Effect of a Polymercaptan Material on the Electro-Optical Properties of Polymer-Dispersed Liquid Crystal Films
Abstract
:1. Introduction
2. Results and Discussion
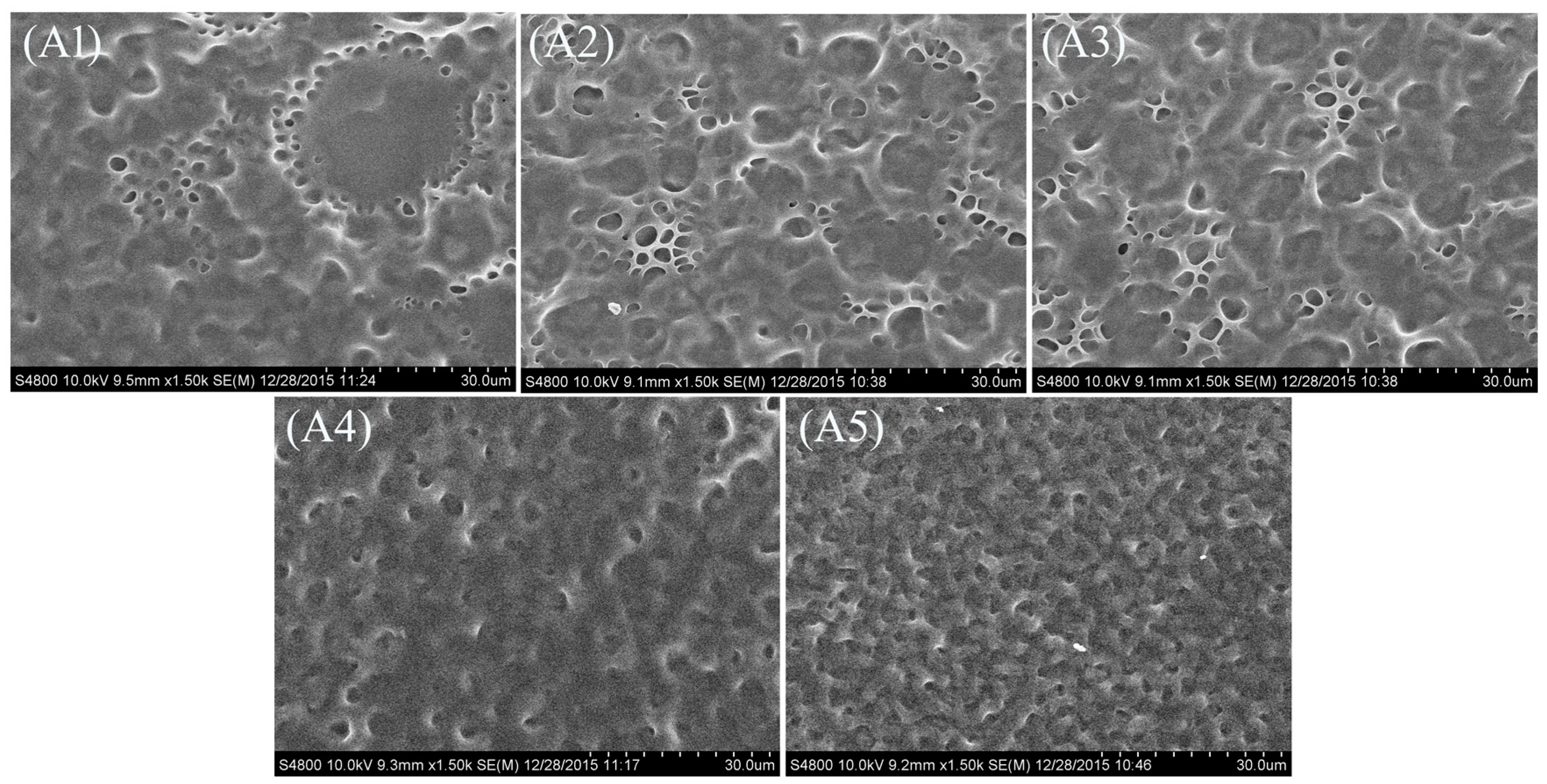
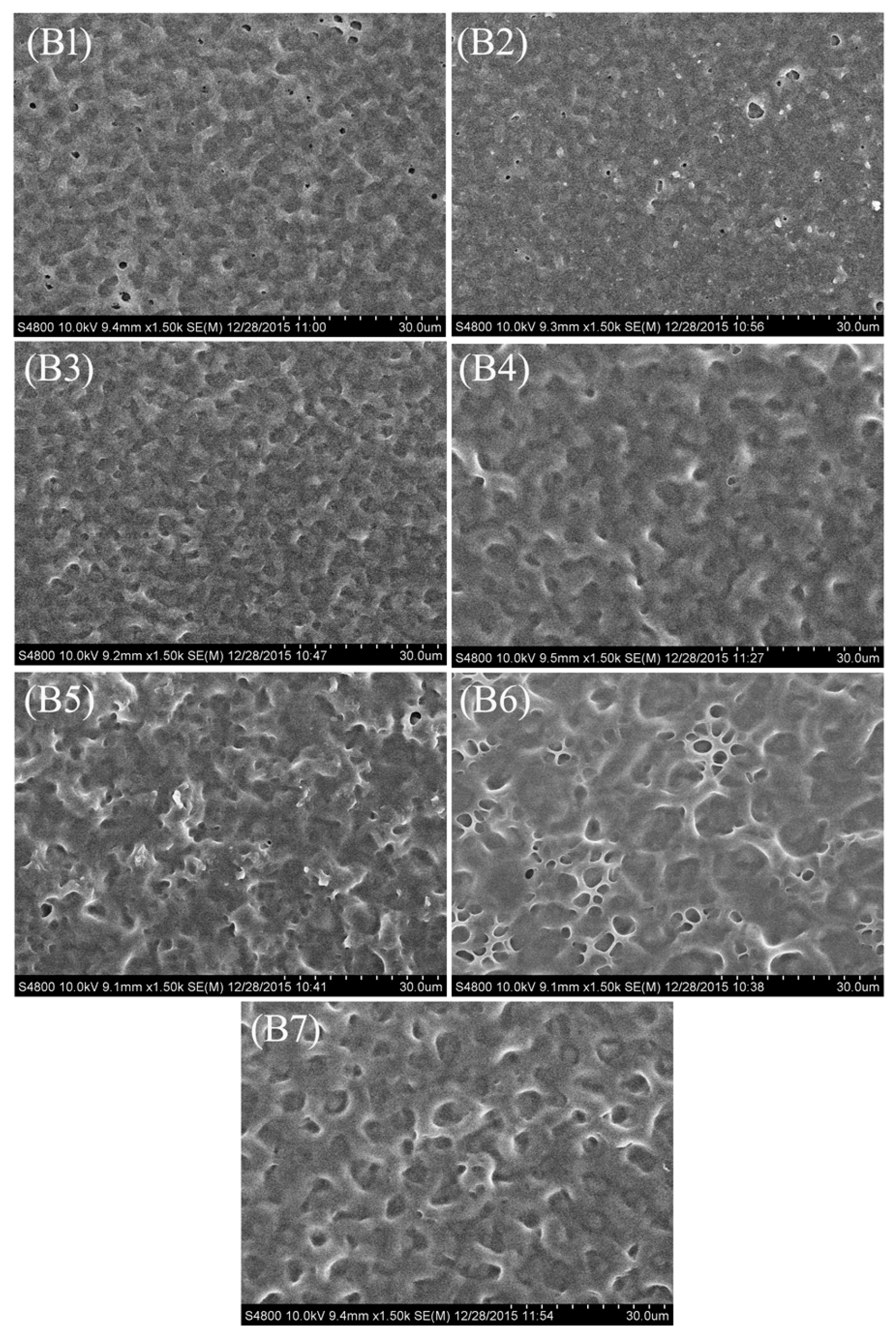
2.1. Morphology of the Polymer Network in the PDLC Films
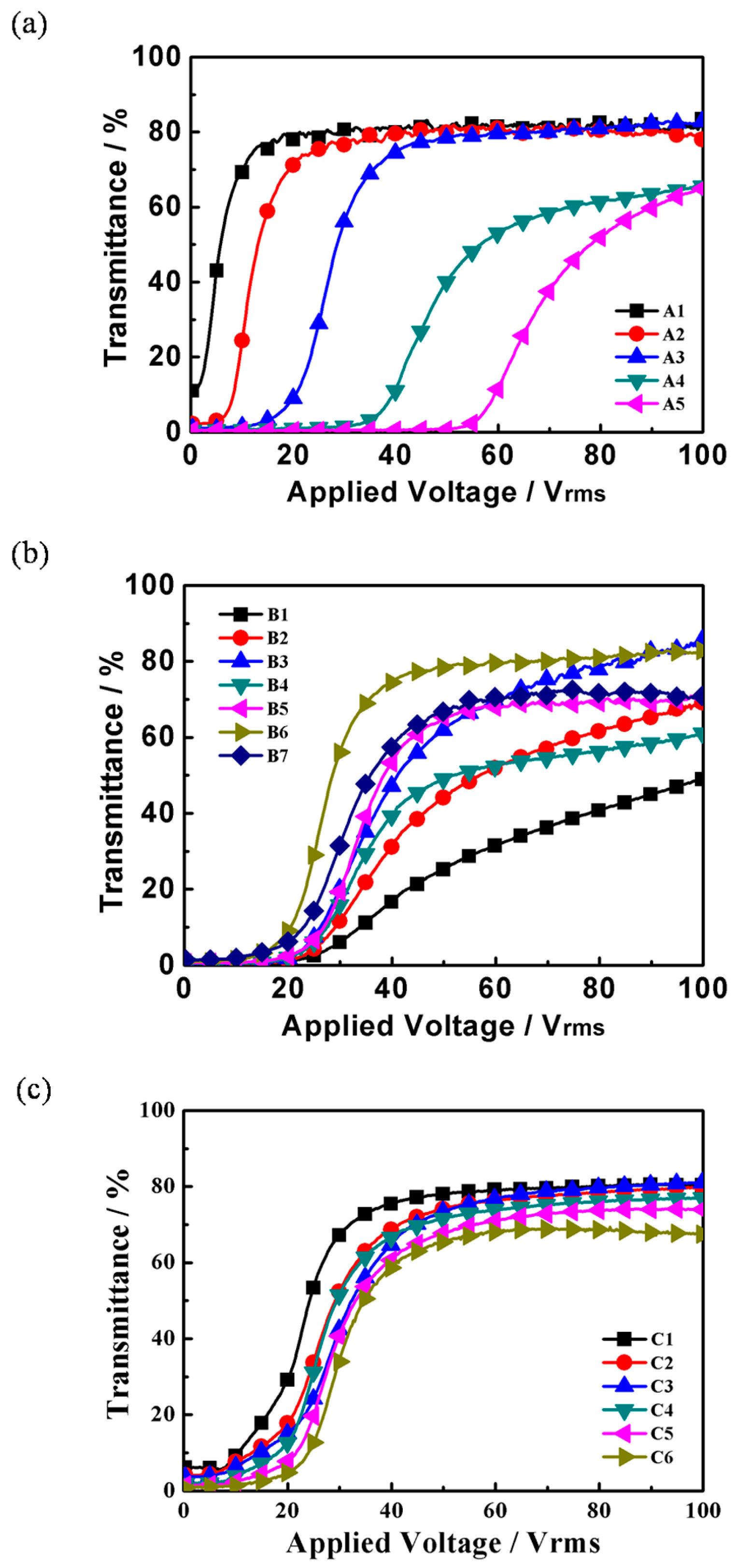
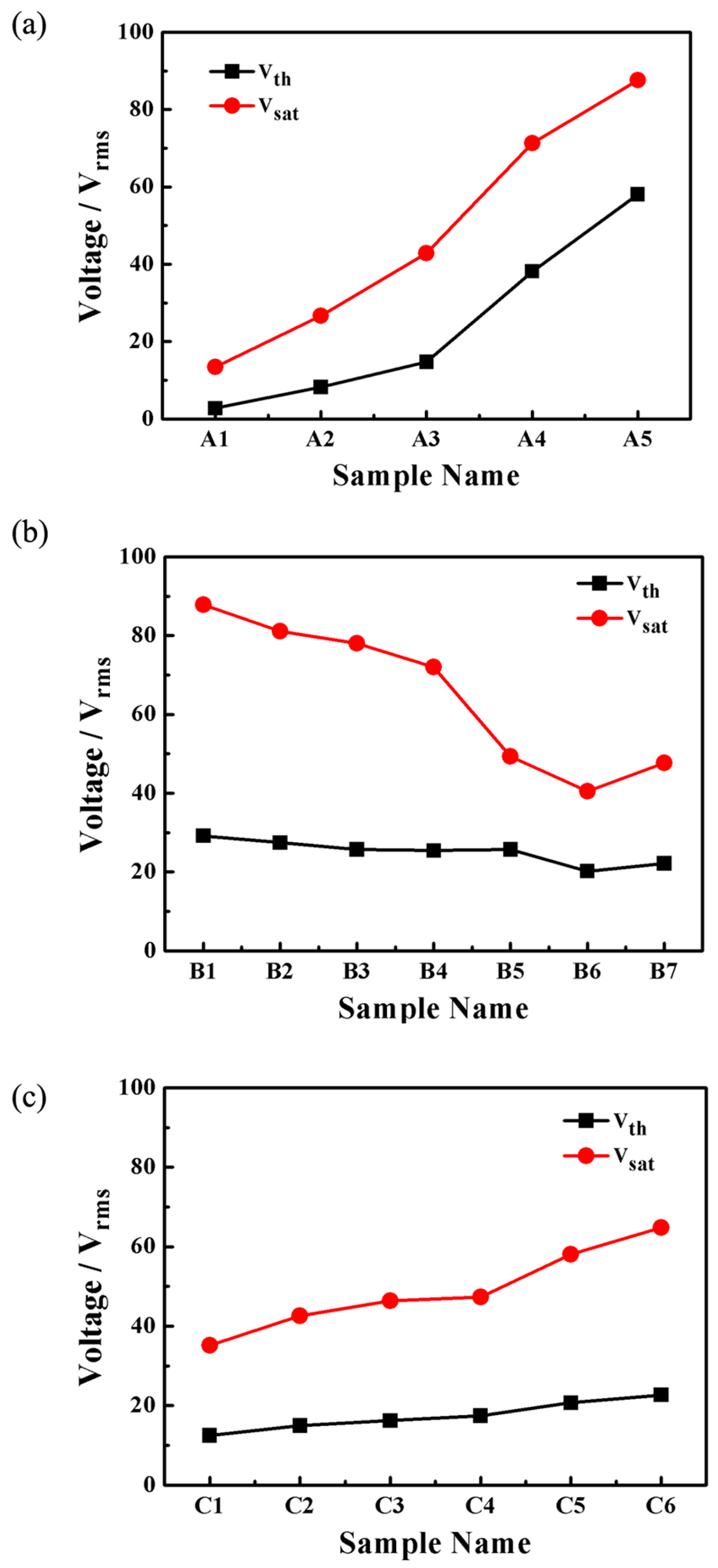
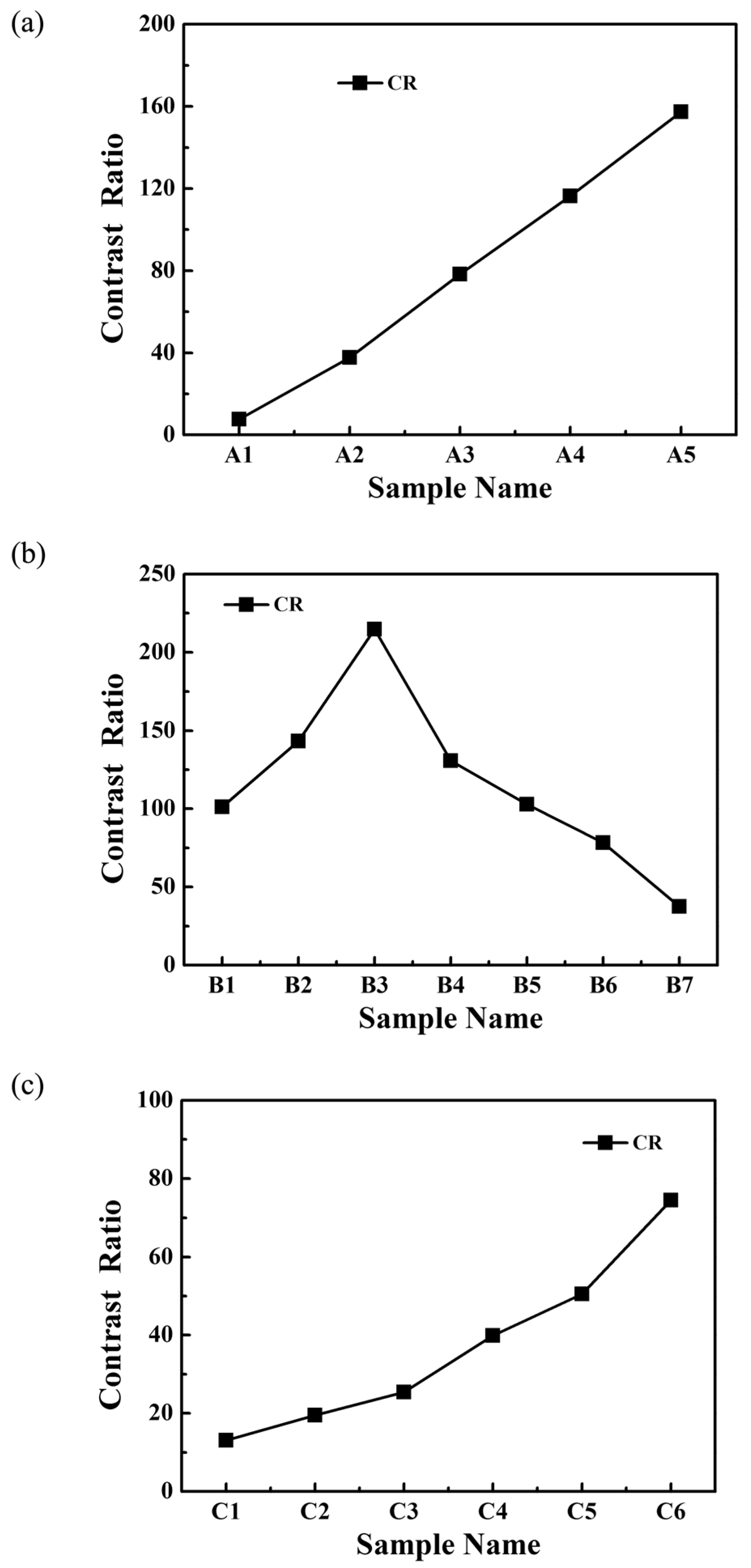
2.2. Electro-Optical Properties of PDLC Films
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation
3.3. Morphological Analysis
3.4. Electro-Optical Measurements
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheng, Z.; Wang, T.; Li, X.; Zhang, Y.; Yu, H.F. NIR-VIS-UV light-responsive actuator films of polymer-dispersed liquid crystal/graphene oxide nanocomposites. ACS Appl. Mater. Interfaces 2015, 7, 27494–27501. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Cheng, Z.; Dong, Z.; Zhang, Y.; Yu, H.F. Photomechanical response of polymer-dispersed liquid crystals/graphene oxide nanocomposites. J. Mater. Chem. C 2014, 2, 8501–8506. [Google Scholar] [CrossRef]
- Doane, J.W.; Golemme, A.; West, J.L.; Whitehead, J.B., Jr.; Wu, B.G. Polymer dispersed liquid crystals for display application. Mol. Cryst. Liq. Cryst. 1988, 165, 511–532. [Google Scholar] [CrossRef]
- Malik, P.; Raina, K.K. Droplet orientation and optical properties of polymer dispersed liquid crystal composite films. Opt. Mater. 2004, 27, 613–617. [Google Scholar] [CrossRef]
- Kovalchuk, A.V.; Kurik, M.V.; Lavrentovich, O.D.; Sergan, V.V. Structural transformations in nematic drops located in an external electric-field. Sov. Phys. JETP 1988, 67, 1065–1073. [Google Scholar]
- Kovalchuk, A.V.; Lavrentovich, O.D.; Sergan, V.V. Kenetic of electrooptical effects in drops of nematic with different structure. Sov. Tech. Phys. Lett. 1989, 15, 529–533. [Google Scholar]
- Doane, J.W.; Vaz, N.A.; Wu, B.G.; Žumer, S. Field controlled light scattering from nematic microdroplets. Appl. Phys. Lett. 1986, 48, 269–271. [Google Scholar] [CrossRef]
- Higgins, D.A.; Hall, J.E.; Xie, A. Optical microscopy studies of dynamics within individual polymer-dispersed liquid crystal droplets. Acc. Chem. Res. 2005, 38, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Lovinger, A.J.; Amundson, K.R.; Davis, D.D. Morphological investigation of UV-curable polymer-dispersed liquid-crystal (PDLC) materials. Chem. Mater. 1994, 6, 1726–1736. [Google Scholar] [CrossRef]
- Jain, S.C.; Thakur, R.S. Thermo-electro-optic switch based on polymer dispersed liquid crystal composite. Appl. Phys. Lett. 1992, 61, 1641–1642. [Google Scholar] [CrossRef]
- Drzaic, P.S. Polymer dispersed nematic liquid crystal for large area displays and light valves. J. Appl. Phys. 1986, 60, 2142–2148. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, J.; Sun, Y.; Yang, H.; Yu, H.F. Erasable thin-film optical diode based on a photoresponsive liquid crystal polymer. Nanoscale 2014, 6, 3854–3860. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, S.; Masumoto, K.; Nonaka, T. Optical shutter driven photochemically from anisotropic polymer network containing liquid crystalline and azobenzene molecules. Appl. Phys. Lett. 1998, 73, 160–162. [Google Scholar] [CrossRef]
- Wang, L.; He, W.; Xiao, X.; Wang, M.; Wang, M.; Yang, P.; Zhou, Z.; Yang, H.; Yu, H.F.; Lu, Y. Low voltage and hysteresis-free blue phase liquid crystal dispersed by ferroelectric nanoparticles. J. Mater. Chem. 2012, 22, 19629–19633. [Google Scholar] [CrossRef]
- Kim, S.H.; Heo, C.P.; Park, K.S.; Kim, B.K. Effect of prepolymer structure on the electro-optic performance of polymer dispersed liquid crystals. Polym. Int. 1998, 46, 143–149. [Google Scholar] [CrossRef]
- Li, W.; Cao, H.; Kashima, M.; Liu, F.; Cheng, Z.; Yang, Z.; Zhu, S.; Yang, H. Control of the microstructure of polymer network and effects of the microstructures on light scattering properties of UV-cured polymer-dispersed liquid crystal films. J. Phys. Chem. C 2008, 46, 2090–2099. [Google Scholar] [CrossRef]
- Tao, H.J.; Zhang, J.; Wang, X.L.; Gao, J. Phase separation and polymer crystallization in a poly (4-methyl-1-pentene)-dioctylsebacate-dimethylphthalate system via thermally induced phase separation. J. Polym. Sci. Part B Polym. Phys. 2007, 45, 153–161. [Google Scholar] [CrossRef]
- Liu, J.H.; Wu, F.T. Synthesis of photoisomeric azobenzene monomers and model compound effect on electric-optical properties in PDLC films. J. Appl. Polym. Sci. 2005, 97, 721–732. [Google Scholar] [CrossRef]
- Liu, F.; Cao, H.; Mao, Q.; Song, P.; Yang, H. Effects of monomer structure on the morphology of polymer networks and the electro-optical properties of polymer-dispersed liquid crystal films. Liq. Cryst. 2012, 39, 419–424. [Google Scholar] [CrossRef]
- Schulte, M.D.; Clarson, S.J.; Natarajan, L.V.; Tomlin, D.W.; Bunning, T.J. The effect of fluorine-substituted acrylate monomers on the electro-optical and morphological properties of polymer dispersed liquid crystals. Liq. Cryst. 2000, 27, 467–475. [Google Scholar] [CrossRef]
- Senyurt, A.F.; Warren, G.; Whitehead, J.B., Jr.; Hoyle, C.E. Matrix physical structure effect on the electro-optic characteristics of thiol-ene based H-PDLC films. Polymer 2006, 47, 2741–2749. [Google Scholar] [CrossRef]
- White, T.J.; Natarajan, L.V.; Tondiglia, V.P.; Bunning, T.J.; Guymon, C.A. Polymerization kinetics and monomer functionality effects in thiol-ene polymer dispersed liquid crystals. Macromolecules 2007, 40, 1112–1120. [Google Scholar] [CrossRef]
- Hoyle, C.E.; Lee, T.Y.; Roper, T. Thiol–enes: chemistry of the past with promise for the future. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5301–5338. [Google Scholar] [CrossRef]
- Morgan, C.R.; Magnotta, F.; Ketley, A.D. Thlol/ene photocurable polymers. J. Polym. Sci. Part A Polym. Chem. 1977, 15, 627–645. [Google Scholar] [CrossRef]
- Cramer, N.B.; Scott, J.P.; Bowman, C.N. Photopolymerizations of thiol-ene polymers without photoinitiators. Macromolecules 2002, 35, 5361–5365. [Google Scholar] [CrossRef]
- Szczepanski, C.R.; Pfeifer, C.S.; Stansbury, J.W. A new approach to network heterogeneity: polymerization induced phase separation in photo-initiated, free-radical methacrylic systems. Polymer 2012, 53, 4694–4701. [Google Scholar] [CrossRef] [PubMed]
- Kade, M.J.; Burke, D.J.; Hawker, C.J. The power of thiol-ene chemistry. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 743–750. [Google Scholar] [CrossRef]
- Northrop, B.H.; Coffey, R.N. Thiol−ene click chemistry: computational and kinetic analysis of the influence of alkene functionality. J. Am. Chem. Soc. 2012, 134, 13804–13817. [Google Scholar] [CrossRef] [PubMed]
- Mucha, M. Polymer as an important component of blends and composites with liquid crystals. Prog. Polym. Sci. 2003, 28, 837–873. [Google Scholar] [CrossRef]
- Jain, S.C.; Rout, D.K. Electro-optic response of polymer dispersed liquid crystal films. J. Appl. Phys. 1991, 70, 6988–6992. [Google Scholar] [CrossRef]
- Wu, B.G.; Erdmann, J.H.; Doane, J.W. Response times and voltages for PDLC light shutters. Liq. Cryst. 1989, 5, 1453–1465. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the PDLC films are available from the authors.
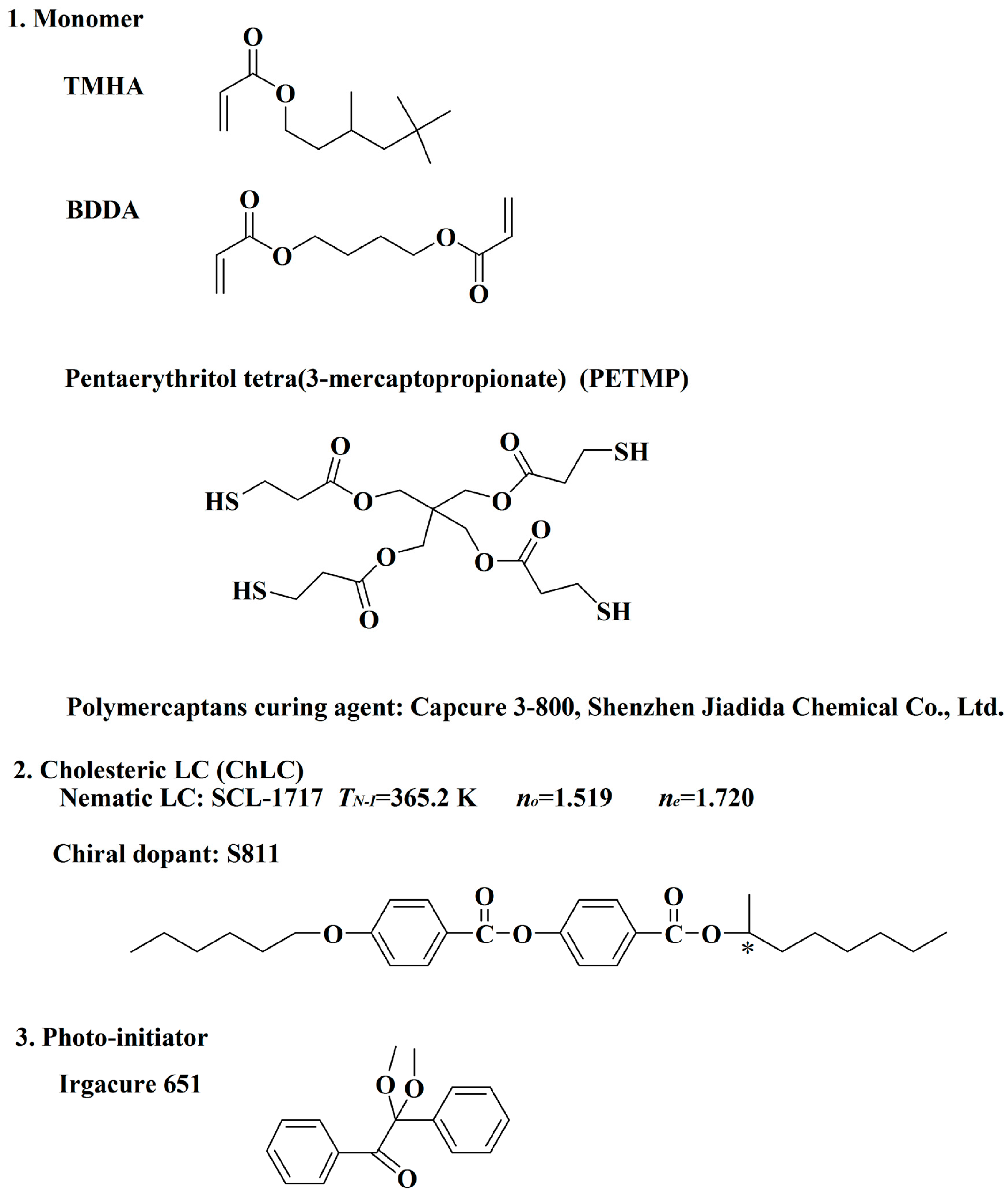

| Sample | Monomers (20.0 wt %) | LC (80.0 wt %) | |
|---|---|---|---|
| Composition of Monomers | |||
| Group A | TMHA + BDDAa/Capcure 3-800 | SLC1717/S811 | |
| A1 | 11.0/9.0 | 80.0/- | |
| A2 | 11.0/9.0 | 79.0/1.0 | |
| A3 | 11.0/9.0 | 77.0/3.0 | |
| A4 | 11.0/9.0 | 75.0/5.0 | |
| A5 | 11.0/9.0 | 73.0/7.0 | |
| Group B | TMHA + BDDA a/Capcure 3-800 | SLC1717/S811 | |
| B1 | 20.0/- | 77.0/3.0 | |
| B2 | 19.0/1.0 | 77.0/3.0 | |
| B3 | 17.0/3.0 | 77.0/3.0 | |
| B4 | 15.0/5.0 | 77.0/3.0 | |
| B5 | 13.0/7.0 | 77.0/3.0 | |
| B6 | 11.0/9.0 | 77.0/3.0 | |
| B7 | 9.0/11.0 | 77.0/3.0 | |
| Group C | TMHA + BDDA a | Capcure 3-800/PETMP | SLC1717/S811 |
| C1 | 10.0 | -/10.0 | 77.0/3.0 |
| C2 | 10.0 | 2.0/8.0 | 77.0/3.0 |
| C3 | 10.0 | 4.0/6.0 | 77.0/3.0 |
| C4 | 10.0 | 6.0/4.0 | 77.0/3.0 |
| C5 | 10.0 | 8.0/2.0 | 77.0/3.0 |
| C6 | 10.0 | 10.0/- | 77.0/3.0 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhang, C.; Zhou, L.; Fang, H.; Huang, J.; Ma, H.; Zhang, Y.; Yang, J.; Zhang, L.-Y.; Song, P.; et al. Effect of a Polymercaptan Material on the Electro-Optical Properties of Polymer-Dispersed Liquid Crystal Films. Molecules 2017, 22, 43. https://doi.org/10.3390/molecules22010043
Sun Y, Zhang C, Zhou L, Fang H, Huang J, Ma H, Zhang Y, Yang J, Zhang L-Y, Song P, et al. Effect of a Polymercaptan Material on the Electro-Optical Properties of Polymer-Dispersed Liquid Crystal Films. Molecules. 2017; 22(1):43. https://doi.org/10.3390/molecules22010043
Chicago/Turabian StyleSun, Yujian, Cuihong Zhang, Le Zhou, Hua Fang, Jianhua Huang, Haipeng Ma, Yi Zhang, Jie Yang, Lan-Ying Zhang, Ping Song, and et al. 2017. "Effect of a Polymercaptan Material on the Electro-Optical Properties of Polymer-Dispersed Liquid Crystal Films" Molecules 22, no. 1: 43. https://doi.org/10.3390/molecules22010043
APA StyleSun, Y., Zhang, C., Zhou, L., Fang, H., Huang, J., Ma, H., Zhang, Y., Yang, J., Zhang, L.-Y., Song, P., Gao, Y., Xiao, J., Li, F., & Li, K. (2017). Effect of a Polymercaptan Material on the Electro-Optical Properties of Polymer-Dispersed Liquid Crystal Films. Molecules, 22(1), 43. https://doi.org/10.3390/molecules22010043








