Semisynthesis, an Anti-Inflammatory Effect of Derivatives of 1β-Hydroxy Alantolactone from Inula britannica
Abstract
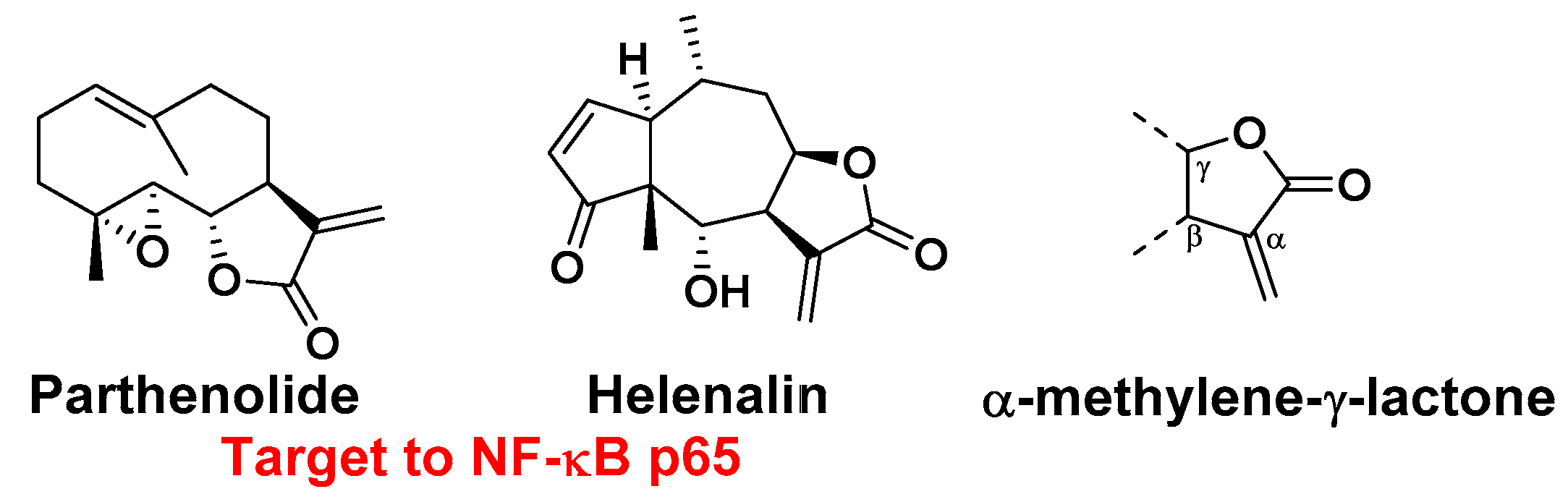
:1. Introduction
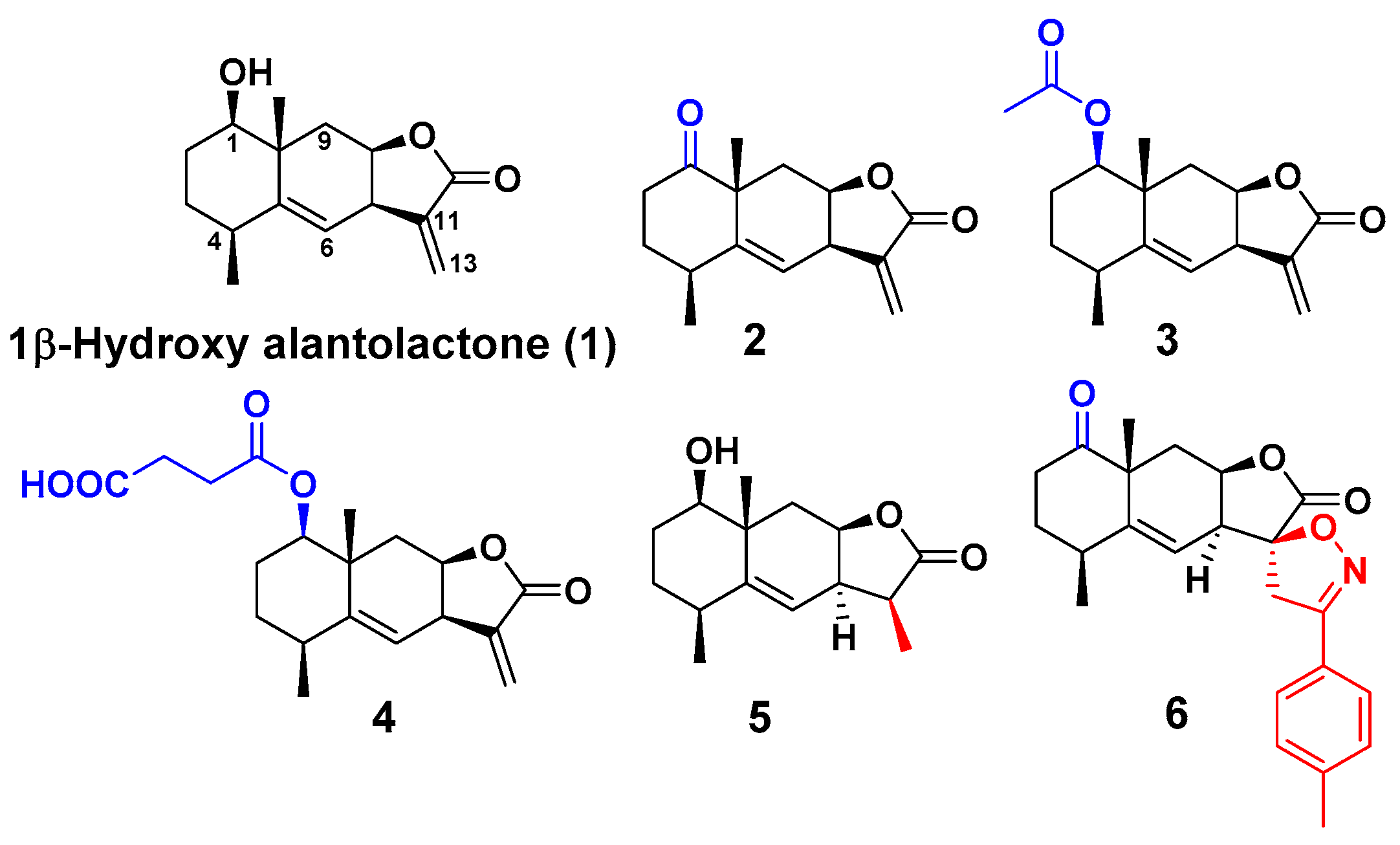
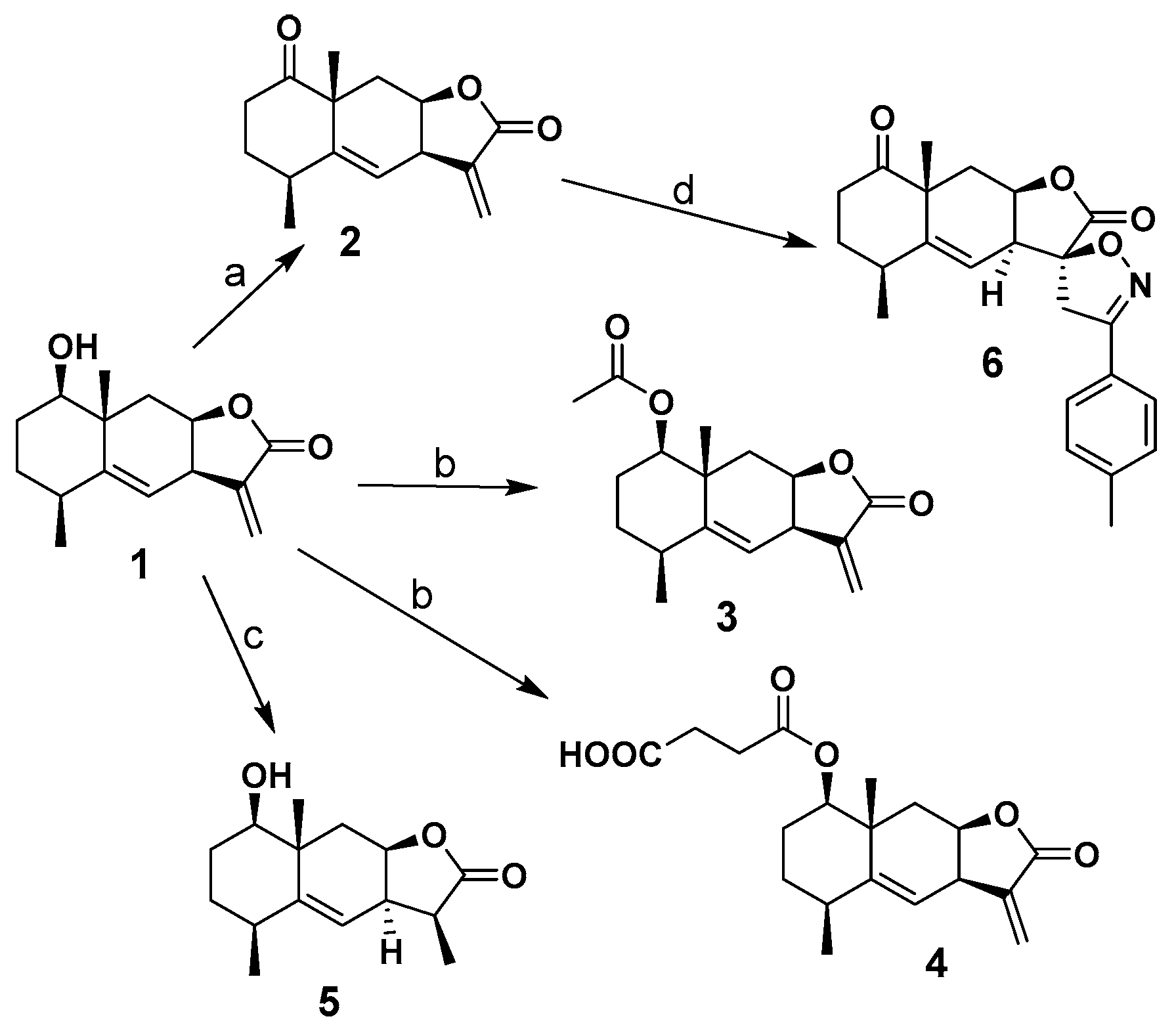
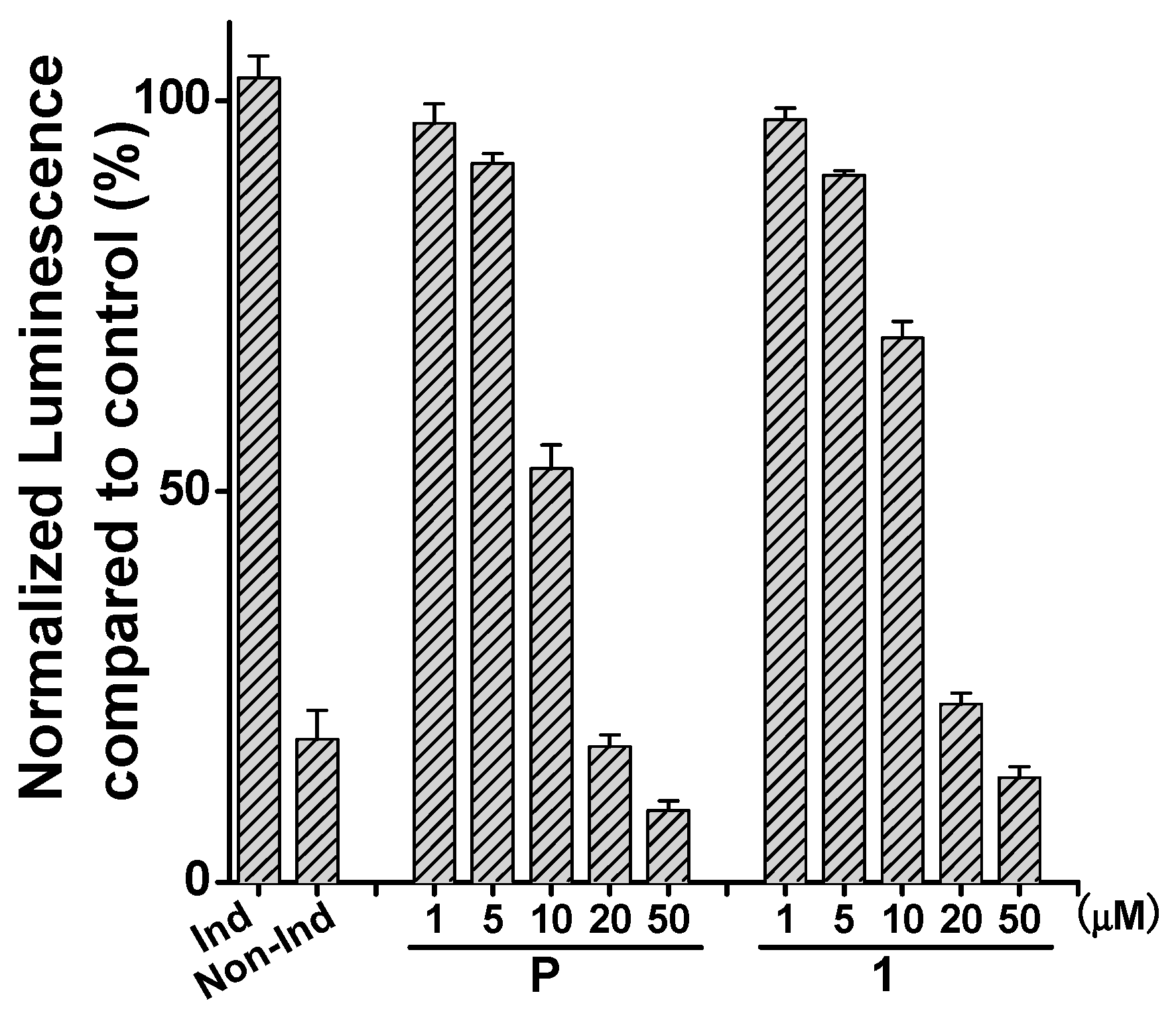
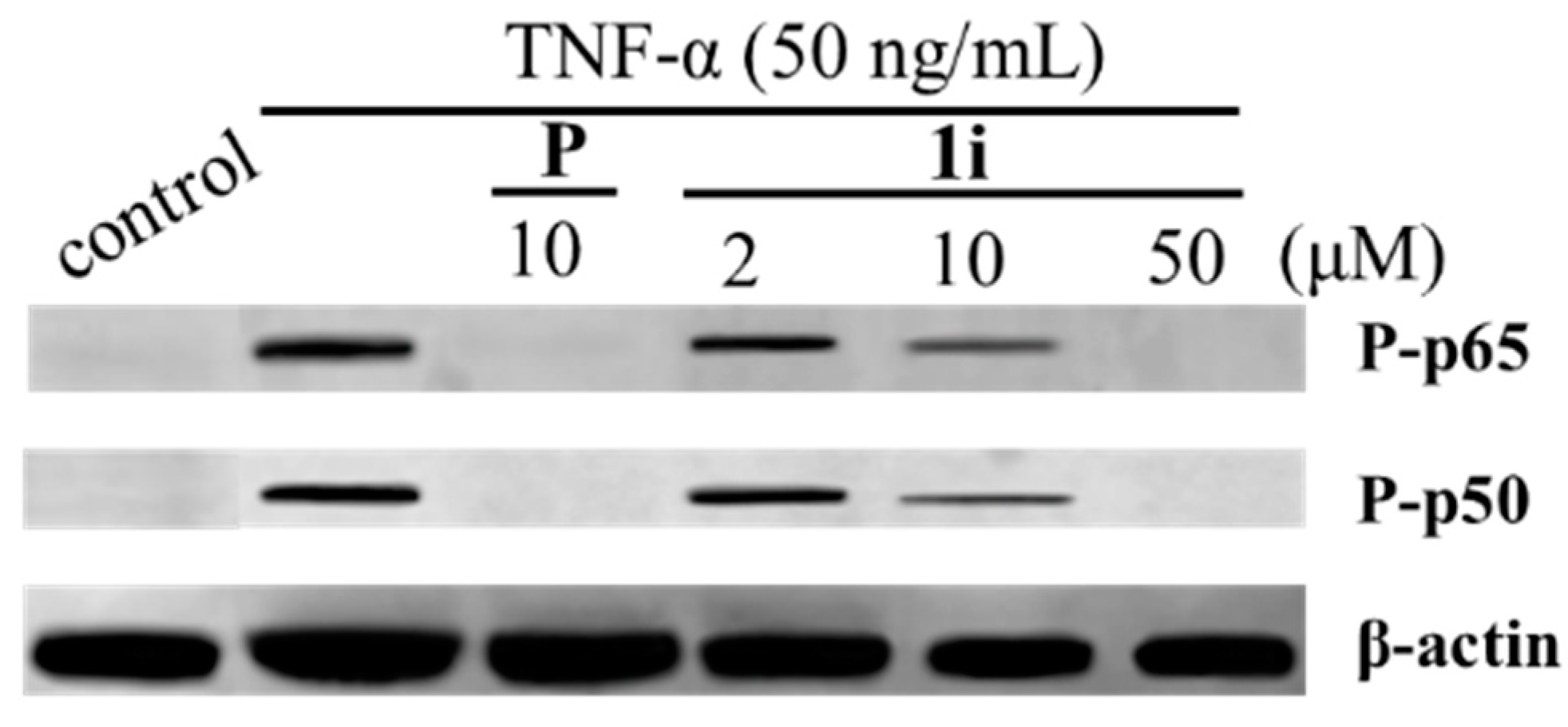
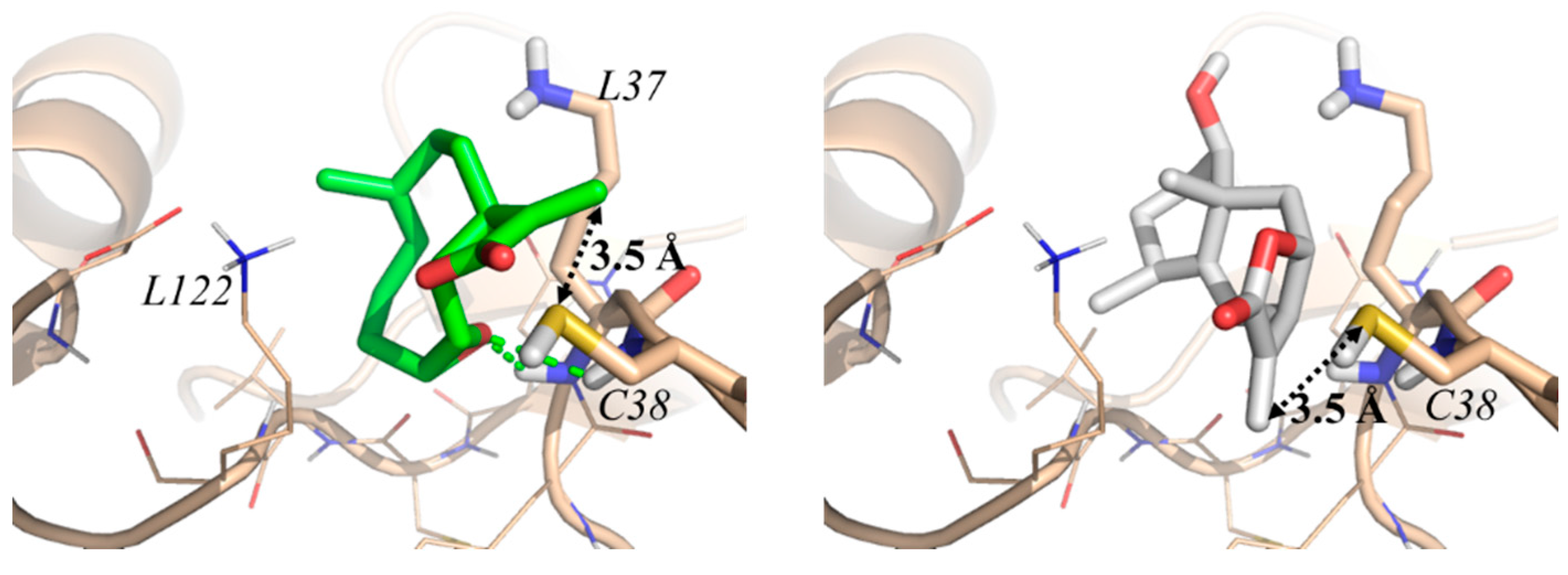
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Extraction and Isolation from Plant Material
3.3. Synthesis of Derivatives
3.4. Cell Culture
3.5. Measurement of NO Production in RAW264.7 Macrophages
3.6. Luciferase NF-κB Reporter Assay
3.7. Western Blotting Analysis
3.8. Molecular Modeling
3.9. Statistics Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, G.W.; Qin, J.J.; Cheng, X.R.; Shen, Y.H.; Shan, L.; Jin, H.Z.; Zhang, W.D. Inula sesquiterpenoids: Structural diversity, cytotoxicity and anti-tumor activity. Expert Opin. Investig. Drugs 2014, 23, 317–345. [Google Scholar] [CrossRef] [PubMed]
- Amorim, M.H.; Gil da Costa, R.M.; Lopes, C.; Bastos, M.M. Sesquiterpene lactones: Adverse health effects and toxicity mechanisms. Crit. Rev. Toxicol. 2013, 43, 559–579. [Google Scholar] [CrossRef] [PubMed]
- Ghantous, A.; Gali-Muhtasib, H.; Vuorela, H.; Saliba, N.A.; Darwiche, N. What made sesquiterpene lactones reach cancer clinical trials? Drug Discov. Today 2010, 15, 668–678. [Google Scholar] [CrossRef] [PubMed]
- Tanasova, M.; Sturla, S.J. Chemistry and biology of acylfulvenes: Sesquiterpene-derived antitumor agents. Chem. Rev. 2012, 112, 3578–3610. [Google Scholar] [CrossRef] [PubMed]
- Merfort, I. Perspectives on sesquiterpene lactones in inflammation and cancer. Curr. Drug Targets 2011, 12, 1560–1573. [Google Scholar] [CrossRef] [PubMed]
- Muangphrom, P.; Seki, H.; Fukushima, E.O.; Muranaka, T. Artemisinin-based antimalarial research: Application of biotechnology to the production of artemisinin, its mode of action, and the mechanism of resistance of plasmodium parasites. J. Nat. Med. 2016, 70, 318–334. [Google Scholar] [CrossRef] [PubMed]
- Zaima, K.; Wakana, D.; Demizu, Y.; Kumeta, Y.; Kamakura, H.; Maruyama, T.; Kurihara, M.; Goda, Y. Isoheleproline: A new amino acid-sesquiterpene adduct from Inula helenium. J. Nat. Med. 2014, 68, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Janecka, A.; Wyrebska, A.; Gach, K.; Fichna, J.; Janecki, T. Natural and synthetic alpha-methylenelactones and alpha-methylenelactams with anticancer potential. Drug Discov. Today 2012, 17, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Aliyu, A.B.; Abdullahi, H.; Solomon, T.; Toko, E.; Garba, A.; Bashir, M.; Habila, N. Lactone-rich fraction from vernonia blumeoides: Antitrypanosomal activity and alleviation of the parasite-induced anemia and organ damage. J. Nat. Med. 2013, 67, 750–757. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, P.; Schultze, N.; Niehs, S.; Albrecht, D.; Methling, K.; Wurster, M.; Wachlin, G.; Lalk, M.; Lindequist, U.; Haertel, B. Differential effects of helenalin, an anti-inflammatory sesquiterpene lactone, on the proteome, metabolome and the oxidative stress response in several immune cell types. Toxicol. In Vitro 2017, 40, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, M.; Trewin, H.; Gawthrop, F.; Wagstaff, C. Sesquiterpenoids lactones: Benefits to plants and people. Int. J. Mol. Sci. 2013, 14, 12780–12805. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Fu, J. Nf-kappab and cancer: A paradigm of yin-yang. Am. J. Cancer Res. 2011, 1, 192–221. [Google Scholar] [PubMed]
- Widen, J.C.; Kempema, A.M.; Villalta, P.W.; Harki, D.A. Targeting nf-kappab p65 with a helenalin inspired bis-electrophile. ACS Chem. Biol. 2017, 12, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pineres, A.J.; Lindenmeyer, M.T.; Merfort, I. Role of cysteine residues of p65/NF-kappaB on the inhibition by the sesquiterpene lactone parthenolide and N-ethyl maleimide, and on its transactivating potential. Life Sci. 2004, 75, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Seca, A.M.L.; Grigore, A.; Pinto, D.C.G.A.; Silva, A.M.S. The genus Inula and their metabolites: From ethnopharmacological to medicinal uses. J. Ethnopharmacol. 2014, 154, 286–310. [Google Scholar] [CrossRef] [PubMed]
- Gong, H.Q.; Wu, Q.X.; Liu, L.L.; Yang, J.L.; Wang, R.; Shi, Y.P. Sesquiterpenoids from the aerial parts of Inula japonica. Helv. Chim. Acta 2011, 94, 1269–1276. [Google Scholar] [CrossRef]
- Xiang, P.; Guo, X.; Han, Y.Y.; Gao, J.M.; Tang, J.J. Cytotoxic and pro-apoptotic activities of sesquiterpene lactones from Inula britannica. Nat. Prod. Commun. 2016, 11, 7–10. [Google Scholar] [PubMed]
- Ward, J.; Li, L.; Regan, F.; Deasy, M.; Kelleher, F. Bis(spirolactam)1,3-double-armed calix[4]arene compounds and their application as extractants for the determination of heavy metal ions. J. Incl. Phenom. Macrocycl. Chem. 2015, 83, 377–386. [Google Scholar] [CrossRef]
- Liu, J.G.; Tian, Y.Y.; Shi, J.L.; Zhang, S.S.; Cai, Q. An enantioselective synthesis of spirobilactams through copper-catalyzed intramolecular double N-arylation and phase separation. Angew. Chem. Int. Ed. 2015, 54, 10917–10920. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Song, S.S.; Shu, S.Q.; Miao, Z.H.; Zhang, A.; Ding, C.Y. Novel spirobicyclic artemisinin analogues (artemalogues): Synthesis and antitumor activities. Eur. J. Med. Chem. 2015, 103, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, M.; Prachyawarakorn, V.; Aree, T.; Wiyakrutta, S.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. Tricyclic and spirobicyclic norsesquiterpenes from the endophytic fungus Pseudolagarobasidium acaciicola. Eur. J. Org. Chem. 2014, 3976–3980. [Google Scholar] [CrossRef]
- Wang, S.S.; Shi, Y.; Tian, W.S. Highly efficient and scalable synthesis of clionamine d. Org. Lett. 2014, 16, 2177–2179. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, C.M.; Jia, Z.J. Sesquiterpenes and other constituents from the aerial parts of Inula japonica. Planta Med. 2003, 69, 662–666. [Google Scholar] [PubMed]
- Qin, J.-J.; Jin, H.-Z.; Zhu, J.-X.; Fu, J.-J.; Zeng, Q.; Cheng, X.-R.; Zhu, Y.; Shan, L.; Zhang, S.-D.; Pan, Y.-X.; et al. New sesquiterpenes from Inula japonica thunb. With their inhibitory activities against LPS-induced no production in raw264.7 macrophages. Tetrahedron 2010, 66, 9379–9388. [Google Scholar] [CrossRef]
- Duarte, J.; Francisco, V.; Perez-Vizcaino, F. Modulation of nitric oxide by flavonoids. Food Funct. 2014, 5, 1653–1668. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Tang, J.-J.; Zhang, C.-C.; Tian, J.-M.; Guo, J.-T.; Zhang, Q.; Li, H.; Gao, J.-M. Semisynthesis and in vitro cytotoxic evaluation of new analogues of 1-o-acetylbritannilactone, a sesquiterpene from Inula britannica. Eur. J. Med. Chem. 2014, 80, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chi, B.; Wang, W.W.; Gao, J.M.; Wan, J. Exploring the possible binding mode of trisubstituted benzimidazoles analogues in silico for novel drug designtargeting Mtb FtsZ. Med. Chem. Res. 2017, 26, 153–169. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |
| Compound | IC50 (μM) | |
|---|---|---|
| NO Inhibition 1 | Cytotoxicity 2 | |
| 1 | 5.61 ± 0.34 | >50 |
| 2 | 36.1 ± 3.8 | 34.5 ± 5.8 |
| 3 | 46.5 ± 5.6 | >50 |
| 4 | 39.6 ± 5.7 | >50 |
| 5 | >1000 | ND 4 |
| 6 | >1000 | ND |
| Aminoguanidine | 10.9 ± 1.5 3 | ND |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhang, J.-P.; Liu, X.; Tang, J.-J.; Xiang, P.; Ma, X.-M. Semisynthesis, an Anti-Inflammatory Effect of Derivatives of 1β-Hydroxy Alantolactone from Inula britannica. Molecules 2017, 22, 1835. https://doi.org/10.3390/molecules22111835
Chen L, Zhang J-P, Liu X, Tang J-J, Xiang P, Ma X-M. Semisynthesis, an Anti-Inflammatory Effect of Derivatives of 1β-Hydroxy Alantolactone from Inula britannica. Molecules. 2017; 22(11):1835. https://doi.org/10.3390/molecules22111835
Chicago/Turabian StyleChen, Lin, Jian-Ping Zhang, Xin Liu, Jiang-Jiang Tang, Ping Xiang, and Xing-Ming Ma. 2017. "Semisynthesis, an Anti-Inflammatory Effect of Derivatives of 1β-Hydroxy Alantolactone from Inula britannica" Molecules 22, no. 11: 1835. https://doi.org/10.3390/molecules22111835
APA StyleChen, L., Zhang, J.-P., Liu, X., Tang, J.-J., Xiang, P., & Ma, X.-M. (2017). Semisynthesis, an Anti-Inflammatory Effect of Derivatives of 1β-Hydroxy Alantolactone from Inula britannica. Molecules, 22(11), 1835. https://doi.org/10.3390/molecules22111835






