NMR Detection of Semi-Specific Antibody Interactions in Serum Environments
Abstract
:1. Introduction
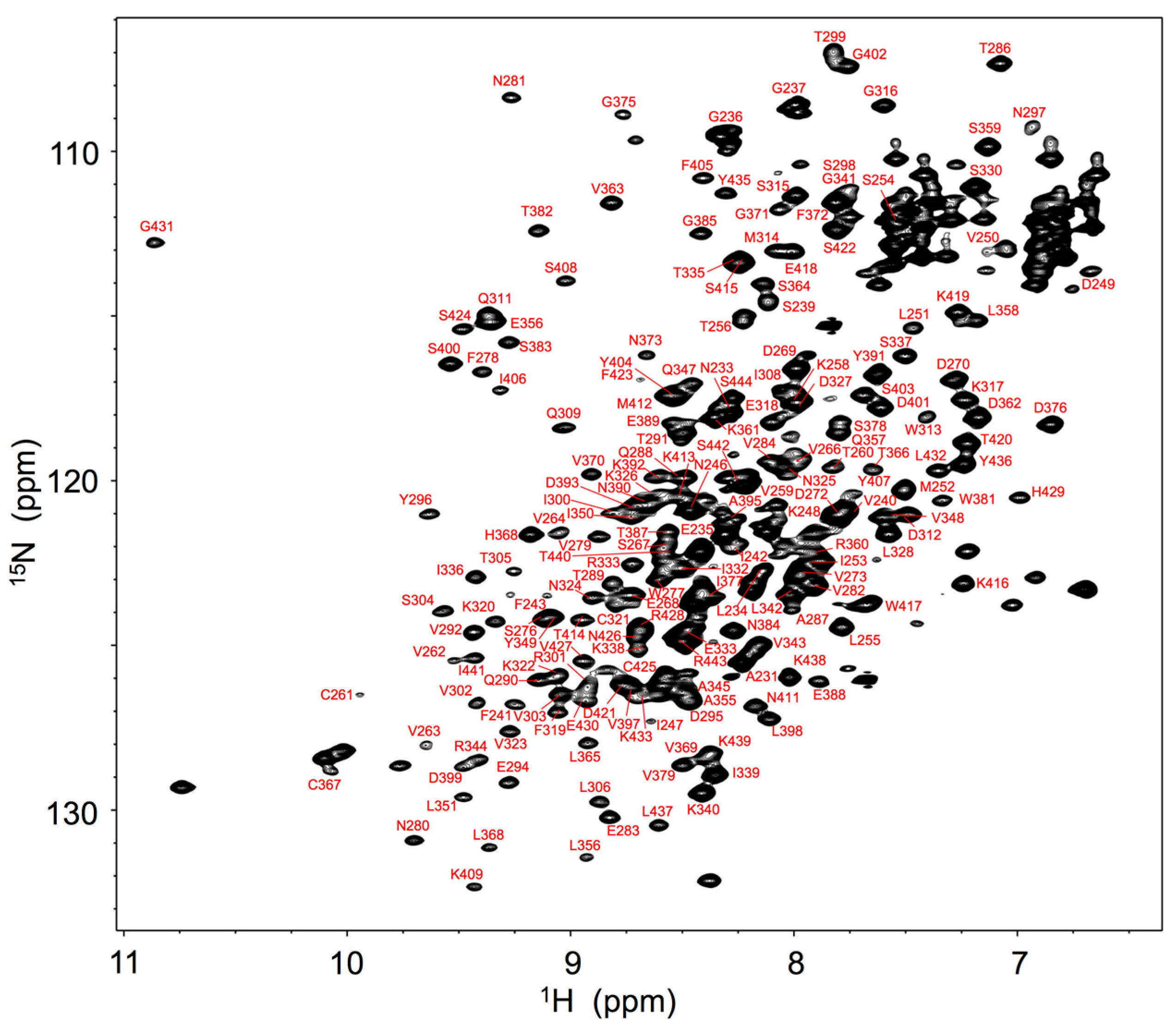
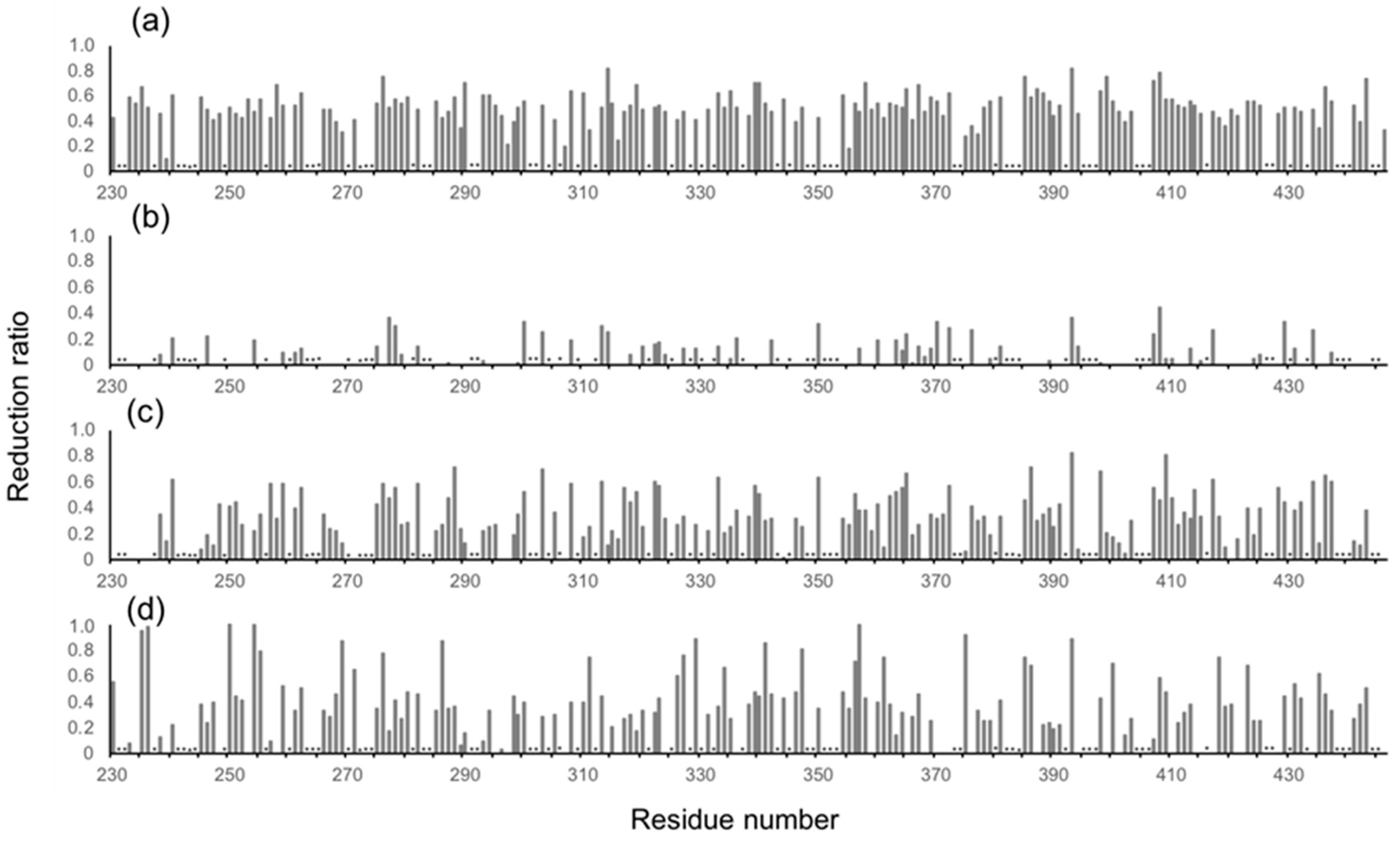
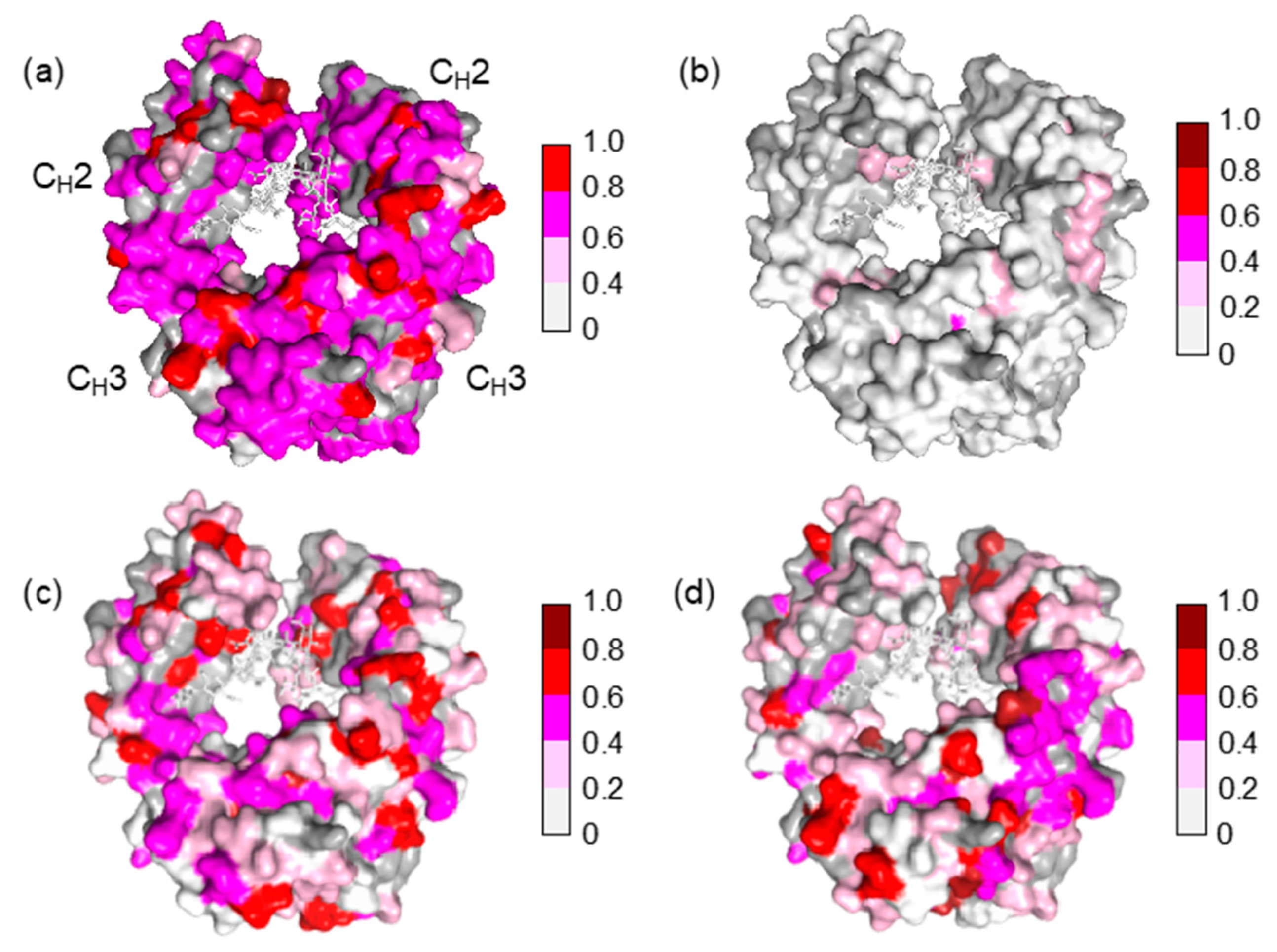
2. Results and Discussion
3. Materials and Methods
3.1. Human Serum
3.2. Preparation of Isotope-Labeled Fc
3.3. Viscosity Measurements
3.4. NMR Measurements and Spectral Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jerne, N.K. Towards a network theory of the immune system. Ann. D’immunologie 1974, 125C, 373–389. [Google Scholar]
- Malhotra, R.; Wormald, M.R.; Rudd, P.M.; Fischer, P.B.; Dwek, R.A.; Sim, R.B. Glycosylation changes of Igg associated with rheumatoid-arthritis can activate complement via the mannose-binding protein. Nat. Med. 1995, 1, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Krayukhina, E.; Noda, M.; Ishii, K.; Maruno, T.; Wakabayashi, H.; Tada, M.; Suzuki, T.; Ishii-Watabe, A.; Kato, M.; Uchiyama, S. Analytical ultracentrifugation with fluorescence detection system reveals differences in complex formation between recombinant human TNF and different biological TNF antagonists in various environments. Mabs-Austin 2017, 9, 664–679. [Google Scholar] [CrossRef] [PubMed]
- Reichert, J.M. Antibodies to watch in 2017. Mabs-Austin 2017, 9, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Dillman, R.O.; Shawler, D.L.; McCallister, T.J.; Halpern, S.E. Human anti-mouse antibody response in cancer patients following single low-dose injections of radiolabeled murine monoclonal antibodies. Cancer Biother. Radiopharm. 1994, 9, 17–28. [Google Scholar] [CrossRef]
- Ritter, G.; Cohen, L.S.; Williams, C., Jr.; Richards, E.C.; Old, L.J.; Welt, S. Serological analysis of human anti-human antibody responses in colon cancer patients treated with repeated doses of humanized monoclonal antibody A33. Cancer Res. 2001, 61, 6851–6859. [Google Scholar] [PubMed]
- Hansel, T.T.; Kropshofer, H.; Singer, T.; Mitchell, J.A.; George, A.J. The safety and side effects of monoclonal antibodies. Nat. Rev. Drug Discov. 2010, 9, 325–338. [Google Scholar] [CrossRef] [PubMed]
- Narciso, J.E.; Uy, I.D.; Cabang, A.B.; Chavez, J.F.; Pablo, J.L.; Padilla-Concepcion, G.P.; Padlan, E.A. Analysis of the antibody structure based on high-resolution crystallographic studies. New Biotechnol. 2011, 28, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Sela-Culang, I.; Kunik, V.; Ofran, Y. The structural basis of antibody-antigen recognition. Front. Immunol. 2013, 4, 302. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Yamaguchi, Y. Glycoproteins and Antibodies: Solution NMR Studies. In Encyclopedia of Magnetic Resonance; Harris, R.K., Wasylishen, R.E., Eds.; John Wiley: Chichester, UK, 2011; pp. 1779–1790. [Google Scholar]
- Inomata, K.; Ohno, A.; Tochio, H.; Isogai, S.; Tenno, T.; Nakase, I.; Takeuchi, T.; Futaki, S.; Ito, Y.; Hiroaki, H.; et al. High-resolution multi-dimensional NMR spectroscopy of proteins in human cells. Nature 2009, 458, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Theillet, F.X.; Binolfi, A.; Bekei, B.; Martorana, A.; Rose, H.M.; Stuiver, M.; Verzini, S.; Lorenz, D.; van Rossum, M.; Goldfarb, D.; et al. Structural disorder of monomeric α-synuclein persists in mammalian cells. Nature 2016, 530, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.L.; Anderson, N.G. The human plasma proteome: history, character, and diagnostic prospects. Mol. Cell. Proteom. 2002, 1, 845–867. [Google Scholar] [CrossRef]
- Solá, R.J.; Griebenow, K. Glycosylation of therapeutic proteins: An effective strategy to optimize efficacy. BioDrugs Clin. Immunother. Biopharma. Gene Ther. 2010, 24, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Solá, R.J.; Griebenow, K. Effects of glycosylation on the stability of protein pharmaceuticals. J. Pharm. Sci. 2009, 98, 1223–1245. [Google Scholar] [CrossRef] [PubMed]
- Mimura, Y.; Katoh, T.; Saldova, R.; O’Flaherty, R.; Izumi, T.; Mimura-Kimura, Y.; Utsunomiya, T.; Mizukami, Y.; Yamamoto, K.; Matsumoto, T.; et al. Glycosylation engineering of therapeutic IgG antibodies: Challenges for the safety, functionality and efficacy. Protein Cell 2017. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Yamaguchi, Y.; Arata, Y. Stable-isotope-assisted NMR approaches to glycoproteins using immunoglobulin G as a model system. Prog. Nucl. Magn. Reson. Spectrosc. 2010, 56, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Zhang, Y.; Yagi-Utsumi, M.; Yamaguchi, T.; Iida, S.; Yamaguchi, Y.; Kato, K. Backbone 1H, 13C, and 15N resonance assignments of the Fc fragment of human immunoglobulin G glycoprotein. Biomol. NMR Assign. 2015, 9, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Kato, K. Dynamics and interactions of glycoconjugates probed by stable-isotope-assisted NMR spectroscopy. Methods Enzymol. 2010, 478, 305–322. [Google Scholar] [PubMed]
- Yagi, H.; Fukuzawa, N.; Tasaka, Y.; Matsuo, K.; Zhang, Y.; Yamaguchi, T.; Kondo, S.; Nakazawa, S.; Hashii, N.; Kawasaki, N.; et al. NMR-based structural validation of therapeutic antibody produced in Nicotiana benthamiana. Plant Cell Rep. 2015, 34, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Yagi, H.; Kato, K. Stable isotope labeling of glycoproteins for NMR study. In NMR in Glycoscience and Glycotechnology; Kato, K., Peters, T., Eds.; RSC Publishing: Cambridge, UK, 2017; pp. 194–205. [Google Scholar]
- Yagi, H.; Nakamura, M.; Yokoyama, J.; Zhang, Y.; Yamaguchi, T.; Kondo, S.; Kobayashi, J.; Kato, T.; Park, E.Y.; Nakazawa, S.; et al. Stable isotope labeling of glycoprotein expressed in silkworms using immunoglobulin G as a test molecule. J. Biomol. NMR 2015, 62, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Driscoll, P.C. Macrimolecular Complexes. In Protein NMR Spectroscopy: Principal Techniques and Applications; Lian, L., Roberts, G., Eds.; John Wiley: Chichester, UK, 2011; pp. 270–317. [Google Scholar]
- Idusogie, E.E.; Presta, L.G.; Gazzano-Santoro, H.; Totpal, K.; Wong, P.Y.; Ultsch, M.; Meng, Y.G.; Mulkerrin, M.G. Mapping of the C1q binding site on rituxan, a chimeric antibody with a human IgG1 Fc. J. Immunol. 2000, 164, 4178–4184. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, T.; Yagi, H.; Takemoto, E.; Shibata-Koyama, M.; Isoda, Y.; Iida, S.; Masuda, K.; Satoh, M.; Kato, K. Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans. Genes Cells 2011, 16, 1071–1080. [Google Scholar] [CrossRef] [PubMed]
- Kiyoshi, M.; Caaveiro, J.M.; Kawai, T.; Tashiro, S.; Ide, T.; Asaoka, Y.; Hatayama, K.; Tsumoto, K. Structural basis for binding of human IgG1 to its high-affinity human receptor FcγRI. Nat. Commun. 2015, 6, 6866. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.L.; West, A.P., Jr.; Gan, L.; Bjorkman, P.J. Crystal structure at 2.8 Å of an FcRn/heterodimeric Fc complex: Mechanism of pH-dependent binding. Mol. Cell 2001, 7, 867–877. [Google Scholar] [CrossRef]
- Ferrara, C.; Grau, S.; Jäger, C.; Sondermann, P.; Brünker, P.; Waldhauer, I.; Hennig, M.; Ruf, A.; Rufer, A.C.; Stihle, M.; et al. Unique carbohydrate-carbohydrate interactions are required for high affinity binding between FcγRIII and antibodies lacking core fucose. Proc. Natl. Acad. Sci. USA 2011, 108, 12669–12674. [Google Scholar] [CrossRef] [PubMed]
- Sun, P. Structural Recognition of Immunoglobulins by Fcγ Receptors. In Antibody Fc: Linking Adaptive and Innate Immunity; Ackerman, M.E., Nimmerjahn, F., Eds.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Kolenko, P.; Dohnálek, J.; Dusková, J.; Skálová, T.; Collard, R.; Hasek, J. New insights into intra- and intermolecular interactions of immunoglobulins: Crystal structure of mouse IgG2b-Fc at 2.1-Å resolution. Immunology 2009, 126, 378–385. [Google Scholar] [CrossRef] [PubMed]
- DeLano, W.L. The PyMOL Molecular Graphics System; DeLano Scientific: San Carlos, CA, USA, 2002. [Google Scholar]
- Yagi, H.; Takahashi, N.; Yamaguchi, Y.; Kato, K. Temperature-dependent isologous Fab-Fab interaction that mediates cryocrystallization of a monoclonal immunoglobulin G. Mol. Immunol. 2004, 41, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Sawada, J.; Terao, T.; Itoh, S.; Maeda, M.; Tsuji, A.; Hosoda, H.; Nambara, T. Production and characterization of monoclonal antibodies to 17 α-hydroxyprogesterone. J. Steroid Biochem. 1987, 28, 405–410. [Google Scholar] [PubMed]
- Yamaguchi, Y.; Kim, H.; Kato, K.; Masuda, K.; Shimada, I.; Arata, Y. Proteolytic fragmentation with high specificity of mouse immunoglobulin G. Mapping of proteolytic cleavage sites in the hinge region. J. Immunol. Methods 1995, 181, 259–267. [Google Scholar] [CrossRef]
- Delaglio, F.; Grzesiek, S.; Vuister, G.W.; Zhu, G.; Pfeifer, J.; Bax, A. Nmrpipe—A multidimensional spectral processing system based on Unix Pipes. J. Biomol. NMR 1995, 6, 277–293. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Iwahara, J.; Koshiba, S.; Tomizawa, T.; Tochio, N.; Güntert, P.; Kigawa, T.; Yokoyama, S. KUJIRA, a package of integrated modules for systematic and interactive analysis of NMR data directed to high-throughput NMR structure studies. J. Biomol. NMR 2007, 39, 31–52. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Harano, Y.; Tochio, N.; Nakatani, E.; Kigawa, T.; Yokoyama, S.; Mading, S.; Ulrich, E.L.; Markley, J.L.; Akutsu, H.; et al. An automated system designed for large scale NMR data deposition and annotation: Application to over 600 assigned chemical shift data entries to the BioMagResBank from the Riken Structural Genomics/Proteomics Initiative internal database. J. Biomol. NMR 2012, 53, 311–320. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples are not available from the authors. |

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yanaka, S.; Yamazaki, T.; Yogo, R.; Noda, M.; Uchiyama, S.; Yagi, H.; Kato, K. NMR Detection of Semi-Specific Antibody Interactions in Serum Environments. Molecules 2017, 22, 1619. https://doi.org/10.3390/molecules22101619
Yanaka S, Yamazaki T, Yogo R, Noda M, Uchiyama S, Yagi H, Kato K. NMR Detection of Semi-Specific Antibody Interactions in Serum Environments. Molecules. 2017; 22(10):1619. https://doi.org/10.3390/molecules22101619
Chicago/Turabian StyleYanaka, Saeko, Toshio Yamazaki, Rina Yogo, Masanori Noda, Susumu Uchiyama, Hirokazu Yagi, and Koichi Kato. 2017. "NMR Detection of Semi-Specific Antibody Interactions in Serum Environments" Molecules 22, no. 10: 1619. https://doi.org/10.3390/molecules22101619
APA StyleYanaka, S., Yamazaki, T., Yogo, R., Noda, M., Uchiyama, S., Yagi, H., & Kato, K. (2017). NMR Detection of Semi-Specific Antibody Interactions in Serum Environments. Molecules, 22(10), 1619. https://doi.org/10.3390/molecules22101619




