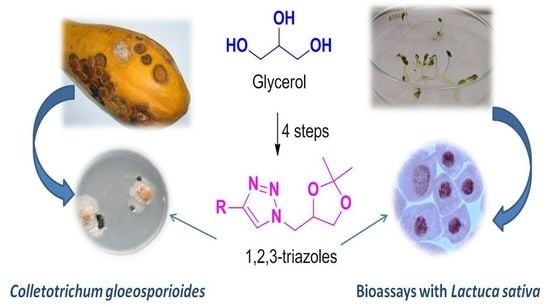
Synthesis of Novel Glycerol-Derived 1,2,3-Triazoles and Evaluation of Their Fungicide, Phytotoxic and Cytotoxic Activities
Abstract
:1. Introduction
2. Results and Discussion
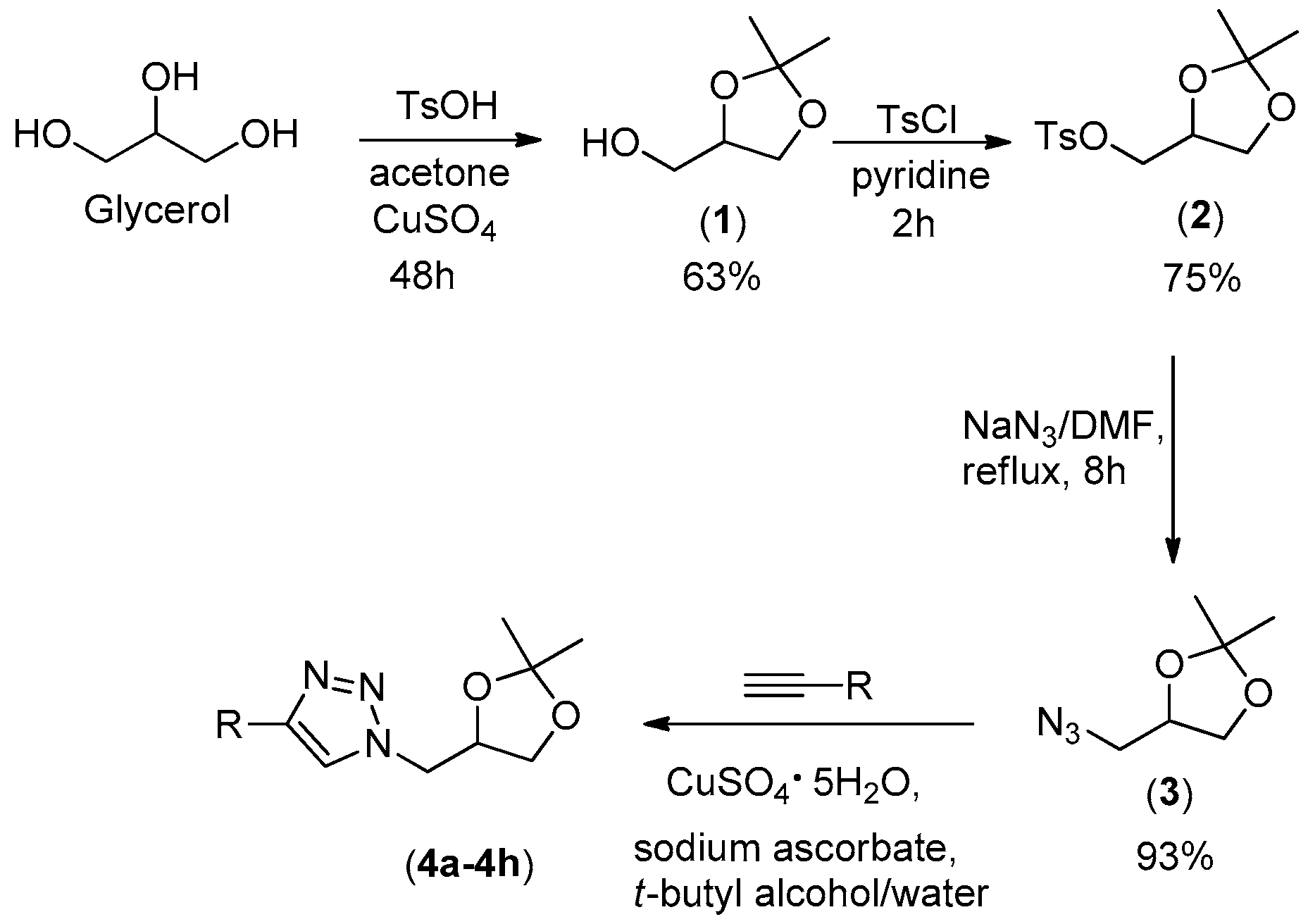
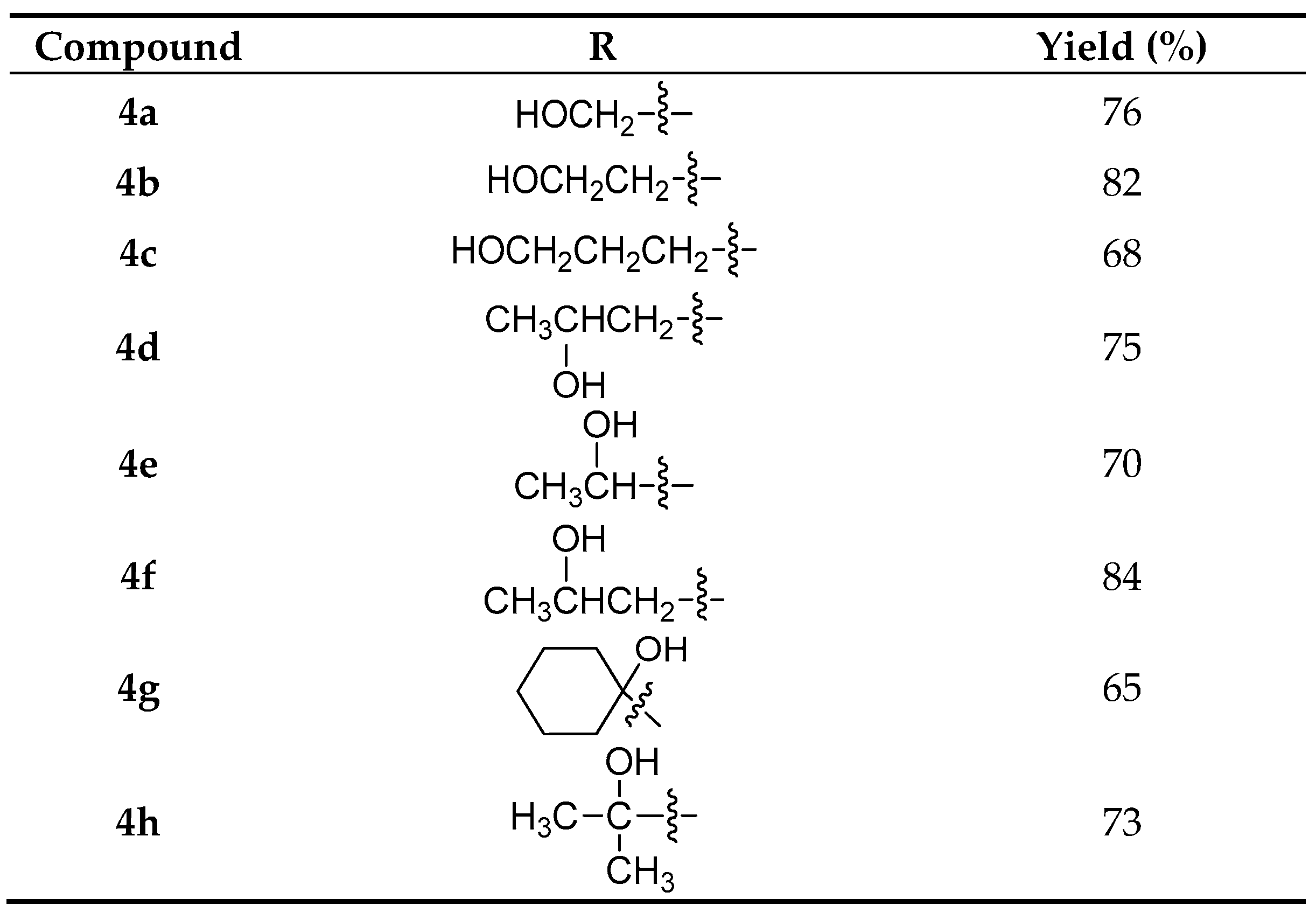
2.1. Synthesis
2.2. Biological Evaluation
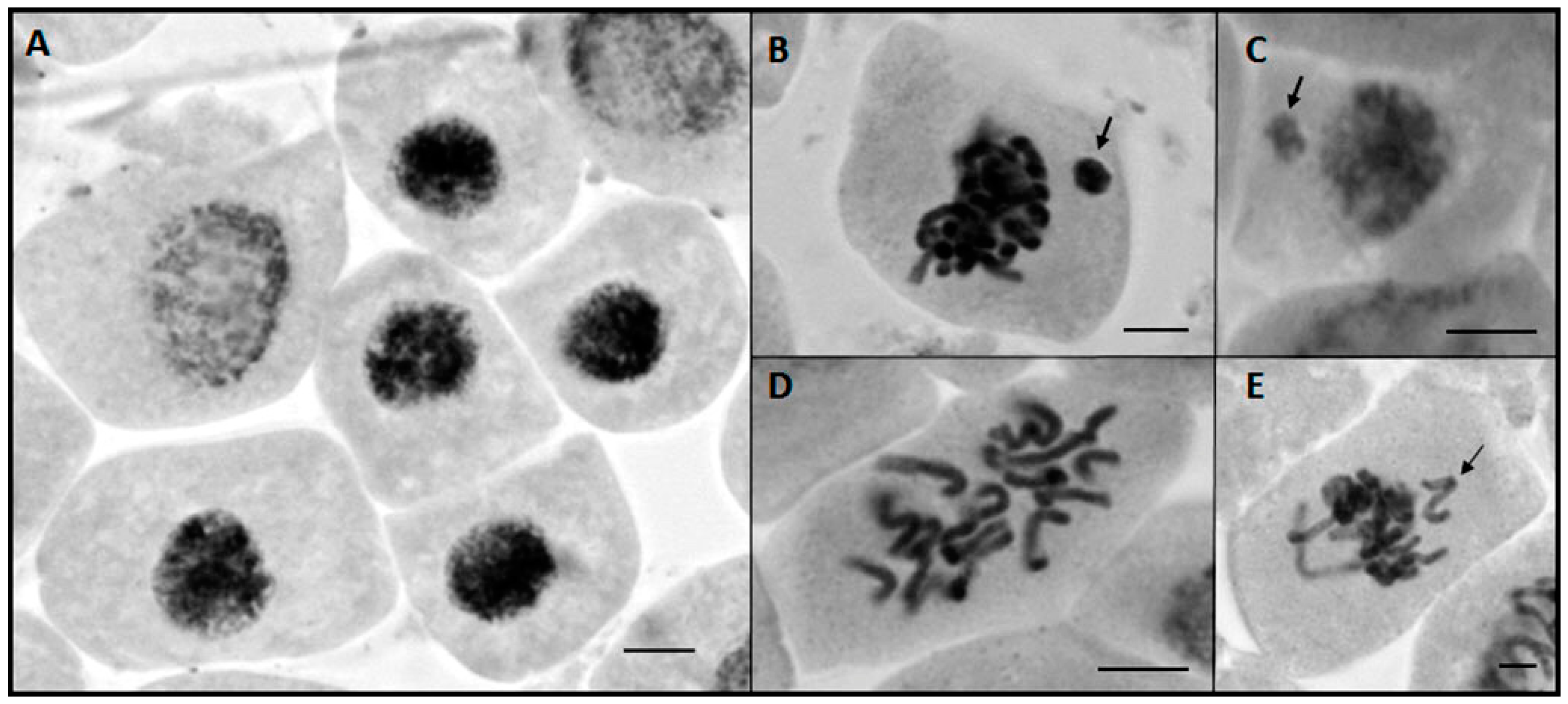
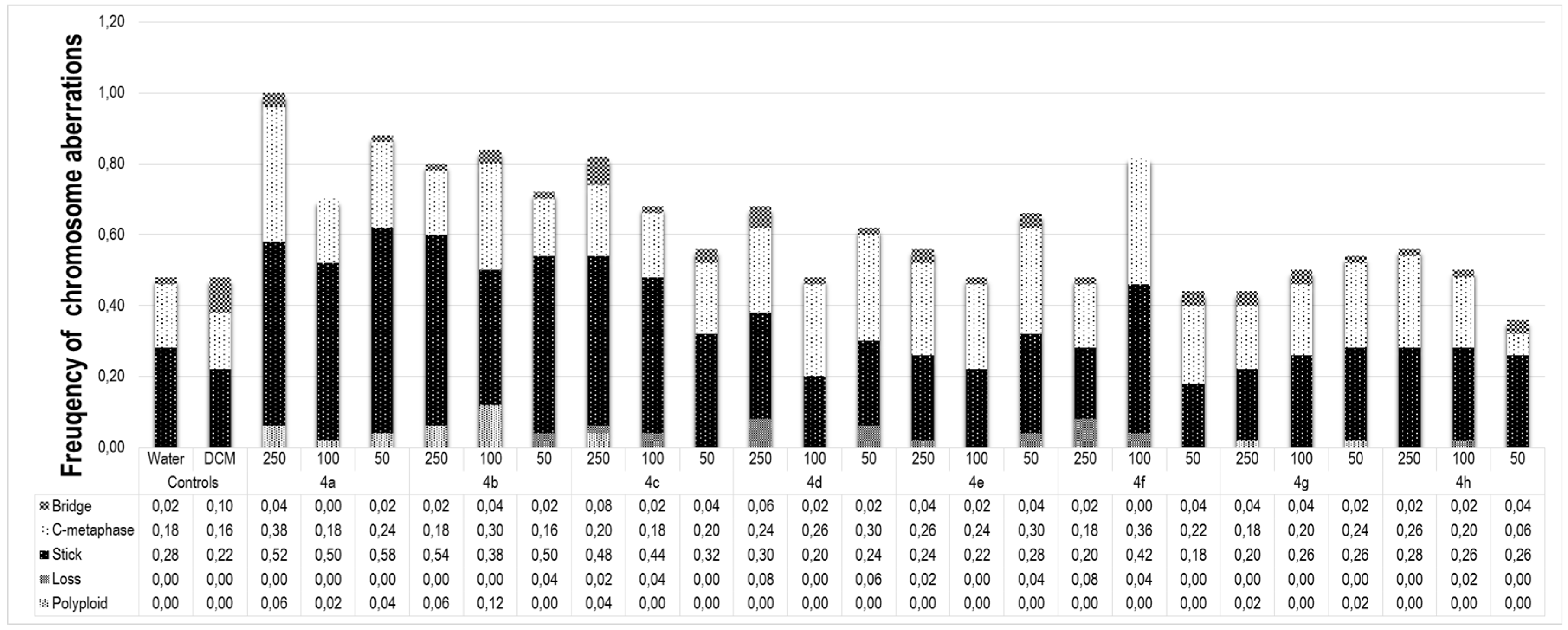
2.3. Cytotoxic and Phytotoxic Effect
3. Materials and Methods
3.1. General Information
3.2. Synthesis of (2,2-dimethyl-1,3-dioxolan-4-yl)methanol (1)
3.3. Synthesis of (2,2-dimethyl-1,3-dioxolan-4-yl)methyl 4-methylbenzenesulfonate (2)
3.4. Synthesis of 4-(azidomethyl)-2,2-dimethyl-1,3-dioxolane (3)
3.5. General Procedure for Copper(I)-Catalyzed Azide-Alkyne Cycloaddition Reactions for the Preparation of Triazoles 4a–4h
3.6. Evaluation of Fungicidal Activity
3.7. Evaluation of Phytotoxicity and Cytotoxicity
3.8. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Struthers, H.; Mindt, T.L.; Schibli, R. Metal chelating systems synthesized using the copper(I) catalyzed azide-alkyne cycloaddition. Dalton Trans. 2010, 39, 675–696. [Google Scholar] [CrossRef] [PubMed]
- Boechat, N.; Ferreira, V.F.; Ferreira, S.B.; Ferreira, M.L.G.; Silva, F.C.; Bastos, M.M.; Costa, M.S.; Lourenço, M.C.S.; Pinto, A.C.; Krettli, A.U.; et al. Novel 1,2,3-triazole derivatives for use against Mycobacterium tuberculosis H37Rv (ATCC 27294) strain. J. Med. Chem. 2011, 54, 5988–5999. [Google Scholar] [CrossRef] [PubMed]
- Anjos, J.V.; Filho, R.A.W.N.; Nascimento, S.C.; Srivastava, R.M.; Melo, S.J.; Sinou, D. Synthesis and cytotoxic profile of glycosyl-triazole linked to 1,2,4-oxadiazole moiety at C5 through a straight-chain carbon and oxigen atoms. Eur J. Med. Chem. 2009, 44, 3571–3576. [Google Scholar] [CrossRef] [PubMed]
- Kamal, A.; Shankaraiah, N.; Devaiah, V.; Reddy, K.L.; Juvekar, A.; Sen, S.; Kurian, N.; Zingde, S. Synthesis of 1,2,3-triazole-linked pyrrolobenzodiazepine conjugates employing ‘click’ chemistry: DNA-binding affinity and anticancer activity. Bioorg. Med. Chem. Lett. 2008, 18, 1468–1473. [Google Scholar] [CrossRef] [PubMed]
- Bakunov, S.A.; Bakunova, S.M.; Wenzler, T.; Ghebru, M.; Werbovetz, K.A.; Brun, R.; Tidwell, R.R. Synthesis and antiprotozoal activity of cationic 1,4-diphenyl-1H-1,2,3-triazoles. J. Med. Chem. 2010, 53, 254–272. [Google Scholar] [CrossRef] [PubMed]
- Aher, N.G.; Pore, V.S.; Mishra, N.N.; Kumar, A.; Shukla, P.K.; Sharma, A.; Bhat, M.K. Synthesis and antifungal activity of 1,2,3-triazole containing fluconazole analogues. Bioorg. Med. Chem. Lett. 2009, 19, 759–763. [Google Scholar] [CrossRef] [PubMed]
- Shichong, Y.; Xiaoyun, C.; Honggang, H.; Yongzheng, Y.; Zhongjun, G.; Yan, Z.; Qingyan, S.; Qiuye, W. Synthesis and antifungal evaluation of novel triazole derivatives as inhibitors of cytochrome P450 14α-demethylase. Eur. J. Med. Chem. 2010, 45, 4435–4445. [Google Scholar]
- Guantai, E.M.; Ncokazi, K.; Egan, T.J.; Gut, J.; Rosenthal, P.J.; Smith, P.J.; Chibale, K. Design, synthesis and in vitro antimalarial evaluation of triazole-linked chalcone and dienone hybrid compounds. Bioorg. Med. Chem. Lett. 2010, 18, 8243–8256. [Google Scholar] [CrossRef] [PubMed]
- Silva, E.M., Jr.; Barreto, R.F.S.M.; Pinto, M.C.F.R.; Silva, R.S.F.; Teixeira, D.V.; Souza, M.C.B.V.; Simone, C.A.; Castro, S.L.; Ferreira, V.F.; Pinto, A.V. Naphthoquinoidal [1,2,3]-triazole, a new structural moiety active against Trypanosoma cruzi. Eur. J. Med. Chem. 2008, 43, 1774–1780. [Google Scholar]
- Borgati, T.F.; Alves, R.B.; Teixeira, R.R.; Freitas, R.P.; Perdigão, T.G.; Silva, S.F.; Santos, A.A.; Bastidas, A.J.O. Synthesis and phytotoxic activity of 1,2,3-triazole derivatives. J. Braz. Chem. Soc. 2013, 24, 953–961. [Google Scholar] [CrossRef]
- Sharpless, K.B.; Finn, M.G.; Kolb, H.C. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. In. Ed. 2011, 40, 2004–2021. [Google Scholar]
- Wikipedia, the free encyclopedia. Available online: http://en.wikipedia.org/wiki/Glycerol (accessed on 28 September 2017).
- Wikipedia, the free encyclopedia. Available online: http://en.wikipedia.org/wiki/Nitroglycerin (accessed on 28 September 2017).
- The chemistry hall of fame. Available online: http://www.chem.yorku.ca/hall_of_fame/essays96/glycerol.htm (accessed on 28 September 2017).
- Vivek, N.; Sindhu, R.; Madhavan, A.; Anju, A.J.; Castro, E.; Faraco, V.; Pandey, A.; Binod, P. Recent advances in the production of value added chemicals and lipids utilizing biodiesel industry generated crude glycerol as a substrate—Metabolic aspects, challenges and possibilities: An overview. Bioresour. Technol. 2017, 239, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Anitha, M.; Kamarudin, S.K.; Kofli, N.T. The potential of glycerol as a value-added commodity. Chem. Eng. J. 2016, 295, 119–130. [Google Scholar] [CrossRef]
- Bagheri, S.; Julkapli, N.M.; Yehye, W.A. Catalytic conversion of biodiesel derived raw glycerol to value added products. Renew. Sustain. Energy Rev. 2015, 41, 113–127. [Google Scholar] [CrossRef]
- García, J.I.; García-Marína, H.; Piresa, E. Glycerol based solvents: Synthesis, properties and applications. Green Chem. 2010, 12, 426–434. [Google Scholar] [CrossRef]
- Gu, J.; Jérôme, F. Glycerol as a sustainable solvent for green chemistry. Green Chem. 2010, 12, 1127–1138. [Google Scholar] [CrossRef]
- Aragão, F.B.; Andrade-Vieira, L.F.; Ferreira, A.; Costa, A.V.; Queiroz, V.T.; Pinheiro, P.F. Phytotoxic and cytotoxic effects of Eucalyptus essential oil on Lactuca sativa L. Allelopathy J. 2015, 35, 259–272. [Google Scholar]
- Zauza, E.A.V.; Couto, M.M.F.; Maffia, L.A.; Alfenas, A.C. Eficiência de fungicidas sistêmicos no controle da ferrugem do Eucalyptus. Rev. Árv. 2008, 32, 829–835. [Google Scholar] [CrossRef]
- Chen, G.; Zhou, Y.; Cai, C.; Lu, J.; Zhang, X. Synthesis and antifungal activity of benzamidine derivatives carrying 1,2,3-triazole moieties. Molecules 2014, 19, 5674–5691. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, I.F.; Martins, P.R.C.; da Silva, E.G.; Ferreira, S.B.; Ferreira, V.F.; da Costa, K.R.C.; de Vasconcellos, M.C.; Lima, E.S.; da Silva, F.C. Synthesis of 1H-1,2,3-triazoles and study of their antifungal and cytotoxicity activities. Med. Chem. 2013, 9, 1085–1090. [Google Scholar] [CrossRef]
- Zhang, J.; Debets, A.J.; Verweij, P.E.; Melchers, W.J.; Zwaan, B.J.; Schoustra, S.E. Asexual sporulation facilitates adaptation: The emergence of azole resistance in Aspergillus fumigatus. Evolution 2015, 9, 2573–2586. [Google Scholar] [CrossRef] [PubMed]
- Valerio, M.E.; Garcia, J.F.; Peinado, F.M. Determination of phytotoxicity of soluble elements in soils, based on a bioassay with lettuce (Lactuca sativa L.). Sci. Total Environ. 2007, 378, 63–66. [Google Scholar] [CrossRef] [PubMed]
- Mauro, M.O.; Monreal, M.T.F.D.; Silva, M.T.P.; Pesarini, J.R.; Mantovani, M.S.; Ribeiro, L.R.; Dichi, J.B.; Carreira, C.M.; Oliveira, R.J. Evaluation of the antimutagenic and anticarcinogenic effects of inulin in vivo. Genet. Mol. Res. 2014, 12, 2281–2293. [Google Scholar] [CrossRef] [PubMed]
- Bernardes, P.M.; Andrade-Vieira, L.F.; Aragão, F.B.; Ferreira, A.; Ferreira, M.F.S. Toxicity of difenoconazole and tebuconazole in Allium cepa. Water Air Soil Pollut. 2015, 226, 207–218. [Google Scholar] [CrossRef]
- Rodrigues, B.N.; Almeida, F.S. Guia de Herbicidas. 2005. Available online: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0006-87052010000200019 (accessed on 2 October 2017).
- Reddy, K.N.; Locke, M.A. Sulfentrazone sorption, desorption, and mineralization in soils from two tillage systems. Weed Sci. 1998, 46, 494–500. [Google Scholar]
- Palmieri, M.J.; Luberb, J.; Andrade-Vieira, L.F.; Davide, L.C. Cytotoxic and phytotoxic effects of the main chemical components of spent pot-liner: A comparative approach. Mutat. Res. 2014, 763, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Vieira, L.F.; Gedraite, L.S.; Campos, J.M.; Davide, L.C. Spent Pot Liner (SPL) induced DNA damage and nuclear alterations in root tip cells of Allium cepa as a consequence of programmed cell death. Ecotoxicol. Environ. Saf. 2011, 74, 822–828. [Google Scholar] [CrossRef] [PubMed]
- Leme, D.M.; Marin-Morales, M.A. Alliumcepa test in environmental monitoring: A review on its application. Mutat. Res. 2009, 82, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Vieira, L.F. Toxicity of landfills assessed by plant cytogenetic approaches. In Landfills: Waste Management, Regional Practices and Environmental Impact; Cabral, G.B.C., Botelho, B.A.E., Eds.; Nova science Publishers: New York, NY, USA, 2012; pp. 319–330. [Google Scholar]
- Sharma, A.; Sen, S. Chromosome Botany; Science Publishers: Enfield, NH, USA, 2002; p. 155. [Google Scholar]
- Campos, J.M.S.; Davide, L.C.; Soares, G.L.G.; Viccini, L.F. Mutagenic effects due to allelopathic action of fern (Gleicheniaceae) extracts. Allelopathy Journal 2008, 1, 143–152. [Google Scholar]
- Vidakovic-Cifrek, Z.; Pavlica, M.; Regula, I.; Papes, D. Cytogenetic Damage in Shallot (Allium cepa) Root Meristems Induced by Oil Industry “HighDensity Brines”. Arch. Environ. Contam. Toxicol. 2002, 43, 284–291. [Google Scholar] [CrossRef] [PubMed]
- EL-Ghamery, A.A.; EL-Kholy, M.A.; EL-Yousser, M.A.A. Evaluation of cytological effects of Zn2+ in relation to germination and root growth of Nigella sativa L. and Triticum aestivum L. Mutat. Res. 2003, 53, 29–41. [Google Scholar] [CrossRef]
- Edgington, L.V.; Khew, K.L.; Barron, G.L. Fungitoxic spectrum of benzimidazoles compounds. Phytopathology 1971, 61, 42–44. [Google Scholar] [CrossRef]
- Rampersad, S.N.; Teelucksingh, L.D. Differential responses of Colletotrichum gloeosporioides and C. truncatum isolates from different hosts to multiple fungicides based on two assays. Plant. Dis. 2012, 96, 1526–1536. [Google Scholar] [CrossRef]
- Dias, L.C.; Rubinger, M.M.M.; Barolli, J.P.; Ardisson, J.D.; Mendes, I.C.; Lima, G.M.; Zambolim, L.; Oliveira, M.R.L. Syntheses, crystal structure, spectroscopic characterization and antifungal activity of novel dibutylbis(N-R-sulfonyldithiocarbimato)stannate(IV) complexes. Polyhedron 2012, 47, 30–36. [Google Scholar] [CrossRef]
- Matoba, H.; Mizutani, T.; Nagano, K.; Hoshi, Y.; Uchiyama, H. Chromosomal study of lettuce and its allied species (Lactuca spp., Asteraceae) by means of karyotype analysis and fluorescence in situ hybridization. Hereditas 2007, 144, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, P.F.; Costa, A.V.; Alves, T.A.; Galter, I.N.; Pinheiro, C.A.; Pereira, A.F.; Oliveira, C.M.R.; Fontes, M.M.P. Phytotoxicity and cytotoxicity of essential oil from leaves of Plectranthus amboinicus, carvacrol, and thymol in plant bioassays. J. Agric. Food Chem. 2015, 63, 8981–8990. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds all are available from the authors. |
| Compounds | Concentrations (µg mL−1) | ||||
|---|---|---|---|---|---|
| 1 | 10 | 100 | 500 | 1000 | |
| 4a | 5.39 e * | 5.36 d | 5.01 e | 4.02 c | 1.74 d |
| 4b | 5.72 d | 5.53 d | 5.29 d | 3.86 d | 2.64 b |
| 4c | 5.75 d | 5.73 c | 5.65 c | 4.88 b | 2.33 c |
| 4d | 5.93 d | 5.90 b | 4.92 e | 3.72 d | 1.66 d |
| 4e | 6.15 c | 6.15 b | 5.27 d | 4.07 c | 1.05 e |
| 4f | 6.17 c | 6.10 b | 5.97 b | 1.50 g | 0.46 g |
| 4g | 6.57 b | 6.19 b | 5.63 c | 2.86 f | 0.85 f |
| 4h | 6.06 c | 6.18 b | 5.64 c | 3.27 e | 1.22 e |
| Tebuconazole | 1.83 f | 0.30 e | 0.00 f | 0.00 h | 0.00 h |
| Control | 7.14 a | 7.14 a | 7.14 a | 7.14 a | 7.14 a |
| Compounds | Concentrations (µg mL−1) | ||||
|---|---|---|---|---|---|
| 1 | 10 | 100 | 500 | 1000 | |
| 4a | 148.28 b * | 145.33 b | 78.78 b | 37.40 b | 18.61 b |
| 4b | 145.15 b | 125.40 c | 71.61 c | 37.66 b | 17.51 b |
| 4c | 128.30 c | 99.06 d | 45.69 f | 37.04 b | 19.83 b |
| 4d | 102.16 e | 85.48 e | 66.66 d | 13.79 e | 2.49 d |
| 4e | 114.46 d | 74.59 g | 58.13 e | 28.10 c | 19.00 b |
| 4f | 112.30 d | 79.54 f | 49.54 f | 19.34 d | 4.68 c |
| 4g | 86.72 f | 46.18 h | 30.28 h | 12.26 e | 2.99 d |
| 4h | 83.04 f | 74.41 i | 41.60 g | 10.20 e | 3.45 c |
| Tebuconazole | 9.35 g | 3.28 m | 0.00 i | 0.00 f | 0.00 e |
| Control | 206.70 a | 206.70 a | 206.70 a | 206.70 a | 206.70 a |
| Solutions | Regression Equations | ED50 (µg mL−1) | ED100 (µg mL−1) | |||
|---|---|---|---|---|---|---|
| MG | SP | MG | SP | MG | SP | |
| 4a | Y = 23,742 + 0.049x ** R2 = 0.98 | Y = 4145 + 0.6421 logx ** R2 = 0.91 | 529.50 | 21.48 | 1537.77 | 1439.60 |
| 4b | Y = 16,937 + 0.046x ** R2 = 0.92 | Y = 4295 + 0.608 logx * R2 = 0.99 | 715.72 | 14.45 | 1798.08 | 1384.10 |
| 4c | Y = 17,159 + 0.048x ** R2 = 0.90 | Y = 4642 + 0.515 logx ** R2 = 0.91 | 682.63 | 4.97 | 1.72194 | 1.42583 |
| 4d | Y = 19,419 + 0.057x ** R2 = 0.97 | Y = 4033 + 0.494 logx * R2 = 0.81 | 529.94 | 0.98 | 1396.40 | 1048.40 |
| 4e | Y = 14,677 + 0.070x ** R 2= 0.99 | Y = 4823 + 0.463 logx ** R2 = 0.99 | 502.20 | 2.41 | 1213.07 | 1425.00 |
| 4f | Y = 19,205 + 0.078x * R2 = 0.88 | Y = 25.6 + 20.7x − 1.2x2 ** R2 = 0.99 | 394.80 | 10.70 | 1035.83 | 1112.17 |
| 4g | Y = 8334 + 0.084x ** R2 = 0.97 | Y = 5183 + 0.517 logx ** R2 = 0.99 | 496.02 | 0.44 | 1091.26 | 1069.37 |
| 4h | Y = 14,334 + 0.069x ** R2 = 0.98 | Y = 5043 + 0.514 logx2 ** R2 = 0.95 | 519.76 | 0.83 | 1248.41 | 1098.15 |
| Tebuconazole | Y = 5627 + 1.097 logxns R2 = 0.55 | Y = 6598 + 0.640 logx2 ** R2 = 0.99 | 0.26 | <1 | 35.32 | 13.71 |
| Compounds | Concentrations (µg mL−1) | G% | GSI | RG | MI% | CA% | NA% |
|---|---|---|---|---|---|---|---|
| 4a | 50 | 98.40 a * | 11.13 ab | 7.76 ab | 6.88 c | 0.88 a | 0.36 c |
| 100 | 100.00 a | 11.12 ab | 8.08 ab | 6.70 d | 0.78 a | 0.40 c | |
| 250 | 98.40 a | 11.70 ab | 6.81 ab | 6.78 d | 1.00 b | 0.36 c | |
| 4b | 50 | 98.40 a | 11.43 ab | 7.22 ab | 6.76 d | 0.72 a | 0.38 c |
| 100 | 98.40 a | 10.97 ab | 7.13 ab | 6.26 d | 0.96 b | 0.38 c | |
| 250 | 100.00 a | 11.35 a | 9.05 a | 6.90 c | 0.80 a | 0.34 c | |
| 4c | 50 | 100.00 a | 11.27 a | 8.18 a | 6.94 c | 0.80 a | 0.30 c |
| 100 | 98.40 a | 11.01 a | 8.54 a | 6.26 d | 0.68 a | 0.40 c | |
| 250 | 100.00 a | 10.43 a | 9.91 a | 7.36 c | 0.82 a | 0.52 d | |
| 4d | 50 | 100.00 a | 11.28 a | 8.20 a | 7.10 c | 0.62 a | 0.20 a |
| 100 | 98.40 a | 10.98 a | 8.77 a | 7.16 c | 0.64 a | 0.28 c | |
| 250 | 99.20 a | 10.57 a | 8.80 a | 7.20 c | 0.68 a | 0.30 c | |
| 4e | 50 | 100.00 a | 11.37 a | 7.37 a | 7.3 c | 0.66 a | 0.34 c |
| 100 | 99.20 a | 10.73 a | 7.88 a | 6.88 c | 0.48 a | 0.36 c | |
| 250 | 99.20 a | 11.46 a | 6.92 a | 7.20 c | 0.56 a | 0.16 a | |
| 4f | 50 | 100.00 a | 11.51 a | 8.13 a | 7.88 a | 0.44 a | 0.06 b |
| 100 | 97.60 a | 10.77 a | 8.17 a | 7.76 a | 0.82 a | 0.20 a | |
| 250 | 99.20 a | 11.31 a | 7.84 a | 7.26 c | 0.48 a | 0.24 a | |
| 4g | 50 | 100.00 a | 11.05 a | 7.66 a | 7.9 a | 0.54 a | 0.20 a |
| 100 | 100.00 a | 11.53 a | 7.87 a | 8.14 b | 0.50 a | 0.08 b | |
| 250 | 96.80 a | 10.35 a | 7.95 a | 7.96 a | 0.44 a | 0.06 b | |
| 4h | 50 | 100.00 a | 11.66 a | 8.07 a | 8.00 a | 0.36 c | 0.02 b |
| 100 | 99.20 a | 11.47 a | 7.95 a | 8.04 a | 0.50 a | 0.08 b | |
| 250 | 100.00 a | 11.27 a | 9.26 a | 8.12 b | 0.56 a | 0.12 a | |
| water | 0 | 98.40 a | 10.90 a | 7.52 a | 7.88 a | 0.48 a | 0.16 a |
| dichloromethane | 0 | 100.00 a | 11.31 b | 7.48 b | 8.10 b | 0.48 a | 0.14 a |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.V.; Oliveira, M.V.L.d.; Pinto, R.T.; Moreira, L.C.; Gomes, E.M.C.; Alves, T.d.A.; Pinheiro, P.F.; Queiroz, V.T.d.; Vieira, L.F.A.; Teixeira, R.R.; et al. Synthesis of Novel Glycerol-Derived 1,2,3-Triazoles and Evaluation of Their Fungicide, Phytotoxic and Cytotoxic Activities. Molecules 2017, 22, 1666. https://doi.org/10.3390/molecules22101666
Costa AV, Oliveira MVLd, Pinto RT, Moreira LC, Gomes EMC, Alves TdA, Pinheiro PF, Queiroz VTd, Vieira LFA, Teixeira RR, et al. Synthesis of Novel Glycerol-Derived 1,2,3-Triazoles and Evaluation of Their Fungicide, Phytotoxic and Cytotoxic Activities. Molecules. 2017; 22(10):1666. https://doi.org/10.3390/molecules22101666
Chicago/Turabian StyleCosta, Adilson Vidal, Marcos Vinicius Lacerda de Oliveira, Roberta Tristão Pinto, Luiza Carvalheira Moreira, Ediellen Mayara Corrêa Gomes, Thammyres de Assis Alves, Patrícia Fontes Pinheiro, Vagner Tebaldi de Queiroz, Larissa Fonseca Andrade Vieira, Robson Ricardo Teixeira, and et al. 2017. "Synthesis of Novel Glycerol-Derived 1,2,3-Triazoles and Evaluation of Their Fungicide, Phytotoxic and Cytotoxic Activities" Molecules 22, no. 10: 1666. https://doi.org/10.3390/molecules22101666
APA StyleCosta, A. V., Oliveira, M. V. L. d., Pinto, R. T., Moreira, L. C., Gomes, E. M. C., Alves, T. d. A., Pinheiro, P. F., Queiroz, V. T. d., Vieira, L. F. A., Teixeira, R. R., & Júnior, W. C. d. J. (2017). Synthesis of Novel Glycerol-Derived 1,2,3-Triazoles and Evaluation of Their Fungicide, Phytotoxic and Cytotoxic Activities. Molecules, 22(10), 1666. https://doi.org/10.3390/molecules22101666