, J.M., Jr.; Nixon, J.A.; Ott, C.; Savin, K.A.; et al. Preparation of 1,3,4-Oxadiazoles and Related Compounds for Use as Melanin Concentrating Hormone Antagonists in the Treatment of Obesity and Diabetes. WO2003097047A1, 27 November 2003. [Google Scholar]
- Hartman, G.D.; Kuduk, S. Preparation of Azepane Derivatives and Methods of Treating Hepatitis B Infections. WO2015109130A1, 23 July 2015. [Google Scholar]
- Wilson, F.X.; Nash, R.J.; Horne, G.; Storer, R.; Tinsley, J.M.; Roach, A.G. Compounds, Including Alkaloids and Iminosugars, for the Treatment of Flaviviral Infections. WO2010015815A2, 26 August 2010. [Google Scholar]
- Núñez-Villanueva, D.; Infantes, L.; García-López, M.T.; González-Muñiz, R.; Martín-Martínez, M. Azepane Quaternary Amino Acids As Effective Inducers of 310 Helix Conformations. J. Org. Chem. 2012, 77, 9833–9839. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Reddy, D.R.; Reddy, P.M.M. Synthesis and DNA-Binding Ability of Pyrrolo[2,1-c][1,4]benzodiazepine-Azepane Conjugates. Bioorg. Med. Chem. Lett. 2006, 16, 1160–1163. [Google Scholar] [CrossRef] [PubMed]
- Johnson, H.A.; Thomas, N.R. Polyhydroxylated Azepanes as New Motifs for DNA Minor Groove Binding Agents. Bioorg. Med. Chem. Lett. 2002, 12, 237–241. [Google Scholar] [CrossRef]
- Psarra, V.; Fousteris, M.A.; Hennig, L.; Bantzi, M.; Giannis, A.; Nikolaropoulos, S.S. Identification of Azepinone Fused Tetracyclic Heterocycles as New Chemotypes with Protein Kinase Inhibitory Activities. Tetrahedron 2016, 72, 2376–2385. [Google Scholar] [CrossRef]
- Pellegrino, S.; Bonetti, A.; Clerici, F.; Contini, A.; Moretto, A.; Soave, R.; Gelmi, M.L. 1H-Azepine-2-oxo-5-amino-5-carboxylic Acid: A 310 Helix Inducer and an Effective Tool for Functionalized Gold Nanoparticles. J. Org. Chem. 2015, 80, 5507–5516. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, S.; Contini, A.; Clerici, F.; Gori, A.; Nava, D.; Gelmi, M.L. 1H-Azepine-4-amino-4-carboxylic Acid: A New α,α-Disubstituted Ornithine Analogue Capable of Inducing Helix Conformations in Short Ala-Aib Pentapeptides. Chem. Eur. J. 2012, 18, 8705–8715. [Google Scholar] [CrossRef] [PubMed]
- Draghici, C.; Wang, T.; Spiegel, D.A. Concise Total Synthesis of Glucosepane. Science 2015, 350, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Andersen, S.M.; Ekhart, C.; Lundt, I.; Stutz, A.E. Syntheses of Sugar-Related Trihydroxyazepanes from Simple Carbohydrates and Their Activities as Reversible Glycosidase Inhibitors. Carbohydr. Res. 2000, 326, 22–33. [Google Scholar] [CrossRef]
- Shih, T.-L.; Liang, M.-T.; Wu, K.-D.; Lin, C.-H. Synthesis of polyhydroxy 7- and N-alkyl-azepanes as potent glycosidase inhibitors. Carbohydr. Res. 2011, 346, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Otero, J.M.; Estevez, A.M.; Soengas, R.G.; Estevez, J.C.; Nash, R.J.; Fleet, G.W.J.; Estevez, R.J. Studies on the Transformation of Nitro-Sugars into Branched Chain Imino-Sugars. Part II: Synthesis of (3R,4R,5R,6S)-2,2-bis(hydroxymethyl)azepane-3,4,5,6-tetraol. Tetrahedron Asymmetry 2008, 19, 2443–2446. [Google Scholar] [CrossRef]
- Dhavale, D.D.; Markad, S.D.; Karanjule, N.S.; PrakashaReddy, J. Asymmetric Dihydroxylation of D-Glucose Derived α,β-Unsaturated Ester: Synthesis of Azepane and Nojirimycin Analogues. J. Org. Chem. 2004, 69, 4760–4766. [Google Scholar] [CrossRef] [PubMed]
- Dhavale, D.D.; Chaudhari, V.D.; Tilekar, J.N. An Expeditious Synthesis of a (3S,4S,5R)-Trihydroxyazepane. Tetrahedron Lett. 2003, 44, 7321–7323. [Google Scholar] [CrossRef]
- Zamoner, L.O.B.; Aragao-Leoneti, V.; Mantoani, S.P.; Rugen, M.D.; Nepogodiev, S.A.; Field, R.A.; Carvalho, I. CuAAC Click Chemistry with N-Propargyl-1,5-dideoxy-1,5-imino-d-gulitol and N-propargyl 1,6-dideoxy-1,6-imino-d-mannitol Provides Access to Triazole-Linked Piperidine and Azepane Pseudo-Disaccharide Iminosugars Displaying Glycosidase Inhibitory Properties. Carbohydr. Res. 2016, 429, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Adams, C.; Fairway, S.; Hardy, C.; Hibbs, D.; Hursthouse, M.; Morley, A.; Sharp, B.; Vicker, N.; Warner, I. Total Synthesis of Balanol: A Potent Protein Kinase C Inhibitor of Fungal Origin. J. Chem. Soc. Perkin Trans. 1995, 2355–2362. [Google Scholar] [CrossRef]
- Fürstner, A.; Thiel, O.R. Formal Total Synthesis of (-)-Balanol: Concise Approach to the Hexahydroazepine Segment Based on RCM. J. Org. Chem. 2000, 65, 1738–1742. [Google Scholar] [CrossRef] [PubMed]
- Goumain, S.; Taghzouti, H.; Portella, C.; Behr, J.-B.; Plantier-Royon, R. Convenient Strategy for the Synthesis of Highly Functionalizable Hydroxylated Unsaturated Azepanes. Tetrahedron Lett. 2012, 53, 4440–4443. [Google Scholar] [CrossRef]
- Li, H.; Bleriot, Y.; Chantereau, C.; Mallet, J.-M.; Sollogoub, M.; Zhang, Y.; Rodriguez-Garcia, E.; Vogel, P.; Jimenez-Barbero, J.; Sinay, P. The First Synthesis of Substituted Azepanes Mimicking Monosaccharides: A New Class of Potent Glycosidase Inhibitors. Org. Biomol. Chem. 2004, 2, 1492–1499. [Google Scholar] [CrossRef] [PubMed]
- Moutel, S.; Shipman, M.; Martin, O.R.; Ikeda, K.; Asano, N. Synthesis of a Trihydroxylated Azepane from D-Arabinose By Way of an Intramolecular Alkene Nitrone Cycloaddition. Tetrahedron Asymmetry 2005, 16, 487–491. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Vogel, P.; Sinay, P.; Bleriot, Y. Tandem Staudinger-azaWittig Mediated Ring Expansion: Rapid Access to New Isofagomine-Tetrahydroxyazepane Hybrids. Chem. Commun. 2007, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Ball, G.; Hunter, L.; Liu, F. Conformational Regulation of Substituted Azepanes Through Selective Monofluorination. Org. Biomol. Chem. 2013, 11, 3781–3785. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Hunter, L.; Bhadbhade, M.M.; Liu, F. Conformational Regulation of Substituted Azepanes through Mono-, Di-, and Trifluorination. Eur. J. Org. Chem. 2014, 2014, 2584–2593. [Google Scholar] [CrossRef]
- Patel, A.R.; Liu, F. Diastereospecific Fluorination of Substituted Azepanes. Tetrahedron 2013, 69, 744–752. [Google Scholar] [CrossRef]
- Corey, E.; Cho, H.; Rücker, C.; Hua, D.H. Studies with Trialkylsilyltriflates: New Syntheses and Applications. Tetrahedron Lett. 1981, 22, 3455–3458. [Google Scholar] [CrossRef]
- Corey, E.; Venkateswarlu, A. Protection of Hydroxyl Groups as tert-Butyldimethylsilyl Derivatives. J. Am. Chem. Soc. 1972, 94, 6190–6191. [Google Scholar] [CrossRef]
- Molecular Operating Environment (MOE); 2016.08; Chemical Computing Group Inc.: Montreal, QC, Canada, 2016.
- TURBOMOLE, V7.1; University of Karlsruhe and Forschungszentrum Karlsruhe GmbH: Karlsruhe, Germany, 2016.
- Männig, D.; Nöth, H. Catalytic Hydroboration with Rhodium Complexes. Angew. Chem. Int. Ed. Engl. 1985, 24, 878–879. [Google Scholar] [CrossRef]
- Evans, D.A.; Fu, G.C.; Hoveyda, A.H. Rhodium (I)-Catalyzed Hydroboration of Olefins. The Documentation of Regio-and Stereochemical Control in Cyclic and Acyclic systems. J. Am. Chem. Soc. 1988, 110, 6917–6918. [Google Scholar] [CrossRef]
- Westcott, S.A.; Blom, H.P.; Marder, T.B.; Baker, R.T. New Homogeneous Rhodium Catalysts for the Regioselective Hydroboration of Alkenes. J. Am. Chem. Soc. 1992, 114, 8863–8869. [Google Scholar] [CrossRef]
- Evans, D.A.; Fu, G.C.; Hoveyda, A.H. Rhodium (I)-and Iridium (I)-Catalyzed Hydroboration Reactions: Scope and Synthetic Applications. J. Am. Chem. Soc. 1992, 114, 6671–6679. [Google Scholar] [CrossRef]
- Burgess, K.; Cassidy, J.; Ohlmeyer, M.J. Substrate-Controlled Diastereoselectivities in Catalyzed and Uncatalyzed Hydroborations of Acyclic Allylic Alcohol Derivatives: Secondary Orbital Effects Involving d.π-p.π. Interactions. J. Org. Chem. 1991, 56, 1020–1027. [Google Scholar] [CrossRef]
- Burgess, K.; Ohlmeyer, M.J. Diastereocontrol in Rhodium-Catalyzed Hydroboration of Chiral Acyclic Allylic Alcohol Derivatives. Tetrahedron Lett. 1989, 30, 395–398. [Google Scholar] [CrossRef]
- Harder, S.; Spielmann, J. Calcium-Mediated Hydroboration of Alkenes: “Trojan horse” or “True” Catalysis? J. Organomet. Chem. 2012, 698, 7–14. [Google Scholar] [CrossRef]
- Crudden, C.M.; Edwards, D. Catalytic Asymmetric Hydroboration: Recent Advances and Applications in Carbon-Carbon Bond-Forming Reactions. Eur. J. Org. Chem. 2003, 2003, 4695–4712. [Google Scholar] [CrossRef]
- Beletskaya, I.; Pelter, A. Hydroborations Catalysed by Transition Metal Complexes. Tetrahedron 1997, 53, 4957–5026. [Google Scholar] [CrossRef]
- Burgess, K.; Ohlmeyer, M.J. Transition-Metal Promoted Hydroborations of Alkenes, Emerging Methodology for Organic Transformations. Chem. Rev. 1991, 91, 1179–1191. [Google Scholar] [CrossRef]
- Carroll, A.-M.; O’Sullivan, T.P.; Guiry, P.J. The Development of Enantioselective Rhodium-Catalyzed Hydroboration of Olefins. Adv. Synth. Catal. 2005, 347, 609–631. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Fujikawa, R.; Umemoto, T.; Miyaura, N. Iridium-Catalyzed Hydroboration of Alkenes with Pinacolborane. Tetrahedron 2004, 60, 10695–10700. [Google Scholar] [CrossRef]
- Lata, C.J.; Crudden, C.M. Dramatic Effect of Lewis Acids on the Rhodium-Catalyzed Hydroboration of Olefins. J. Am. Chem. Soc. 2009, 132, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Noh, D.; Chea, H.; Ju, J.; Yun, J. Highly Regio-and Enantioselective Copper-Catalyzed Hydroboration of Styrenes. Angew. Chem. Int. Ed. Engl. 2009, 48, 6062–6064. [Google Scholar] [CrossRef] [PubMed]
- Noh, D.; Yoon, S.K.; Won, J.; Lee, J.Y.; Yun, J. An Efficient Copper (I)-Catalyst System for the Asymmetric Hydroboration of β-Substituted Vinylarenes with Pinacolborane. Chem. Asian J. 2011, 6, 1967–1969. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).