Residue Dynamics and Risk Assessment of Prochloraz and Its Metabolite 2,4,6-Trichlorophenol in Apple
Abstract
1. Introduction
2. Results and Discussion
2.1. Validation of Derivatization Percent
2.2. Recovery Study
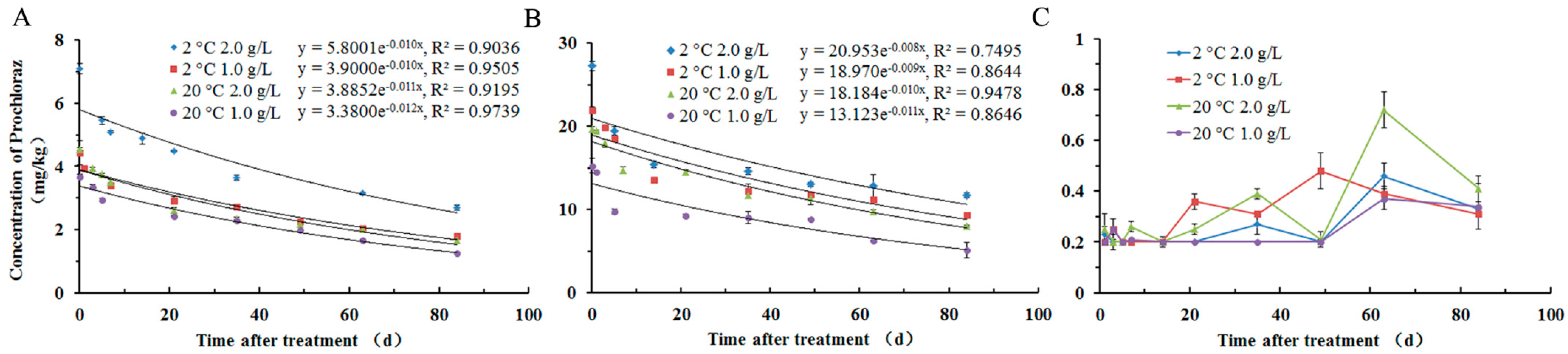
2.3. Residue Dynamics of Prochloraz in Apple
2.4. Residue Dynamics of 2,4,6-TCP in Apple
2.5. Determination of Prochloraz Residue of Apple Samples in Hefei Market
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Postharvest Treatment and Storage
3.3. Sample Extraction, Clean up, and Gas Chromatograpy (GC) Analysis
3.3.1. Sample Preparation
3.3.2. Extraction and Derivatization Process for Prochloraz Determination
3.3.3. Sample Treatment for 2,4,6-TCP Determination
3.3.4. GC Condition
3.4. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Guo, Y.; Zhou, Z.; Yuan, Y.; Yue, T. Survey of patulin in apple juice concentrates in shaanxi (china) and its dietary intake. Food Control 2013, 34, 570–573. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R.H. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Dobrowolskaiwanek, J.; Gąstoł, M.; Adamska, A.; Krośniak, M.; Zagrodzki, P. Traditional versus modern apple cultivars–a comparison of juice composition. Folia Hortic. 2015, 27, 33–41. [Google Scholar]
- Boyer, J.; Liu, R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004, 3, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Verdu, C.F.; Gatto, J.; Freuze, I.; Richomme, P.; Laurens, F.; Guilet, D. Comparison of two methods, UHPLC-UV and UHPLC-MS/MS, for the quantification of polyphenols in cider apple juices. Molecules 2013, 18, 10213–10227. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, J.; Cheng, S. Heavy metals in apple orchard soils and fruits and their health risks in liaodong peninsula, northeast china. Environ. Monit. Assess. 2015, 187, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mateo, E.M.; Valle-Algarra, F.M.; Jiménez, M.; Magan, N. Impact of three sterol-biosynthesis inhibitors on growth of fusarium langsethiae, and on t-2 and ht-2 toxin production in oat grain under different ecological conditions. Food Control 2013, 34, 521–529. [Google Scholar] [CrossRef]
- Lundqvist, J.; Hellman, B.; Oskarsson, A. Fungicide prochloraz induces oxidative stress and dna damage in vitro. Food Chem. Toxicol. 2016, 91, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Kastanias, M.A.; Chrysayi-Tokousbalides, M.; Coward, S.; Philippoussis, A.; Diamantopoulou, P. Residue evaluation of the azole fungicides prochloraz and tebuconazole in the white mushroom Agaricus bisporus. Bull. Environ. Contam. Toxicol. 2006, 77, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Dang, K.T.; Singh, Z.; Swinny, E.E. Impact of postharvest disease control methods and cold storage on volatiles, color development and fruit quality in ripe ‘kensington pride’ mangoes. J. Agric. Food Chem. 2008, 56, 10667–10674. [Google Scholar] [CrossRef] [PubMed]
- Menezes Filho, A.; dos Santos, F.N.; Pereira, P.A. Development, validation and application of a methodology based on solid-phase micro extraction followed by gas chromatography coupled to mass spectrometry (SPME/GC-MS) for the determination of pesticide residues in mangoes. Talanta 2010, 81, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Liu, S.; Hua, X.; Xia, F.; Tian, D.; Zhou, C. Highly sensitive detection of 2,4,6-trichlorophenol based on hs-β-cyclodextrin/gold nanoparticles composites modified indium tin oxide electrode. Electrochim. Acta 2015, 167, 372–378. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Hou, C.; Du, S.; Ren, Y.; Yang, Z.; Xu, Q.; Hu, X. A sub-nanomole level electrochemical method for determination of prochloraz and its metabolites based on medical stone doped disposable electrode. Talanta 2010, 83, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Yong, X.U.; Shou, L.; Miao, Y.U.; Xu, Z.N. Uplc-ms/ms method for determination of paclobutrazol, forchlorfenuron and prochloraz residues in fruits. Chin. J. Pestic. Sci. 2012, 14, 61–66. [Google Scholar]
- Blasco, C.; Font, G.; Jordi Mañes, A.; Picó, Y. Solid-phase microextraction liquid chromatography/tandem mass spectrometry to determine postharvest fungicides in fruits. Anal. Chem. 2003, 75, 3606–3615. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xiang, B.R.; Dong, Y.; Xu, J.P. Rapid determination of prochloraz in orange juice by near-infrared spectroscopy. Anal. Lett. 2013, 46, 2739–2751. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, Y.; Xiang, B.; Xu, J. Feasibility research on rapid detection of prochloraz in green tea soft drink by near-infrared spectroscopy. Food Anal. Method. 2015, 8, 343–351. [Google Scholar] [CrossRef]
- Cengiz, M.F.; Catal, M.; Erler, F.; Bilgin, A.K. Rapid and sensitive determination of the prochloraz residues in the cultivated mushroom, agaricus bisporus (lange) imbach. Anal. Methods 2014, 6, 1970–1976. [Google Scholar] [CrossRef]
- Li, T.X.; Wang, Y.J.; Liu, B.; Gao, X.W. Residual detection and degradation dynamics of prochloraz in ya pear. J. South China Agric. Univ. 2010, 31, 36–39. [Google Scholar]
- Zheng, X.; Xie, D. Residual dynamics of 45% prochloraz in storage banana. Chin. J. Trop. Crops 2012, 33, 2273–2278. [Google Scholar]
- Yuan, X.Y.; Peng, Z.; Zhou, H.P.; Liu, C. Degradation dynamics and safety evaluation of prochloraz in banana. J. Food Saf. Q. 2016, 7, 209–214. [Google Scholar]
- Luo, F.J.; Lou, Z.Y.; Tang, F.B.; Zhang, X.Z.; Liu, G.M.; Chen, Z.M. Residue determination and dynamic research of prochloraz and it’s metabolite in orange. J. Instrum. Anal. 2010, 29, 730–734. [Google Scholar]
- Zhao, Y.; Kang, S.; Zhou, L.I.; Luo, J.; Pan, C. Decay and residue dynamics of 25% prochloraz ec in mandarin orange by simulating postharvest treatment at different storage temperatures. J. Food Process. Preserv. 2013, 37, 496–502. [Google Scholar] [CrossRef]
- Xie, L.; Luo, T.; Rangwei, X.; Cheng, Y. Dynamic analyses of prochloraz and imazalil residues in citrus during fruit storage. J. Huazhong Agric. Univ. 2016, 35, 17–23. [Google Scholar]
- Wang, Y.; Wang, C.; Gao, J.; Cui, L.; Lv, Y.B. Residual dynamics and safety evaluation of prochloraz in ginseng and soil. J. Northeast Agric. Univ. 2014, 45, 25–30. [Google Scholar]
- GB 2763-2014. National Standard of the People’s Republic of China: National Food Safety Standard Maximum Residue Limits for Pesticides in Food; Standards Press of China: Beijing, China, 2014. [Google Scholar]
- Chen, L.; ShangGuan, L.M.; Wu, Y.N.; Xu, L.J.; Fu, F.F. Study on the residue and degradation of fluorine-containing pesticides in oolong tea by using gas chromatography–mass spectrometry. Food Control 2012, 25, 433–440. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, X.; Luo, J.; Wu, Z.; Wang, L.; Li, B.; Wang, Y.; Sun, G. Degradation dynamics of glyphosate in different types of citrus orchard soils in China. Molecules 2015, 20, 1161–1175. [Google Scholar] [CrossRef] [PubMed]
- European Communities Regulation 396/2005 on Pesticide Regulation. 2005. Available online: http://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/public/?event=pesticide.residue.CurrentMRL&language=EN (accessed on 17 October 2017).
Sample Availability: Samples of the compounds prochloraz and 2,4,6-TCP are available from the authors. |

| Concentration (mg·kg−1) | Derivatization Percent (%) | Average Percent (%) | RSD a (%) |
|---|---|---|---|
| 0.1 | 94.9 | 101.0 | 4.2 |
| 0.2 | 106.7 | ||
| 0.8 | 102.2 | ||
| 1.0 | 100.2 |
| Analyte | Sample | Spiked Level (mg·kg−1) | Recovery (%) | CV a (%) |
|---|---|---|---|---|
| Prochloraz | apple fruit | 0.2 | 103.4 | 2.85 |
| 1.0 | 109.2 | 3.49 | ||
| 2.0 | 113.7 | 7.00 | ||
| apple pulp | 0.2 | 114.4 | 1.55 | |
| 1.0 | 111.9 | 4.46 | ||
| 2.0 | 82.9 | 5.71 | ||
| apple peel | 0.25 | 93.9 | 8.61 | |
| 1.0 | 85.1 | 5.07 | ||
| 5.0 | 87.4 | 3.52 | ||
| 2,4,6-TCP | apple fruit | 0.02 | 85.0 | 5.19 |
| 0.2 | 89.8 | 0.70 | ||
| 2.0 | 87.8 | 0.65 | ||
| apple pulp | 0.02 | 109.1 | 1.56 | |
| 0.2 | 84.7 | 2.14 | ||
| 2.0 | 86.7 | 4.59 |
| Sample | Treatment | Equation | T1/2 (d) a | R2 b |
|---|---|---|---|---|
| Apple fruit | 2 °C 2.0 g/L | C = 5.8001 × e−0.010t | 69.3 | 0.9036 |
| 2 °C 1.0 g/L | C = 3.9000 × e−0.010t | 69.3 | 0.9505 | |
| 20 °C 2.0 g/L | C = 3.8852 × e−0.011t | 63.0 | 0.9195 | |
| 20 °C 1.0 g/L | C = 3.3800 × e−0.012t | 57.8 | 0.9739 | |
| Apple peel | 2 °C 2.0 g/L | C = 20.953 × e−0.008t | 86.6 | 0.7495 |
| 2 °C 1.0 g/L | C = 18.970 × e−0.009t | 77.0 | 0.8644 | |
| 20 °C 2.0 g/L | C = 18.184 × e−0.010t | 69.3 | 0.9478 | |
| 20 °C 1.0 g/L | C = 13.123 × e−0.011t | 63.0 | 0.8646 |
| Number | Measured ± SD (mg·kg−1) | Number | Measured ± SD (mg·kg−1) | Number | Measured ± SD (mg·kg−1) |
|---|---|---|---|---|---|
| 1 | <LOD a | 8 | <LOD | 15 | <LOD |
| 2 | <LOD | 9 | <LOD | 16 | 0.86 ± 0.03 |
| 3 | 0.86 ± 0.01 | 10 | 0.49 ± 0.02 | 17 | <LOD |
| 4 | <LOD | 11 | 0.33 ± 0.02 | 18 | <LOD |
| 5 | 0.23 ± 0.01 | 12 | 0.65 ± 0.05 | 19 | <LOD |
| 6 | 0.63 ± 0.03 | 13 | <LOD | 20 | <LOD |
| 7 | <LOD | 14 | <LOD |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Q.; Yao, G.; Shi, Y.; Ding, C.; Wang, Y.; Wu, X.; Hua, R.; Cao, H. Residue Dynamics and Risk Assessment of Prochloraz and Its Metabolite 2,4,6-Trichlorophenol in Apple. Molecules 2017, 22, 1780. https://doi.org/10.3390/molecules22101780
Fang Q, Yao G, Shi Y, Ding C, Wang Y, Wu X, Hua R, Cao H. Residue Dynamics and Risk Assessment of Prochloraz and Its Metabolite 2,4,6-Trichlorophenol in Apple. Molecules. 2017; 22(10):1780. https://doi.org/10.3390/molecules22101780
Chicago/Turabian StyleFang, Qingkui, Gengyou Yao, Yanhong Shi, Chenchun Ding, Yi Wang, Xiangwei Wu, Rimao Hua, and Haiqun Cao. 2017. "Residue Dynamics and Risk Assessment of Prochloraz and Its Metabolite 2,4,6-Trichlorophenol in Apple" Molecules 22, no. 10: 1780. https://doi.org/10.3390/molecules22101780
APA StyleFang, Q., Yao, G., Shi, Y., Ding, C., Wang, Y., Wu, X., Hua, R., & Cao, H. (2017). Residue Dynamics and Risk Assessment of Prochloraz and Its Metabolite 2,4,6-Trichlorophenol in Apple. Molecules, 22(10), 1780. https://doi.org/10.3390/molecules22101780







