Discovery of Novel N-Substituted Prolinamido Indazoles as Potent Rho Kinase Inhibitors and Vasorelaxation Agents
Abstract
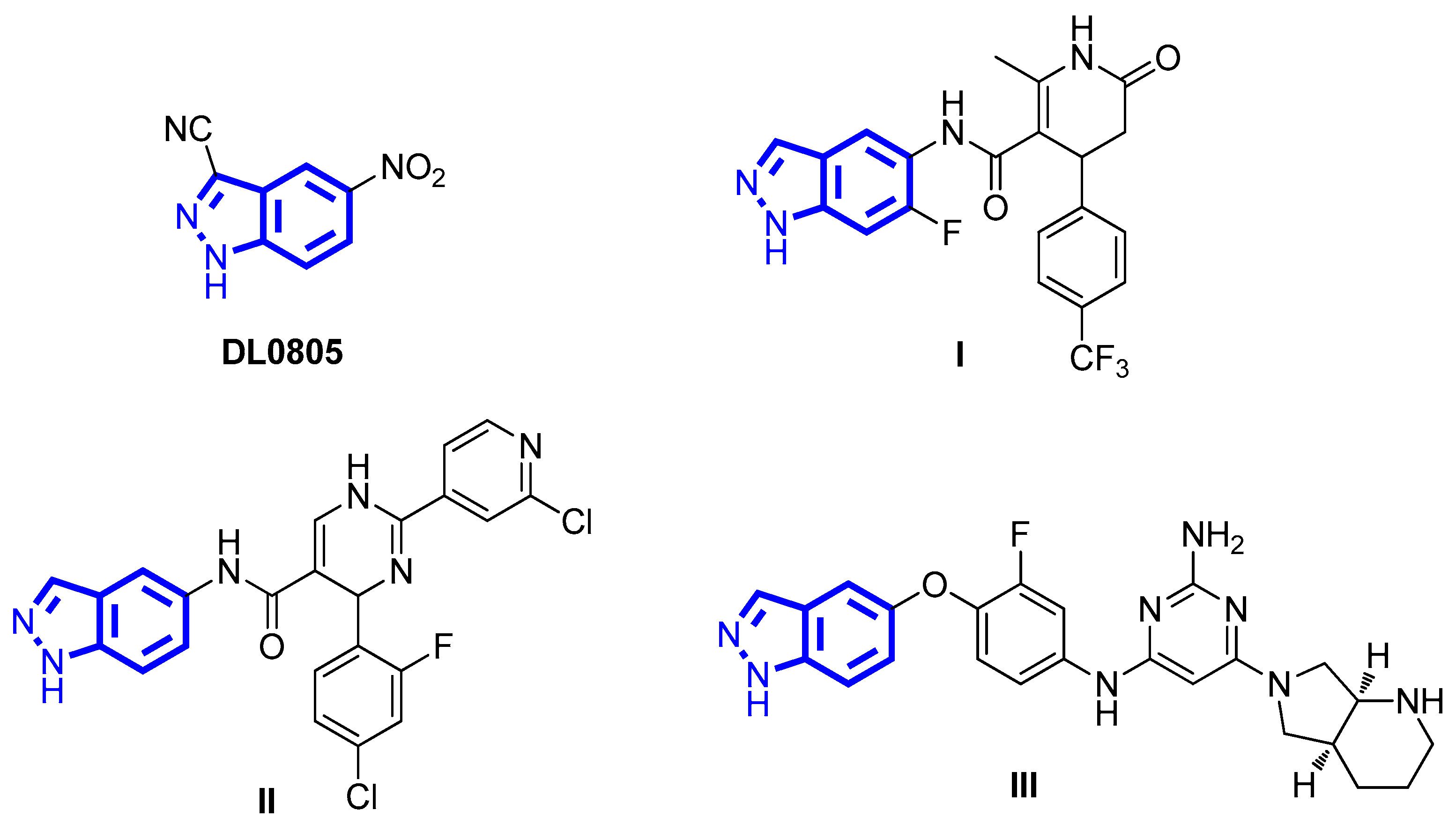
:1. Introduction
2. Results and Discussion
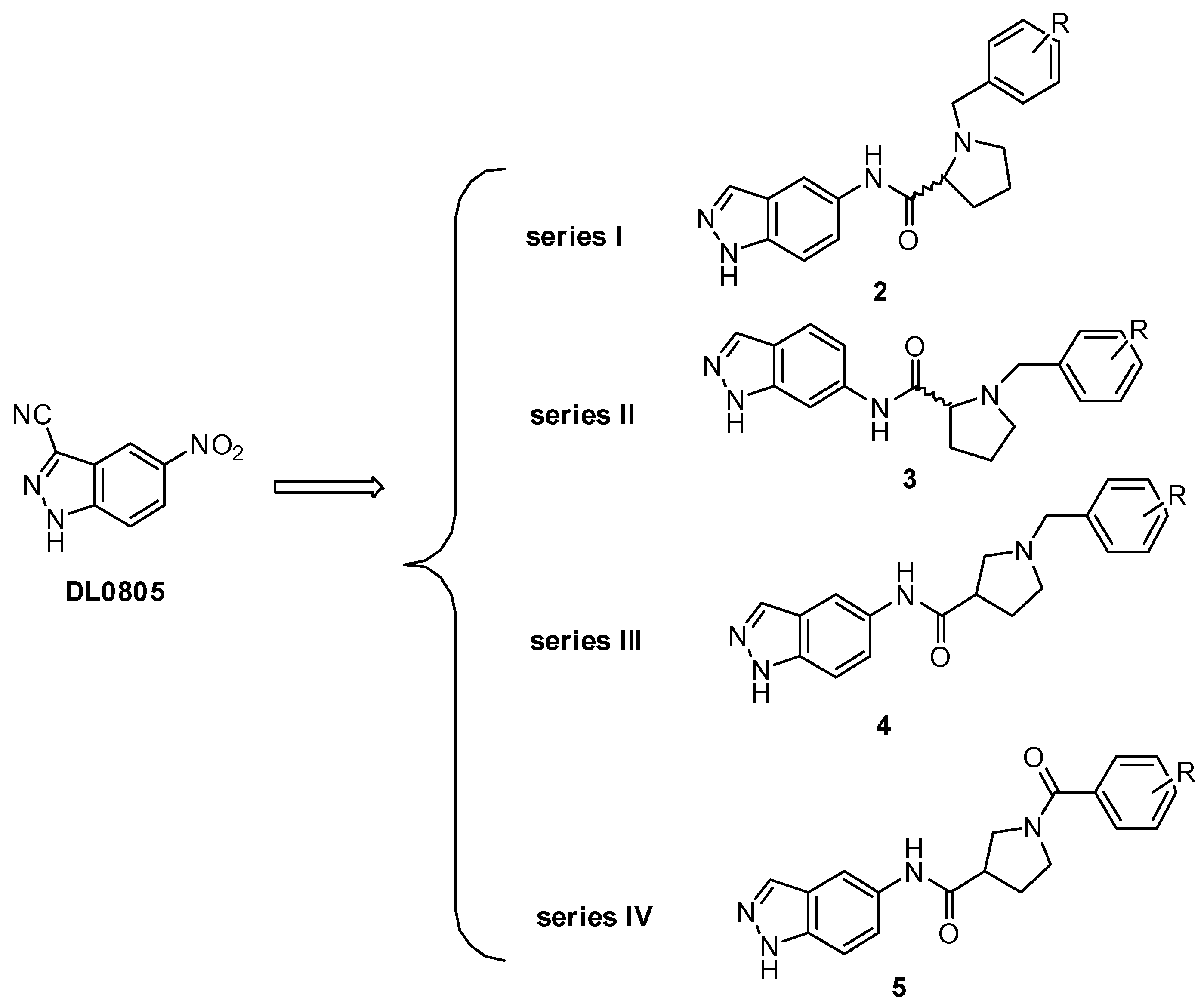
2.1. Molecular Design
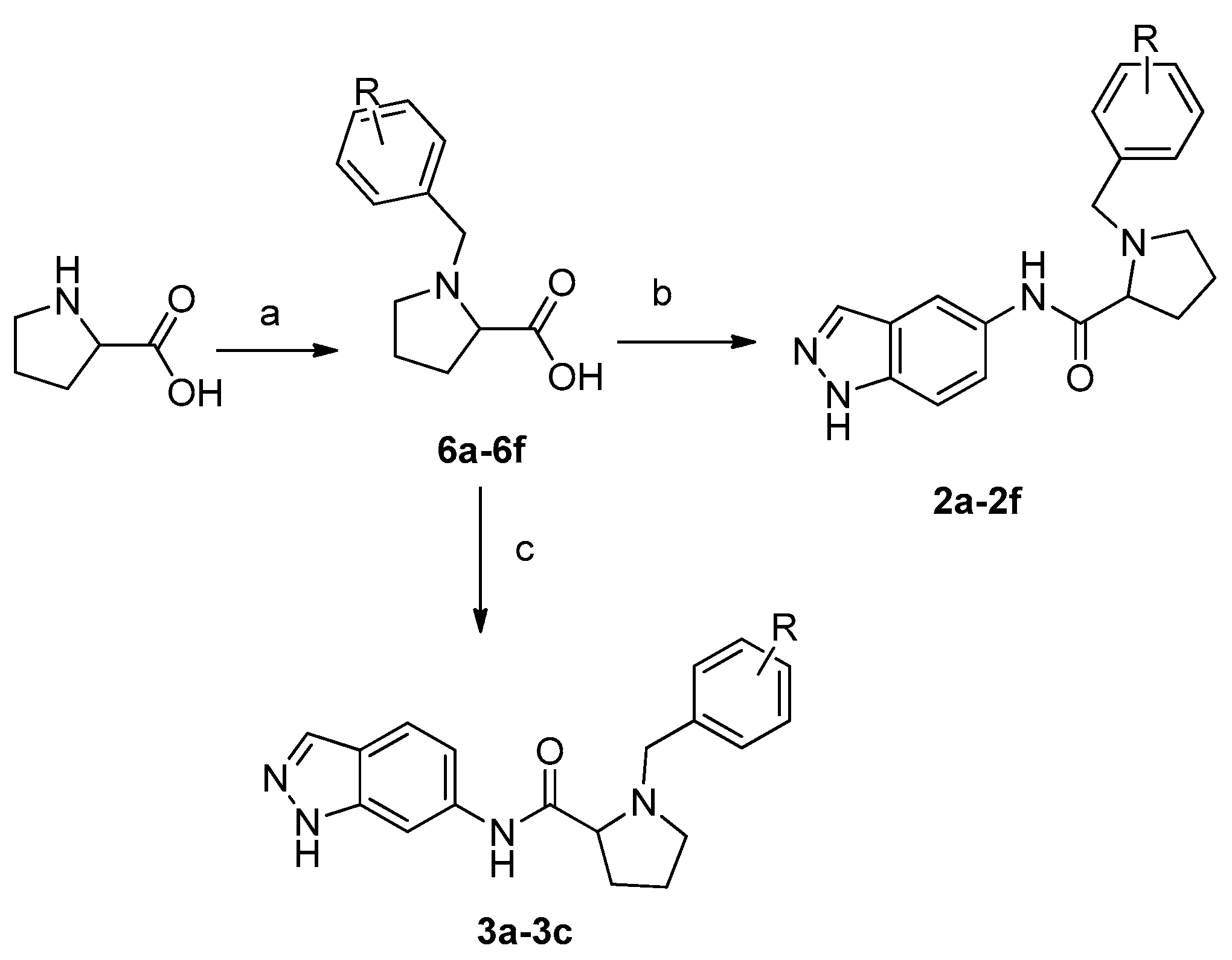
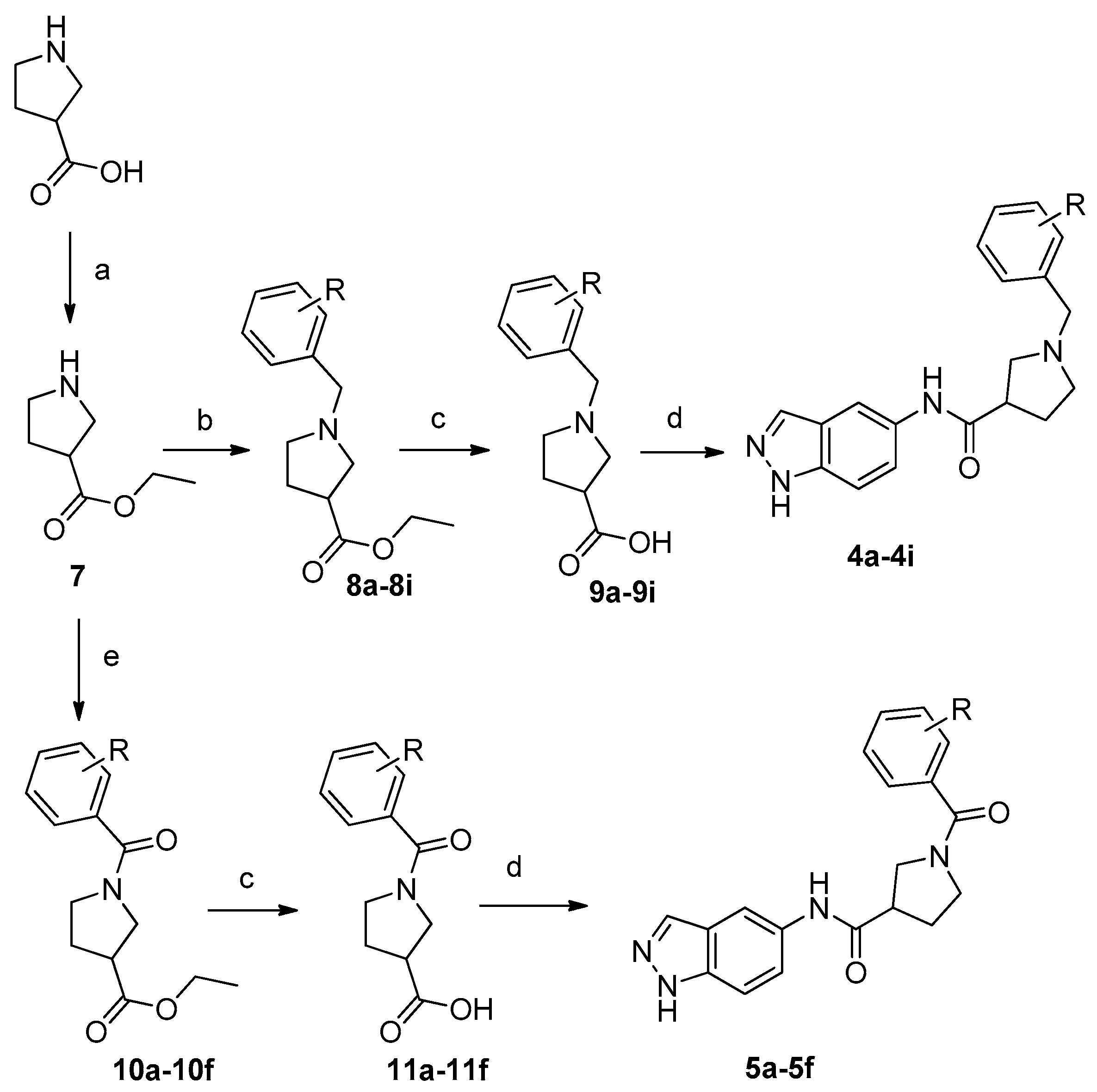
2.2. Chemistry
2.3. Bioassay Studies
2.3.1. ROCK I Inhibitory Activity Evaluation
2.3.2. Vasorelaxant Activity Evaluation
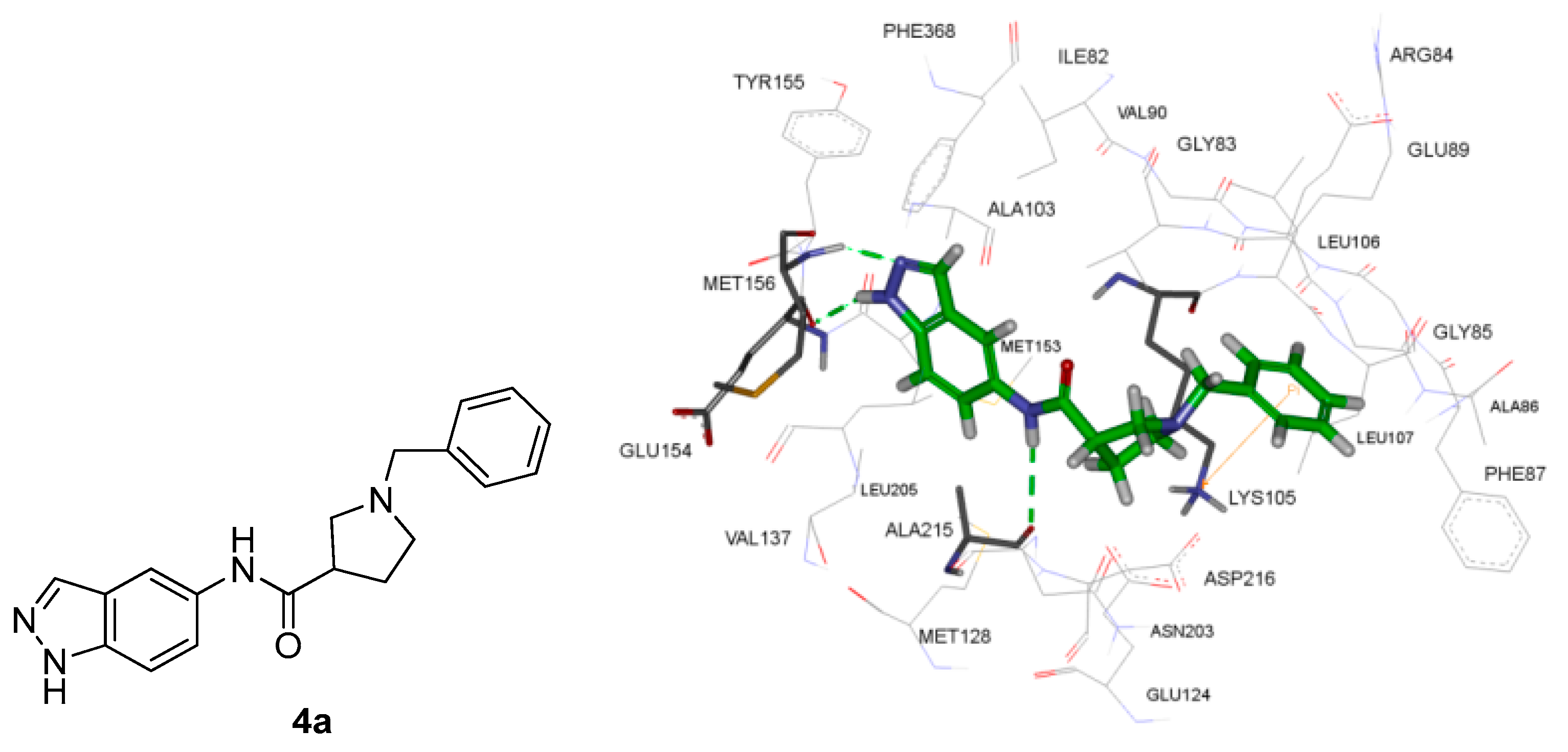
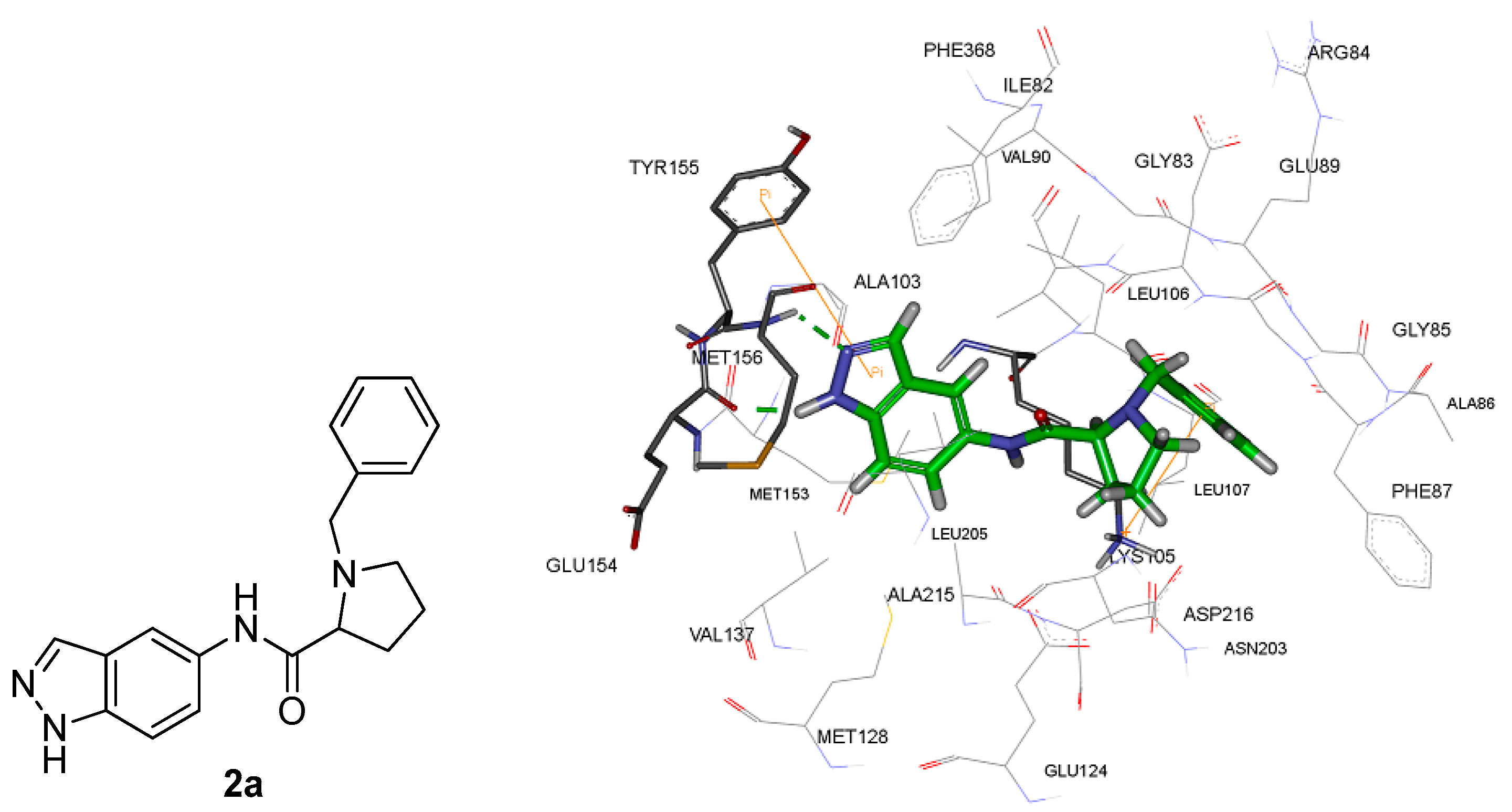
2.4. Molecular Docking Studies
3. Materials and Methods
3.1. Chemistry
3.1.1. General Procedure for the Preparation of Compounds 6
3.1.2. General Procedure for the Preparation of Compounds 7
3.1.3. General Procedure for the Preparation of Compounds 8 or 10
3.1.4. General Procedure for the Preparation of Compounds 9 or 11
3.1.5. General Procedure for the Preparation of Target Compounds 2a–2f; 3a–3c; 4a–4i; 5a–5f
3.2. Bioassay Studies
3.2.1. Rho Kinase Activity Assay
3.2.2. Vasorelaxant Activity Test
3.3. Molecular Docking
4. Conclusions
Acknowledgment
Author Contributions
Conflicts of Interest
References and Notes
- Leung, T.; Manser, E.; Tan, L.; Lim, L. A novel serine/threonine kinase binding the Ras-related RhoA GTPase which translocates the kinase to peripheral membranes. J. Biol. Chem. 1995, 270, 29051–29504. [Google Scholar] [CrossRef] [PubMed]
- Riento, K.; Ridley, A.J. ROCKs: Multifunctional kinases in cell behavior. Nat. Rev. Mol. Cell Biol. 2003, 4, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, O.; Fujisawa, K.; Ishizaki, T.; Saito, Y.; Nakao, K.; Narumiya, S. ROCK-I and ROCK-II, two isoforms of rho-associated coiled-coil forming protein serine/threonine kinase in mice. FEBS Lett. 1996, 392, 189–193. [Google Scholar] [CrossRef]
- Wirth, A. Rho kinase and hypertension. Biochim. Biophys. Acta 2010, 1802, 1276–1284. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Salomone, S.; Ayata, C. Targeting cerebrovascular Rho-kinase in Stroke. Expert Opin. Ther. Targets 2008, 12, 1547–1564. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Moriuchi, H.; Yunoki, M.; Sakamato, K. Y-27632 potentiate relaxant effects of beta 2-adrenoceptor agonists in bovine tracheal smooth muscle. Eur. J. Pharmacol. 2000, 389, 103–106. [Google Scholar] [CrossRef]
- Oka, M.; Fagan, K.; Jone, P.; McMutry, I. Therapeutic potential of Rho A/Rho kinase inhibitors in pulmonary hypertension. Br. J. Pharmacol. 2008, 155, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Ying, H.; Biroc, S.L.; Li, W.W.; Alicke, B.; Xuan, J.A.; Pagila, R.; Ohashi, Y.; Okada, T.; Kamata, Y.; Dinter, H. The Rho kinase inhibitor fasudil inhibits tumor progression in human and rat tumor models. Mol. Cancer Ther. 2005, 5, 2158–2164. [Google Scholar] [CrossRef] [PubMed]
- Somlyo, C.; Phelps, C.; Dipiperro, C.; Eto, M.; Read, P.; Barrett, M.; Gibson, J.J.; Burnitz, M.C.; Myers, C.; Somlyo, A.P. Rho kinase and matrix metalloproteinase inhibitors cooperate to inhibit angiogenesis and growth of human prostate cancer xenotransplants. FASEB J. 2003, 17, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Waki, M.; Yoshida, Y.; Oka, T.; Azuma, M. Reduction of intraocular pressure by topical administration of an inhibitor of the Rho-associated protein kinase. Curr. Eye Res. 2001, 22, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Navak, G.D. Emerging drug for ophthalmic diseases. Exp. Opin. Emerg. Drugs 2003, 8, 251–266. [Google Scholar]
- Tanihara, H.; Inatani, M.; Honjo, M.; Tokushige, H.; Azuma, J.; Araie, M. Intraocular pressure-lowering effects and safety of topical administration of a selective ROCK inhibitor, SNJ-1656, in healthy volunteers. Arch. Ophthalmol. 2008, 3, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Takashima-Hirano, M.; Koyama, H.; Yamaoka, T. Efficient synthesis of [11C] H-1152, a PET probe specific for Rho-kinases, highly potential targets in diagnostic medicine and drug development. Tetrahedron 2012, 68, 2336–2341. [Google Scholar] [CrossRef]
- Chowdhury, S.; Chen, Y.T.; Fang, X.G.; Grant, W.; Pocas, J.; Cameron, M.D.; Ruiz, C.; Lin, L.; Park, H.; Schröter, T.; et al. Amino acid derived quinazolines as Rock/pka inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 1592–1599. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.B.; Cameron, M.D.; Frackowiak, B.; Griffin, E.; Lin, L.; Ruiz, C.; Schroter, T.; Lograsso, P. Structure-activity relationships, and drug metabolism and pharmacokinetic properties for indazolepiperazine and indazolepiperdine inhibitors of ROCK II. Bioorg. Med. Chem. Lett. 2007, 17, 2355–2360. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.; Session, E.H.; Pocas, J.R.; Grant, W.; Schroter, T.; Lin, L.; Ruiz, C.; Cameron, M.D.; Schurer, S.; Lograsso, P. Discovery and optimization of indoles and 7-azaindoles as Rho kinase (ROCK) inhibitors (Part I). Bioorg. Med. Chem. Lett. 2011, 21, 7107–7112. [Google Scholar] [CrossRef] [PubMed]
- Takami, A.; Iwakubo, M.; Okada, Y.; Kawata, T.; Odai, H.; Nobuaki, T.; Kazutoshi, S.; Kaname, K.; Yoshimichi, T.; Mika, M.; et al. Design and synthesis of Rho kinase inhibitors (I). Bioorg. Med. Chem. 2004, 12, 2115–2137. [Google Scholar] [CrossRef] [PubMed]
- Iwakubo, M.; Takami, A.; Okada, Y.; Tagami, Y.; Ohashi, H.; Sato, M.; Sugiyama, T.; Fukushima, K.; Iijima, H. Design and synthesis of Rho kinase inhibitors (II). Bioorg. Med. Chem. 2007, 15, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Iwakubo, M.; Takami, A.; Okada, Y.; Kawata, T.; Tagami, Y.; Sato, M.; Sugiyama, T.; Fukushima, K.; Taya, S.; Amano, M.; et al. Design and synthesis of Rho kinase inhibitors (III). Bioorg. Med. Chem. 2007, 15, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.S.; Oh, B.K.; Park, C.H.; Seo, H.W. Cardiovascular effects of a noval selective Rho kinase inhibitor, 2-(1H-indazole-5-yl)amino-4-methoxy-6-piperazino triazine (DW1865). Eur. J. Pharmacol. 2013, 702, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Session, E.H.; Yin, Y.; Bannister, T.D.; Weiser, A.; Griffin, E.; Pocas, J.; Cameron, M.D.; Ruiz, C.; Lin, L.; Schürer, S.C.; et al. Benzimidazole- and benzoxazole-based inhibitors of Rho kinase. Bioorg. Med. Chem. Lett. 2008, 18, 6390–6393. [Google Scholar] [CrossRef] [PubMed]
- Session, E.H.; Smolinski, M.; Wang, B.; Frackowiak, B.; Chowdhury, S.; Yin, Y.; Chen, Y.T.; Ruiz, C.; Lin, L.; Pocas, J.; et al. The development of Benzimidazole as selective rho kinase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 1939–1943. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Lin, L.; Ruiz, C.; Cameron, M.D.; Pocas, J.R.; Grant, W.; Schröter, T.; Chen, W.; Duckett, D.; Schürer, S.; et al. Benzothiazoles as Rho-associated kinase (ROCK-II) inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 6686–6690. [Google Scholar] [CrossRef] [PubMed]
- Letellier, M.A.; Guillard, J.; Caignard, D.H.; Ferry, G.; Boutin, J.A.; Viaud-Massuard, M.-C. Synthesis of potential Rho-kinase inhibitors based on the chemistry of an original heterocycle: 4,4-dimethyl-3,4-dihydro-1H-quinolin-2-one. Eur. J. Med. Chem. 2008, 43, 1730–1736. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.G.; Chen, Y.T.; Session, E.H.; Chowdhury, S.; Vojkovsky, T.; Yin, Y.; Pocas, J.R.; Grant, W.; Schröter, T.; Lin, L.; et al. Synthesis and biological evalution of 4-quinazolinones as Rho kinase inhibitors. Bioorg. Med. Chem. Lett. 2011, 21, 1844–1848. [Google Scholar] [CrossRef] [PubMed]
- Herderson, A.J.; Hadden, M.; Guo, C.; Douglas, N.; Decornez, H.; Hellberg, M.R.; Rusinko, A.; McLaughlin, M.; Sharif, N.; Drace, C.; et al. 2,3-Diaminopyrazines as rho kinase inhibitors. Bioorg. Med. Chem. Lett. 2010, 20, 1137–1140. [Google Scholar] [CrossRef] [PubMed]
- Rajagopalan, L.E.; Davies, M.S.; Kahn, L.E.; Kornmeier, C.M.; Shimada, H.; Steiner, T.A.; Zweifel, B.S.; Wendling, J.M.; Payne, M.A.; Loeffler, R.F.; et al. Biochemical, cellular, and anti-inflammatory properties of a potent, selective, orally bioavailable benzamide inhibitor of Rho kinase activity. J. Pharmacol. Exp. Ther. 2010, 333, 707–716. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Bannister, T.D.; Weiser, A.; Griffin, E.; Lin, L.; Ruiz, C.; Cameron, M.D.; Schürer, S.; Duckett, D.; Schröter, T.; et al. Chroman-3-amides as potent Rho kinase inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 6406–6409. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Lin, L.; Ruiz, C.; Khan, S.; Cameron, M.D.; Grant, W.; Pocas, J.; Eid, N.; Park, H.; Schroter, T.; et al. Synthesis and biological evalution of urea derivatives as highly potent and selective Rho kinase inhibitors. J. Med. Chem. 2013, 56, 3568–3581. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.B.; LoGrasso, P.V.; Defert, O.; Li, R. Rho kinase (ROCK) inhibitors and their therapeutic potential. J. Med. Chem. 2016, 59, 2269–2300. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.L.; Peng, J.H.; Fang, L.L.; Ping, X.; Jiao, X.-Z.; Xie, P.; Fang, L.-H.; Du, G.-H. The vasorelaxant mechanisms of a Rho kinase inhibitor in rat thoracic aorta. Molecules 2012, 17, 5935–5944. [Google Scholar] [CrossRef] [PubMed]
- Goodman, K.B.; Cui, H.; Dowdell, S.E.; Gaitanopoulos, D.E.; Ivy, R.L.; Sehon, C.A.; Stavenger, R.A.; Wang, G.Z.; Viet, A.Q.; Xu, W.; et al. Development of dihydropyridoneindazole amides as selective rho-kinase inhibitors. J. Med. Chem. 2007, 50, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Sehon, C.A.; Wang, G.Z.; Viet, A.Q.; Goodman, K.B.; Dowdell, S.E.; Elkins, P.A.; Semus, S.F.; Evans, C.; Jolivette, L.J.; Kirkpatrick, R.B.; et al. Potent, selective and orally bioavailable dihydropyrimidine inhibitors of Rho kinase (ROCK i) as potential therapeutic agents for cardiovascular diseases. J. Med. Chem. 2008, 51, 6631–6634. [Google Scholar] [CrossRef] [PubMed]
- Feurer, A.; Bennabi, S.; Heckroth, H.; Schirok, H.; Mittendorf, J. Heteroaryl-Substituted Phenylaminopyridines as Rho Kinase Inhibitors. International Patent WO2004039796, 13 May 2004. [Google Scholar]
- Yuan, T.Y.; Chen, Y.C.; Zhang, H.F.; Li, L.; Jiao, X.-Z.; Xie, P.; Fang, L.-H.; Du, G.-H. A novel indazole derivative, DL0805–2, relaxes the angiotensin II-inducedvascular smooth muscle contration by inhibiting Rho kinase and calcium fluxes. Acta Pharmacol. Sin. 2016, 37, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bosanac, T.; Hickey, E.R.; Ginn, J.; Kashem, M.; Kerr, S.; Kugler, S.; Li, X.; Olague, A.; Schlyer, S.; Young, E.R. Substituted 2H-isoquinolin-1-ones as potent Rho-kinase inhibitors: part 3, aryl substituted pyrrolidines. Bioorg. Med. Chem. Lett. 2010, 20, 3746–3749. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 2a-2f; 3a-3c; 4a-4i; 5a-5f are available from the authors. |
| Compound | R | Configuration | % Inhibition of ROCK I | Compound | R | Configuration | % Inhibition of ROCK I |
|---|---|---|---|---|---|---|---|
| 2a | 4-H | (±) | 27.2 | 4e | 4-NO2 | (±) | 44.7 |
| 2b | 4-H | (R) | 24.3 | 4f | 4-CN | (±) | 24.5 |
| 2c | 4-H | (S) | 24.6 | 4g | 4-F | (±) | 63.8 |
| 2d | 4-CH3 | (±) | 26.6 | 4h | 4-H | (S) | 54.8 |
| 2e | 4-CH3 | (R) | 4i | 4-H | (R) | 73.9 | |
| 2f | 4-CH3 | (S) | 21.3 | 5a | 4-H | (±) | 42.5 |
| 3a | 4-H | (±) | 16.0 | 5b | 4-CH3 | (±) | 54.9 |
| 3b | 4-H | (R) | 15.0 | 5c | 4-OCH3 | (±) | 19.1 |
| 3c | 4-H | (S) | 23.7 | 5d | 4-Br | (±) | 27.9 |
| 4a | 4-H | (±) | 73.2 | 5e | 4-NO2 | (±) | 45.4 |
| 4b | 4-CH3 | (±) | 75.8 | 5f | 4-Cl | (±) | 55.2 |
| 4c | 4-OCH3 | (±) | 53.3 | DL0805 | 72.3 | ||
| 4d | 4-Br | (±) | 62.5 | fasudil | 71.1 |
| Compound | ROCK I (μM) | Compound | ROCK I (μM) | Compound | ROCK I (μM) |
|---|---|---|---|---|---|
| 4a | 0.27 a | 4g | 1.41 b | 5f | 7.26 b |
| 4b | 0.17 a | 4h | 0.42 b | DL0805 | 6.67 b |
| 4c | 1.86 b | 4i | 7.32 b | fasudil | 0.36 a |
| 4d | 0.51 b | 5b | 11.5 b |
| Compound | Vasorelexant Evaluation (μM) | |
|---|---|---|
| In High-Potassium Model | In NE Model | |
| 4a | 4.00 a | 5.62 a |
| 4b | 3.47 a | 4.07 a |
| 4c | 5.80 a | 5.57 a |
| 4d | 58.1 b | 46.8 b |
| 4g | 19.5 b | 8.91 b |
| 4h | 32.36 b | -- |
| 4i | 6.02 b | 11.48 b |
| 5b | 60.6 b | 15.6 b |
| 5f | -- | -- |
| DL0805 | 9.44 b | 28.8 b |
| fasudil | 5.47 a | 4.65 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Li, R.; Liu, X.; Yang, F.; Yang, Y.; Li, X.; Shi, X.; Yuan, T.; Fang, L.; Du, G.; et al. Discovery of Novel N-Substituted Prolinamido Indazoles as Potent Rho Kinase Inhibitors and Vasorelaxation Agents. Molecules 2017, 22, 1766. https://doi.org/10.3390/molecules22101766
Yao Y, Li R, Liu X, Yang F, Yang Y, Li X, Shi X, Yuan T, Fang L, Du G, et al. Discovery of Novel N-Substituted Prolinamido Indazoles as Potent Rho Kinase Inhibitors and Vasorelaxation Agents. Molecules. 2017; 22(10):1766. https://doi.org/10.3390/molecules22101766
Chicago/Turabian StyleYao, Yangyang, Renze Li, Xiaoyu Liu, Feilong Yang, Ying Yang, Xiaoyu Li, Xiang Shi, Tianyi Yuan, Lianhua Fang, Guanhua Du, and et al. 2017. "Discovery of Novel N-Substituted Prolinamido Indazoles as Potent Rho Kinase Inhibitors and Vasorelaxation Agents" Molecules 22, no. 10: 1766. https://doi.org/10.3390/molecules22101766
APA StyleYao, Y., Li, R., Liu, X., Yang, F., Yang, Y., Li, X., Shi, X., Yuan, T., Fang, L., Du, G., Jiao, X., & Xie, P. (2017). Discovery of Novel N-Substituted Prolinamido Indazoles as Potent Rho Kinase Inhibitors and Vasorelaxation Agents. Molecules, 22(10), 1766. https://doi.org/10.3390/molecules22101766





