The Influence of Anionic Initiator on the Selected Properties of Poly-N-Isopropyl Acrylamide Evaluated for Controlled Drug Delivery
Abstract
:1. Introduction
2. Results
2.1. Synthesis Course
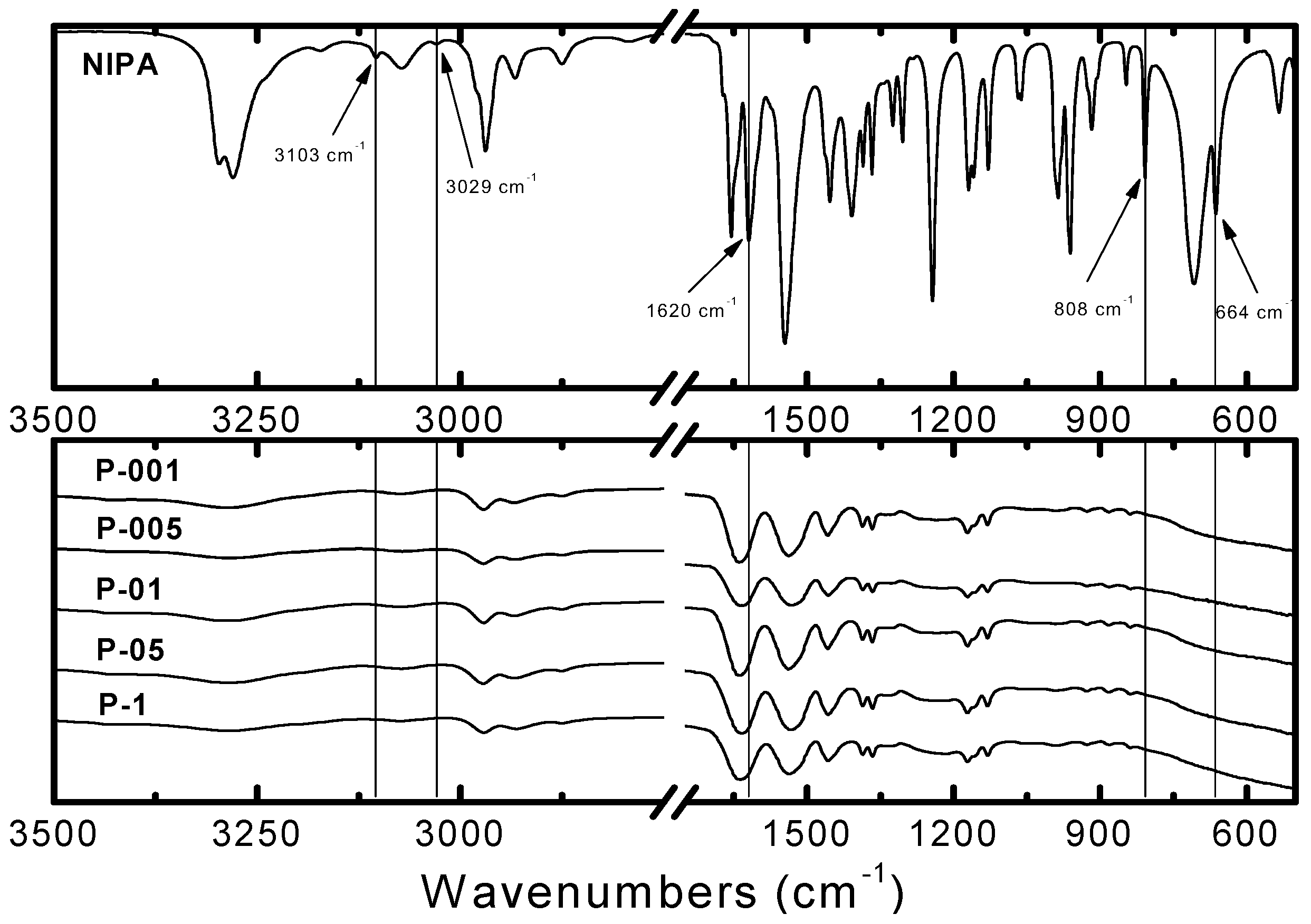
2.2. Fourier Transform Infrared Spectroscopy Measurements
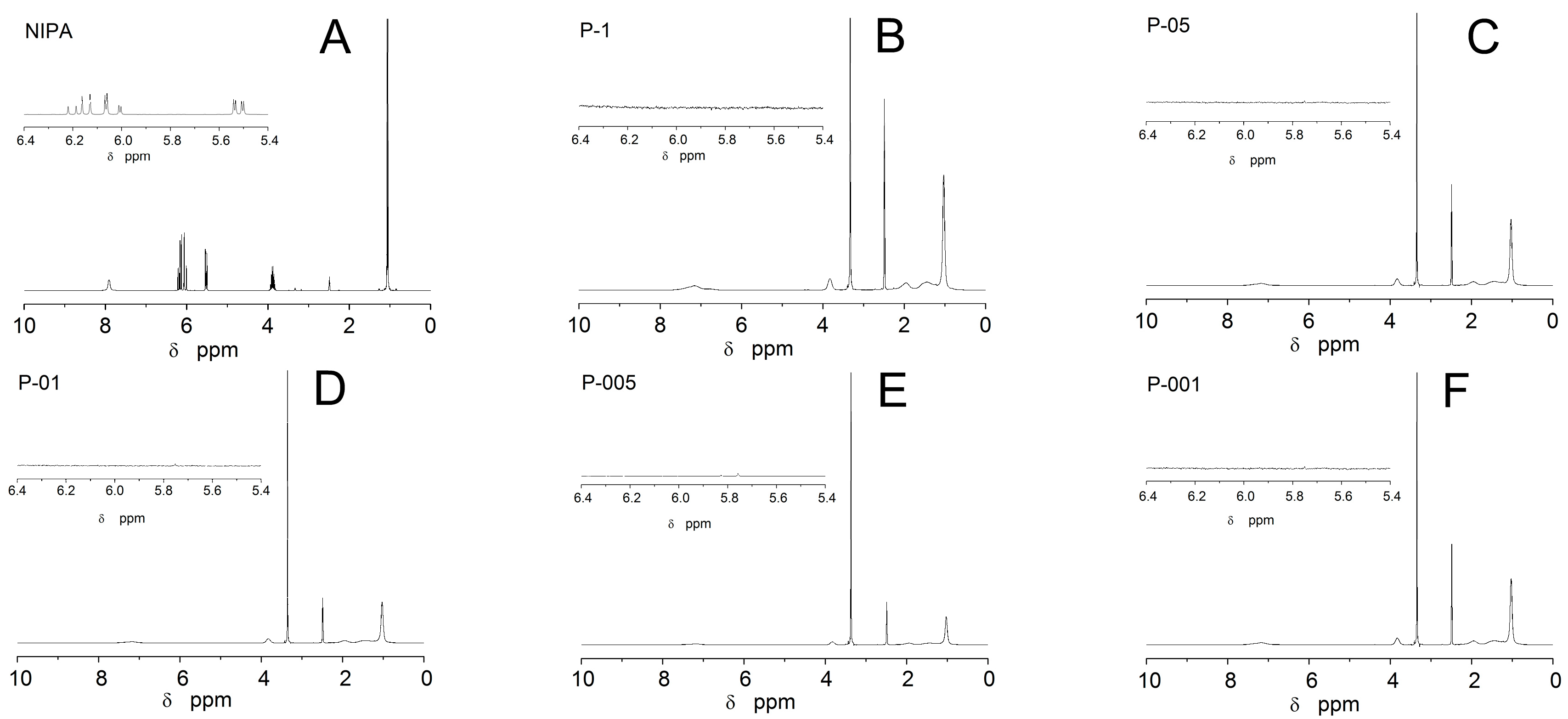
2.3. Nuclear Magnetic Resonance Spectroscopy Measurements
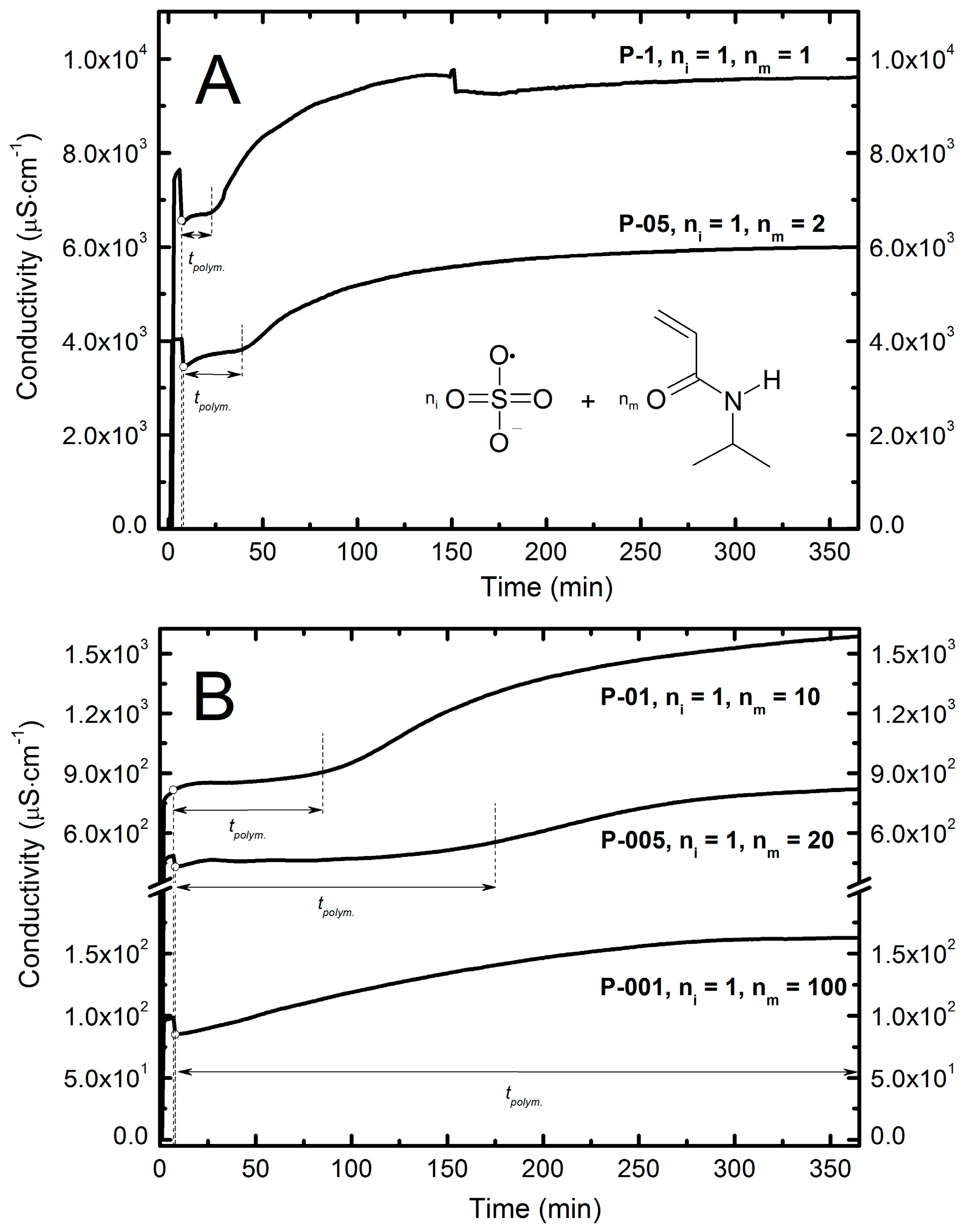
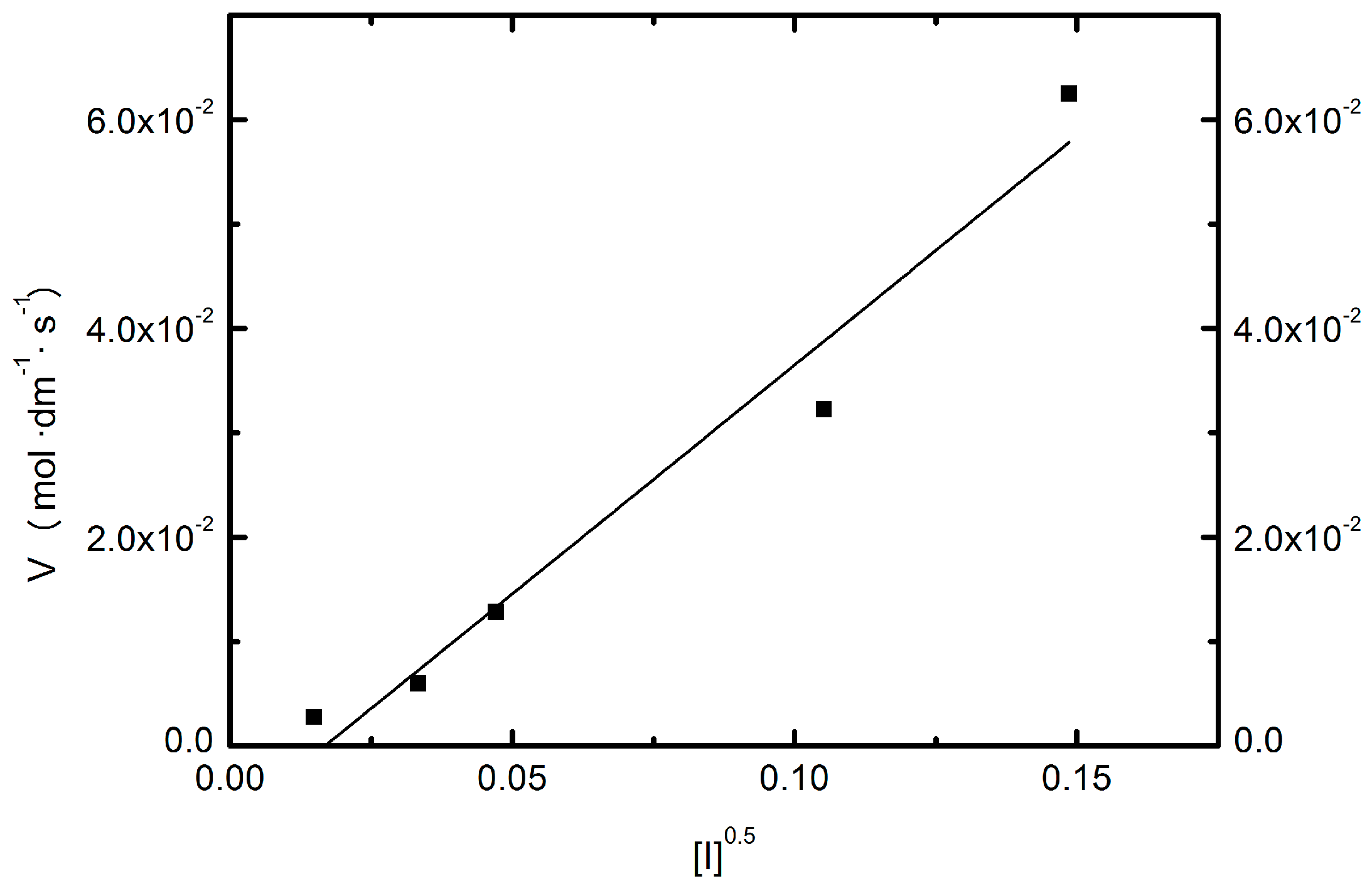
2.4. Conductivity Measurements
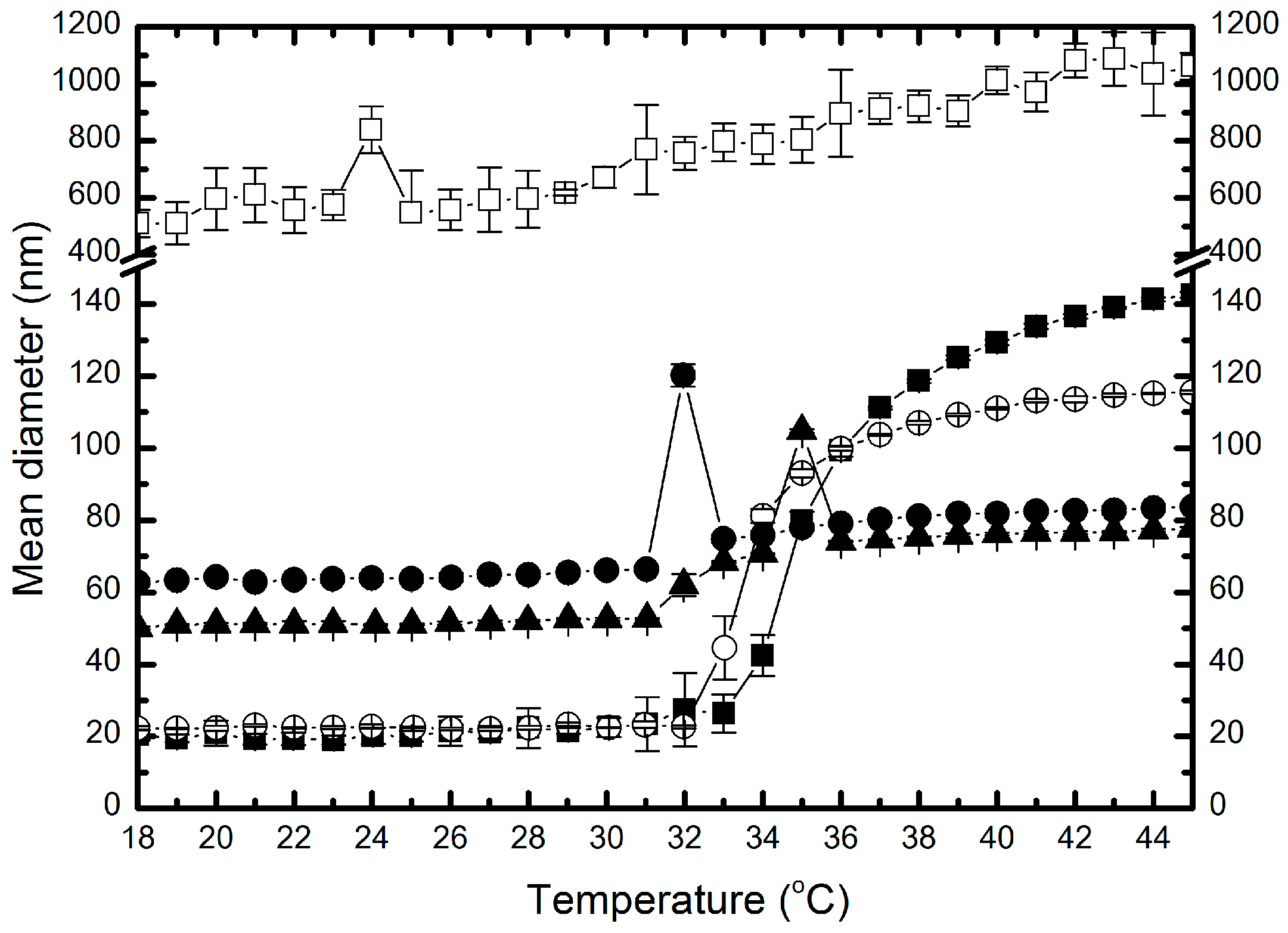
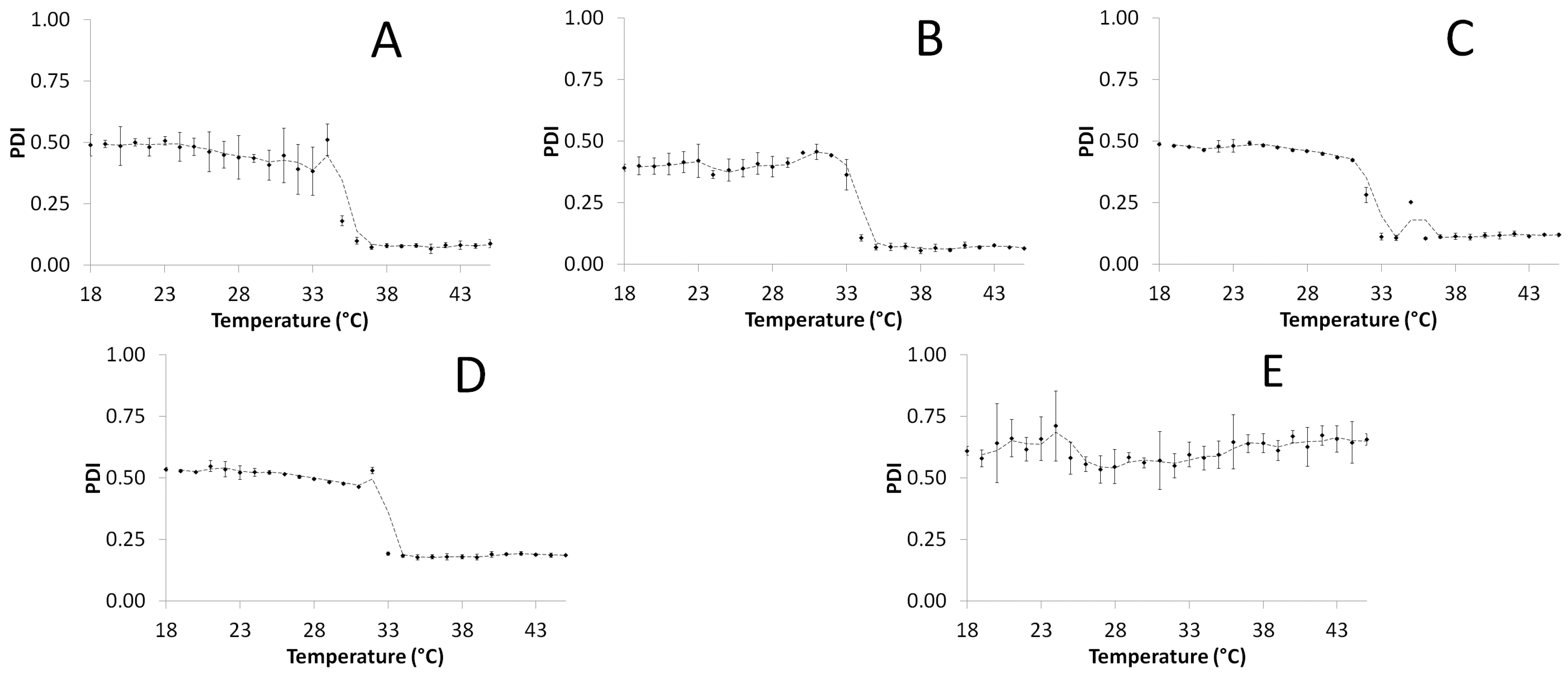
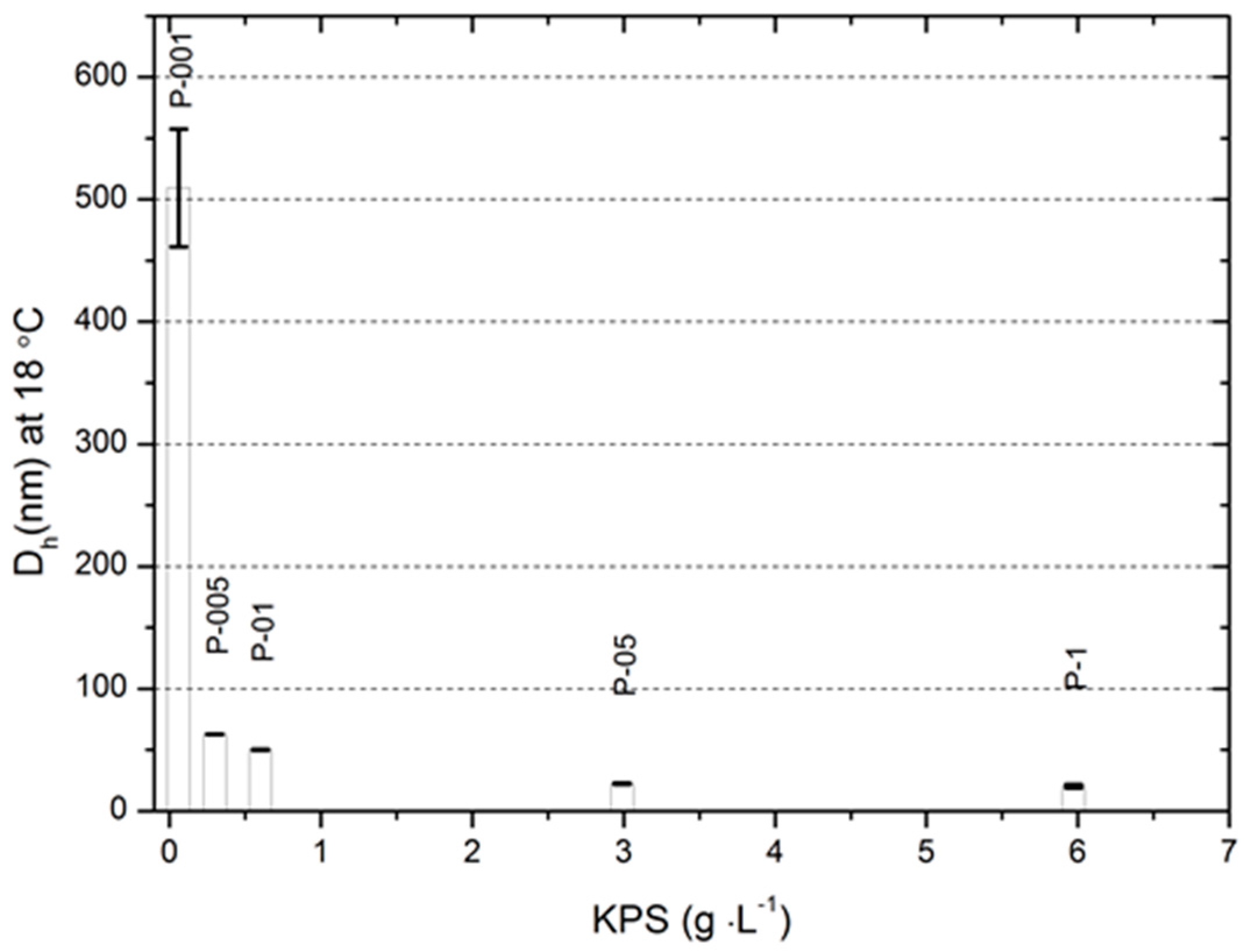
2.5. Hydrodynamic Diameter and Polydispersity Index
2.6. Zeta Potential
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis of Poly-N-isopropylacrylamide Particles
4.3. Fourier Transform Infrared Spectroscopy
4.4. Nuclear Magnetic Resonance Spectroscopy
4.5. Conductivity Measurements
4.6. Dynamic Light Scattering
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of interest
References
- Rodkate, N.; Rutnakornpituk, M. Multi-responsive magnetic microsphere of poly(N-isopropylacrylamide)/carboxymethylchitosan hydrogel for drug controlled release. Carbohydr. Polym. 2016, 151, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.M.; Liu, Q.; Zhang, Y.; Hong, Z.; Wang, H.F. A thermo- and pH-responsive poly(N-isopropylacrylamide)-Mn-ZnS nanocomposite for controlled release and real-time photoluminescence tracking of doxorubicin. RSC Adv. 2016, 6, 50985–50992. [Google Scholar] [CrossRef]
- Vo, T.N.; Tabata, Y.; Mikos, A.G. Effects of cellular parameters on the in vitro osteogenic potential of dual-gelling mesenchymal stem cell-laden hydrogels. J. Biomater. Sci. Polym. Ed. 2016, 27, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Zhang, H.; Shi, L.; Hu, X.; He, Y.; Chen, L.; Zhao, K. In vitro studies of biocompatible thermo-responsive hydrogels with controlled-release basic fibroblast growth factor. React. Funct. Polym. 2016, 100, 73–82. [Google Scholar] [CrossRef]
- Álvarez-Bautista, A.; Mendizábal, E.; Duarte, C.M.M.; Katime, I. Poly (N-Isopropylacrylamide-Co-Acrylic Acid) Smart Nanocarriers for Drug Release: A Study of Theophylline Delivery. J. Mol. Genet. Med. 2016, 9, 196. [Google Scholar]
- Abreu, C.M.; Paula, H.C.; Seabra, V.; Feitosa, J.P.; Sarmento, B.; de Paula, R.C. Synthesis and characterization of non-toxic and thermo-sensitive poly(N-isopropylacrylamide)-grafted cashew gum nanoparticles as a potential epirubicin delivery matrix. Carbohydr. Polym. 2016, 154, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Danyuo, Y.; Dozie-Nwachukwu, S.; Obayemi, J.D.; Ani, C.J.; Odusanya, O.S.; Oni, Y.; Soboyejo, W.O. Swelling of poly(N-isopropylacrylamide) P(NIPA)-based hydrogels with bacterial-synthesized prodigiosin for localized cancer drug delivery. Mater. Sci. Eng. C 2016, 59, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Bölgen, N.; Aguilar, M.R.; Fernández, M.D.M.; Gonzalo-Flores, S.; Villar-Rodil, S.; San Román, J.; Pişkin, E. Thermoresponsive biodegradable HEMA-lactate-dextran-co-NIPA cryogels for controlled release of simvastatin. Artif. Cells Nanomed. Biotechnol. 2015, 43, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Jalili, N.A.; Muscarello, M.; Gaharwar, A.K. Nanoengineered Thermoresponsive Magnetic Hydrogels for Biomedical Applications. Bioeng. Transl. Med. 2016, 77843, 1–9. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Nadagouda, M.N.; More, U.A.; Joshi, S.D.; Kulkarni, V.H.; Noolvi, M.N.; Kulkarni, P.V. Controlled release of therapeutics using interpenetrating polymeric networks. Expert Opin. Drug Deliv. 2015, 12, 669–688. [Google Scholar] [CrossRef] [PubMed]
- Maximilien, J.; Beyazit, S.; Rossi, C.; Haupt, K.; Bui, B.T.S. Nanoparticles in biomedical applications. Bioanal. Rev. 2016, 5, 177–210. [Google Scholar]
- Musiał, W.; Pluta, J.; Michálek, J. Thermosensitive microgels of poly-N-isopropylacrylamide for drug carriers—Practical approach to synthesis. Acta Pol. Pharm. 2015, 72, 409–422. [Google Scholar] [PubMed]
- Ye, T.; Song, Y.; Zheng, Q. Synthesis and solution property of acrylamide-sulfobetaine copolymers. Colloid Polym. Sci. 2015, 293, 797–807. [Google Scholar] [CrossRef]
- Wellert, S.; Radulescu, A.; Carl, A.; Klitzing, R.V.; Gawlitza, K. Evolution of size and structure during the polymerization process: A SANS study on EG-based microgels. Macromolecules 2015, 48, 4901–4909. [Google Scholar] [CrossRef]
- Strachota, B.; Hodan, J.; Matějka, L. Poly(N-isopropylacrylamide)-clay hydrogels: Control of mechanical properties and structure by the initiating conditions of polymerization. Eur. Polym. J. 2016, 77, 1–15. [Google Scholar] [CrossRef]
- Strachota, B.; Matějka, L.; Zhigunov, A.; Konefał, R.; Spěváček, J.; Dybal, J.; Puffr, R. Poly(N-isopropylacrylamide)-clay based hydrogels controlled by the initiating conditions: Evolution of structure and gel formation. Soft Matter 2015, 11, 9291–9306. [Google Scholar] [CrossRef] [PubMed]
- Cuggino, J.C.; Molina, M.; Wedepohl, S.; Igarzabal, C.I.A.; Calderón, M.; Gugliotta, L.M. Responsive nanogels for application as smart carriers in endocytic pH-triggered drug delivery systems. Eur. Polym. J. 2016, 78, 14–24. [Google Scholar] [CrossRef]
- Cirillo, E.N.M.; Nardinocchi, P.; Sciarra, G. Temperature-driven volume transition in hydrogels: Phase-coexistence and interface localization. Int. J. Non-Linear Mech. 2016, 81, 115–121. [Google Scholar] [CrossRef]
- Drozdov, A.D. Volume phase transition in thermo-responsive hydrogels: Constitutive modeling and structure–property relations. Acta Mech. 2015, 226, 1283–1303. [Google Scholar] [CrossRef]
- Mazaheri, H.; Baghani, M.; Naghdabadi, R. Inhomogeneous and homogeneous swelling behavior of temperature-sensitive poly-(N-isopropylacrylamide) hydrogels. J. Intell. Mater. Syst. Struct. 2016, 27, 324–336. [Google Scholar] [CrossRef]
- Coates, J. Interpretation of infrared pectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons: Chichester, UK, 2000; pp. 10815–10837. [Google Scholar]
- Yoshida, H.; Furukawa, Y.; Tasumi, M. Structural studies and vibrational analyses of stable and less stable conformers of 1,3,5-hexatriene based on ab initio MO calculations. J. Mol. Struct. 1989, 194, 279–299. [Google Scholar] [CrossRef]
- Scherer, J.R.; Potts, W.J. Normal Coordinates for the Out-of-Plane Deformations of Vinyl Bromide. J. Chem. Phys. 1959, 30, 1527–1529. [Google Scholar] [CrossRef]
- Guchhait, S.; Banerjee, M.; Konar, R.S. Kinetics and mechanism of potassium persulphate-initiated aqueous polymerization of methacrylonitrile at 50 °C in an inert atmosphere of nitrogen gas. Polymer 1991, 32, 3182–3189. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Xiong, G.L.; Haus, E.; Sackett-Lundeen, L.; Ashkenazi, I.; Maibach, H.I. Time-dependent variations of the skin barrier function in humans: Transepidermal water loss, stratum corneum hydration, skin surface pH, and skin temperature. J. Investig. Dermatol. 1998, 110, 20–23. [Google Scholar] [CrossRef] [PubMed]
- Cavin, L.; Rouge, A.; Meyer, T.; Renken, A. Kinetic modeling of free radical polymerization of styrene initiated by the bifunctional initiator 2, 5-dimethyl-2, 5-bis (2-ethyl hexanoyl peroxy) hexane. Polymer 2000, 41, 3925–3935. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds P-1, P-05, P-01, P-005, P-001 are available from the authors.
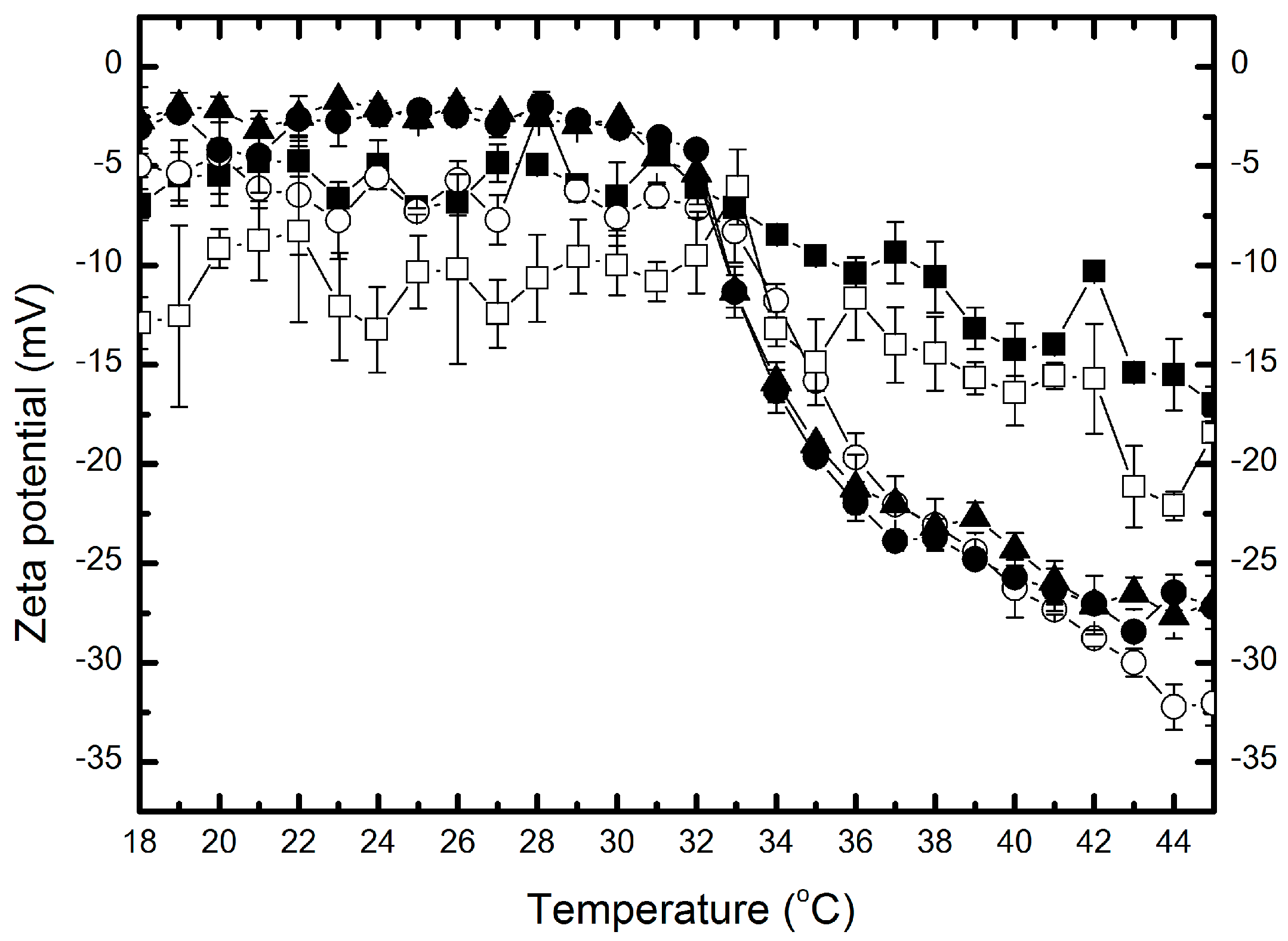
| Type of Polymer | Range of ZP (mV) | ∆ZP (mV) |
|---|---|---|
| P-1 | −7 to −17 | 10 |
| P-05 | −5 to −32 | 27 |
| P-01 | −3 to −30 | 27 |
| P-005 | −3 to −27 | 24 |
| P-001 | −12 to −18 | 6 |
| Type of Polimer | Estimated Time of Polymerization (min) | |
|---|---|---|
| P-1 | 16 | 0.1486 |
| P-05 | 31 | 0.1052 |
| P-01 | 78 | 0.0471 |
| P-005 | 167 | 0.0333 |
| P-001 | 363 | 0.0149 |
| Type of Nanosphere | NIPA (g) | KPS (g) |
|---|---|---|
| P-1 | 5.002 | 5.972 |
| P-05 | 5.004 | 2.986 |
| P-01 | 5.006 | 0.598 |
| P-005 | 5.002 | 0.300 |
| P-001 | 5.006 | 0.061 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gola, A.; Knysak, T.; Musial, W. The Influence of Anionic Initiator on the Selected Properties of Poly-N-Isopropyl Acrylamide Evaluated for Controlled Drug Delivery. Molecules 2017, 22, 23. https://doi.org/10.3390/molecules22010023
Gola A, Knysak T, Musial W. The Influence of Anionic Initiator on the Selected Properties of Poly-N-Isopropyl Acrylamide Evaluated for Controlled Drug Delivery. Molecules. 2017; 22(1):23. https://doi.org/10.3390/molecules22010023
Chicago/Turabian StyleGola, Agnieszka, Tomasz Knysak, and Witold Musial. 2017. "The Influence of Anionic Initiator on the Selected Properties of Poly-N-Isopropyl Acrylamide Evaluated for Controlled Drug Delivery" Molecules 22, no. 1: 23. https://doi.org/10.3390/molecules22010023
APA StyleGola, A., Knysak, T., & Musial, W. (2017). The Influence of Anionic Initiator on the Selected Properties of Poly-N-Isopropyl Acrylamide Evaluated for Controlled Drug Delivery. Molecules, 22(1), 23. https://doi.org/10.3390/molecules22010023






