Anti-Inflammatory Effects and Mechanisms of Action of Coussaric and Betulinic Acids Isolated from Diospyros kaki in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages
Abstract
:1. Introduction
2. Results
2.1. Isolation of CA and BA
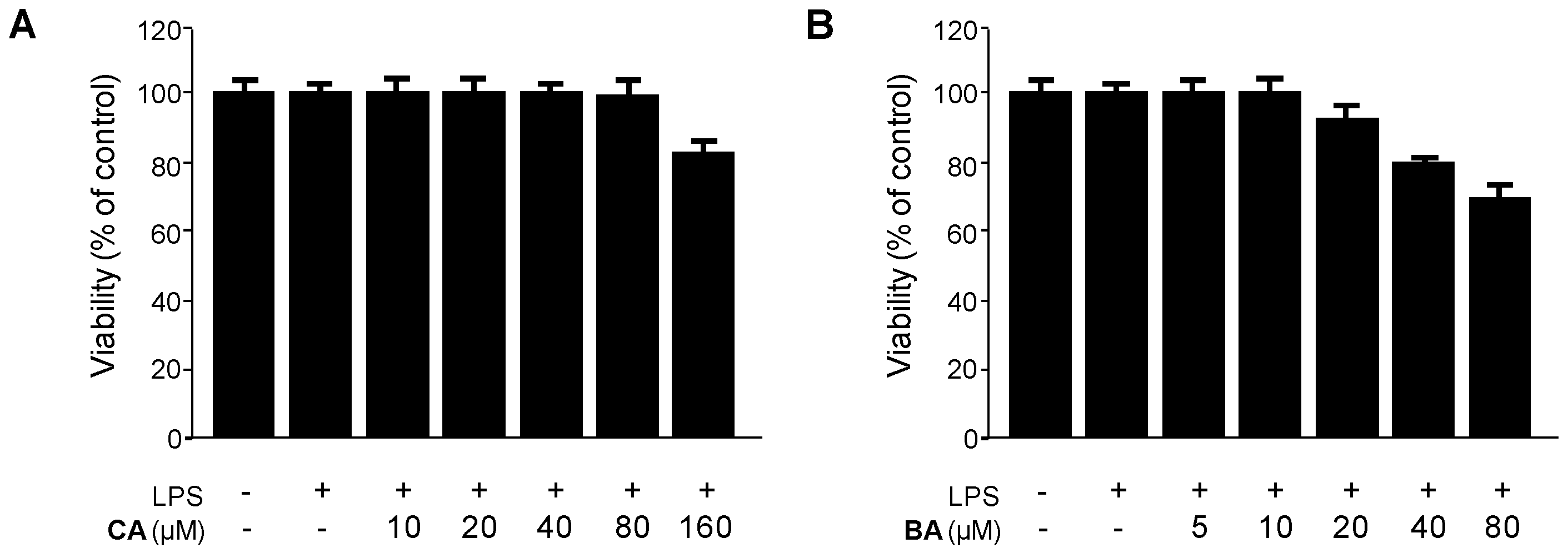
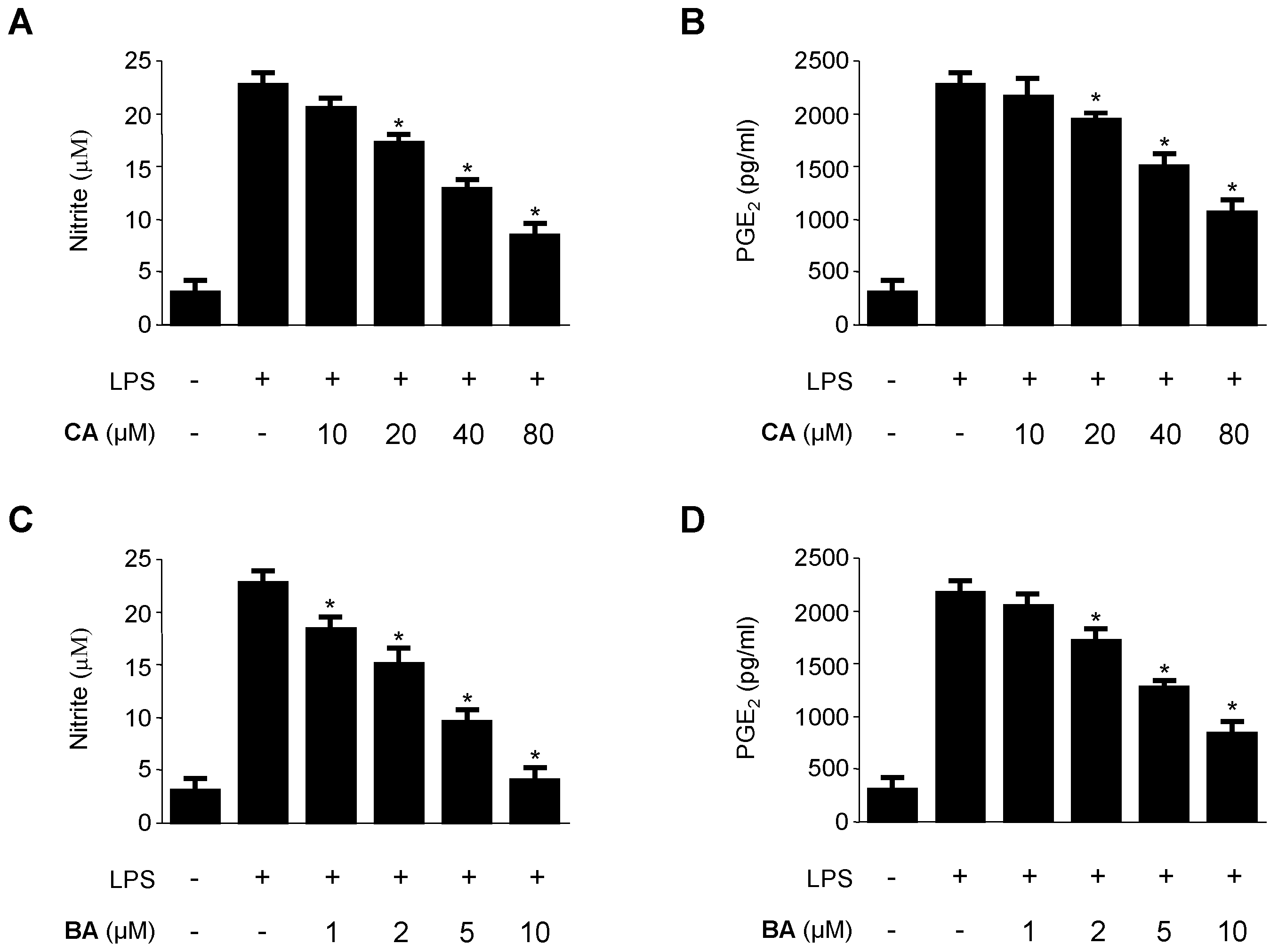
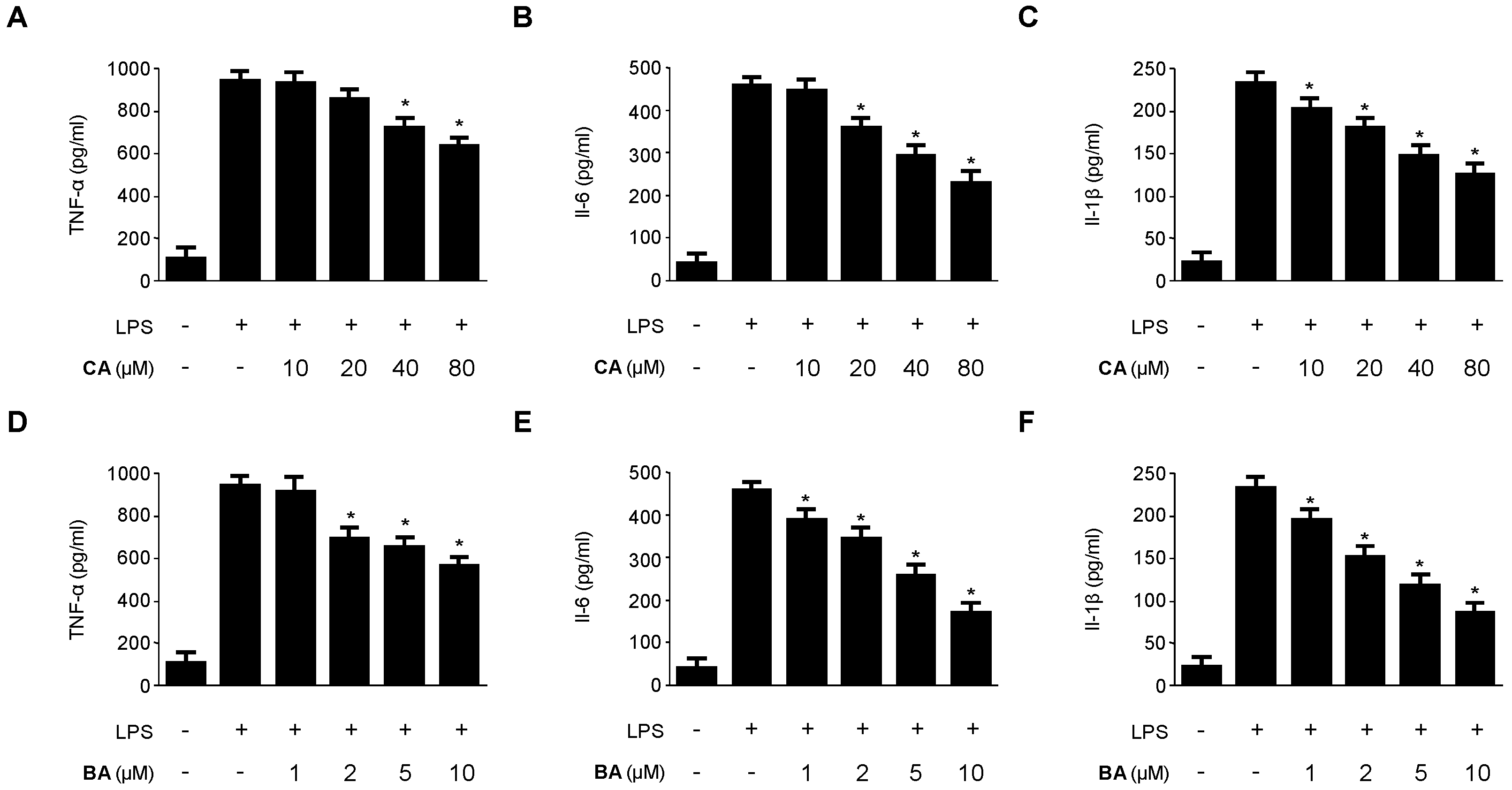
2.2. Inhibitory Effects of CA and BA on the Production of Pro-Inflammatory Mediators and Enzymes in LPS-stimulated RAW 264.7 Macrophages
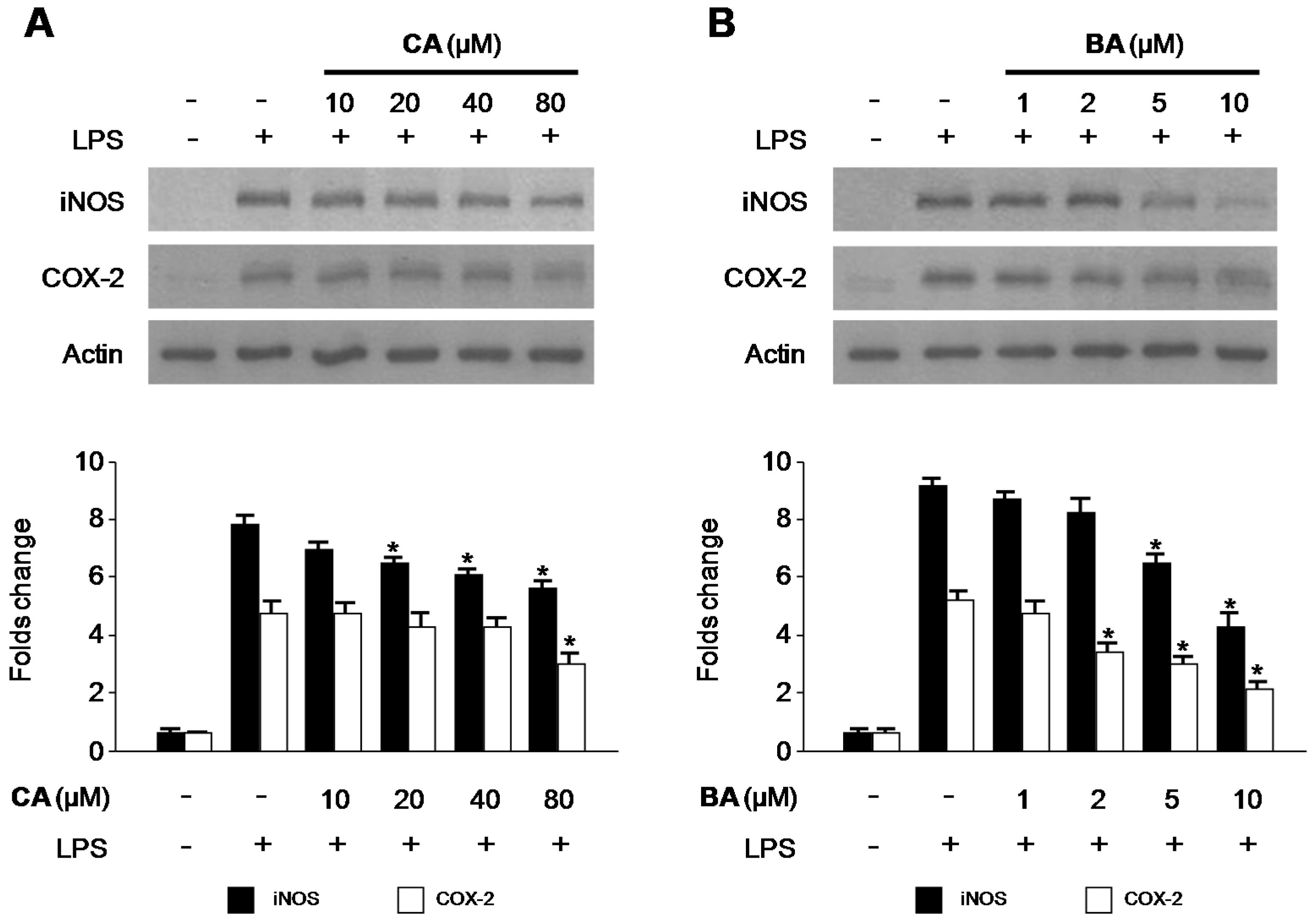
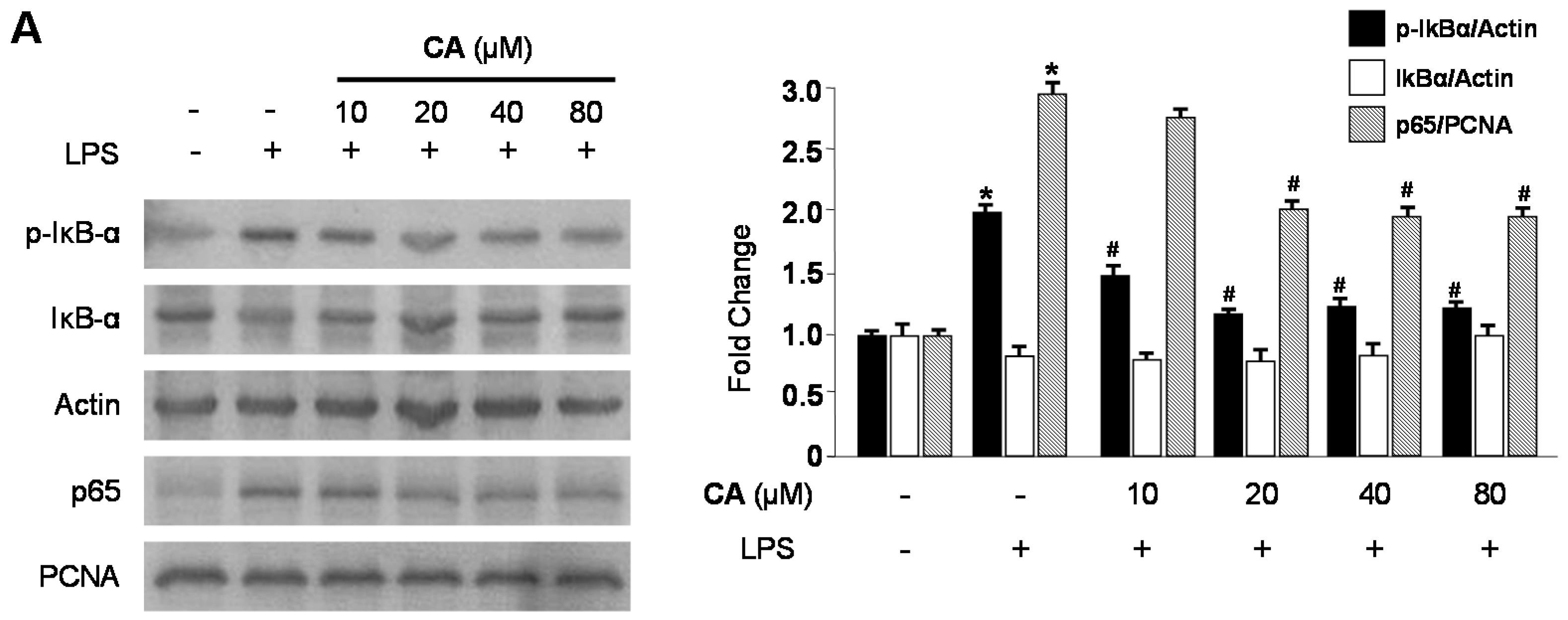
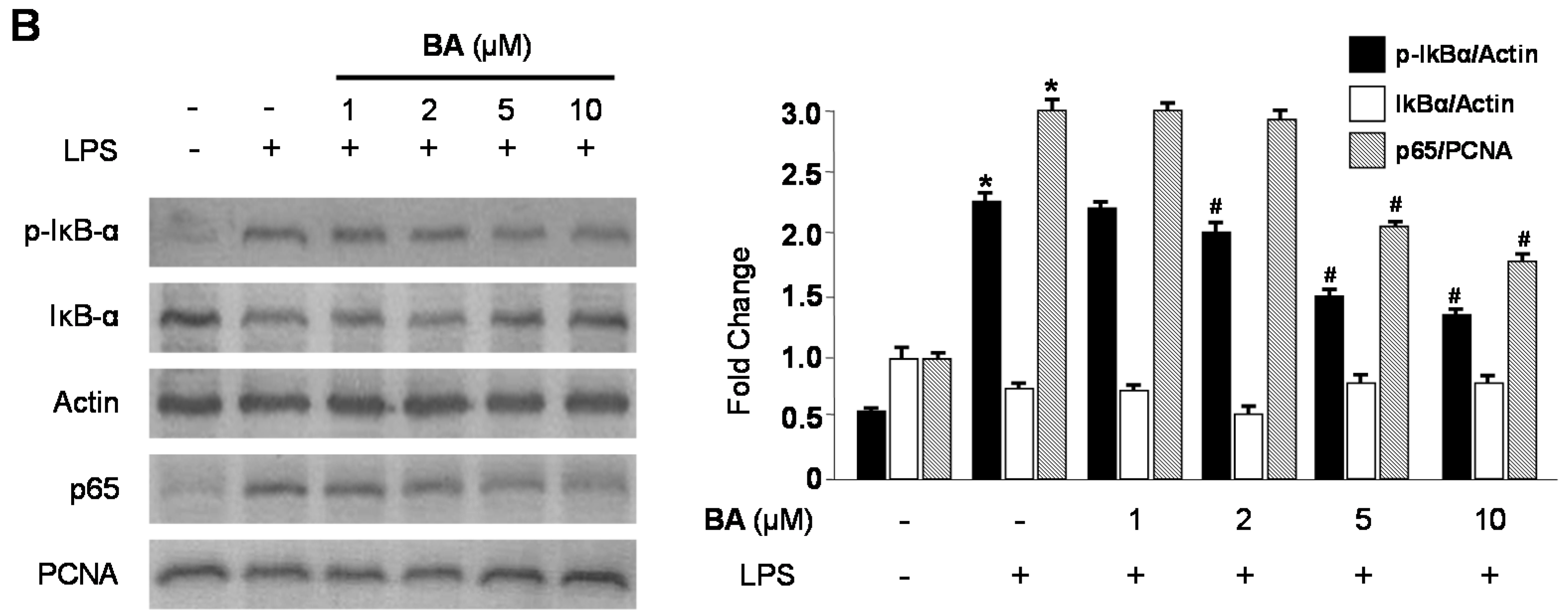
2.3. Effects of CA and BA on iNOS and COX-2 Expression and NF-κB Activation in LPS-Stimulated RAW 264.7 Macrophages
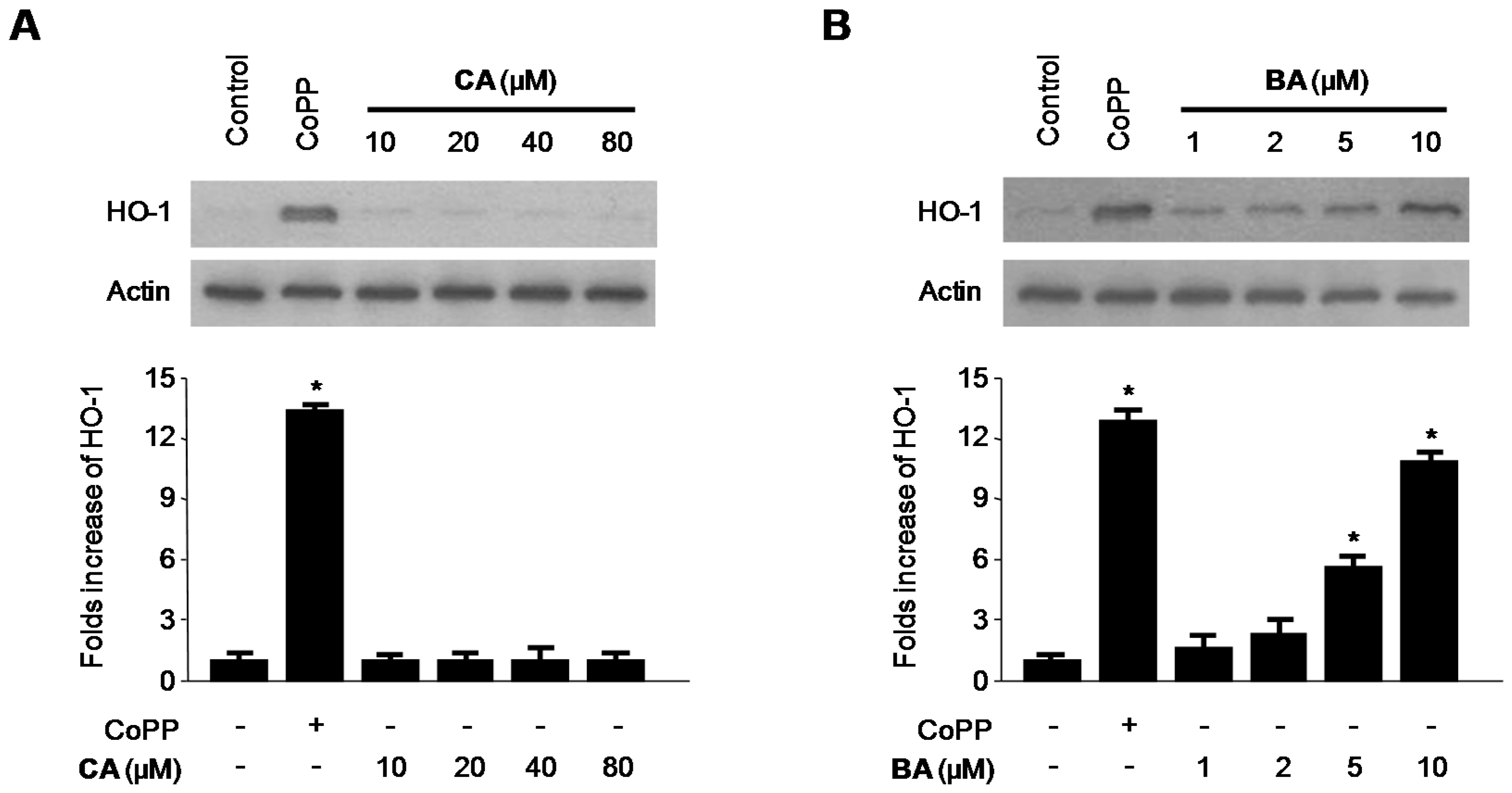
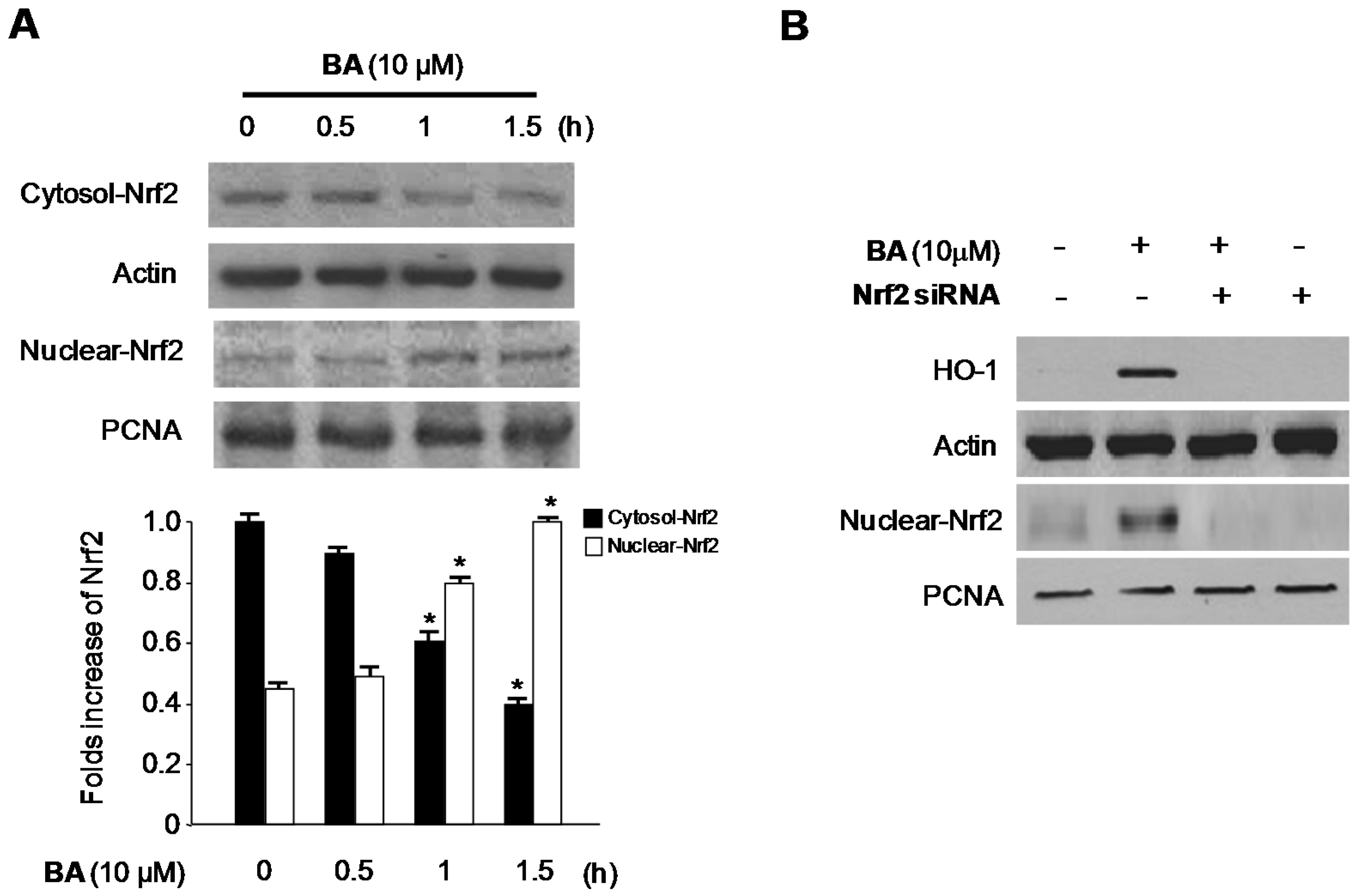
2.4. Effects of CA and BA on HO-1 Expression and Nrf2 Nuclear Translocation in RAW 264.7 Macrophages
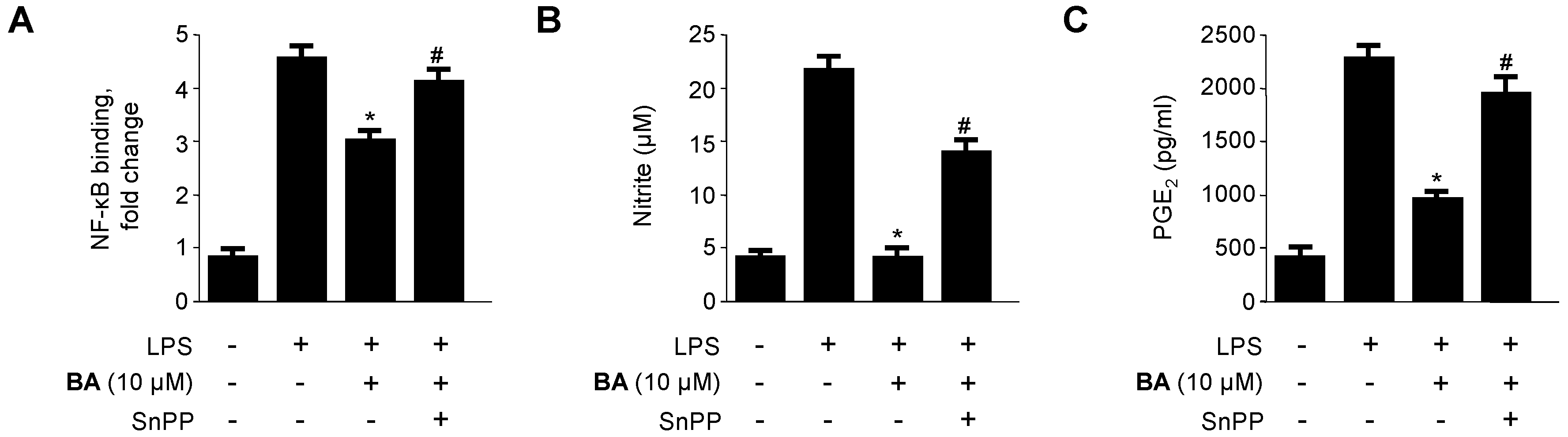
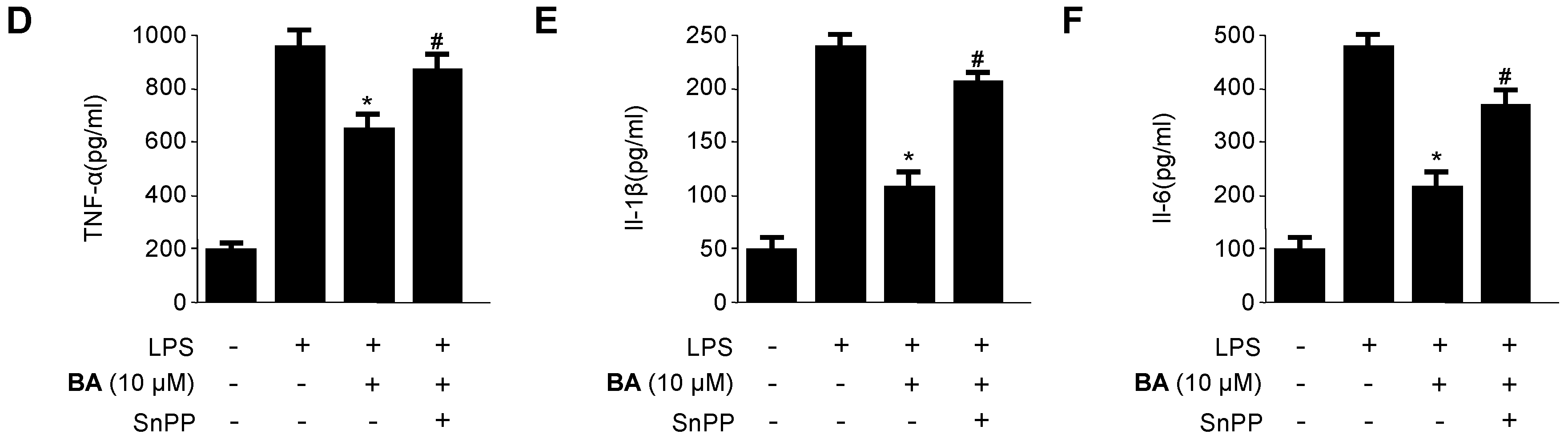
2.5. Effects of HO-1 Expression on the Inhibition of Pro-Inflammatory Mediators, Cytokines, and NF-κB Activity by BA in LPS-Stimulated RAW 264.7 Macrophages
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Sample Preparation
4.3. Cell Culture and Viability Assay
4.4. Determination of Nitrite Production and PGE2, TNF-α, IL-1β, and IL-6 Assays
4.5. Preparation of Cytosolic and Nuclear Fractions
4.6. Western Blot Analysis
4.7. DNA-Binding Activity of NF-κB
4.8. Transfection
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ferrero-Miliani, L.; Nielsen, O.H.; Anderson, P.S.; Girardin, S.E. Chronic inflammation: Importance of NOD2 and NALP3 in interleukin-1beta generation. Clin. Exp. Immunol. 2007, 147, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Berenbaum, F. Proinflammatory cytokines, prostaglandins, and the chondrocyte: Mechanisms of intracellular activation. Jt. Bone Spine 2000, 67, 561–564. [Google Scholar] [CrossRef]
- Karpurapu, M.; Wang, X.; Deng, J.; Park, H.; Xiao, L.; Sadikot, R.T.; Frey, R.S.; Maus, U.A.; Park, G.Y.; Scott, E.W.; et al. Functional PU.1 in macrophages has a pivotal role in NF-κB activation and neutrophilic lung inflammation during endotoxemia. Blood 2011, 118, 5255–5266. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Verma, I.M. NF-kappaB regulation in the immune system. Nat. Rev. Immunol. 2002, 2, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.S.; Tsai, H.L.; Chau, L.Y. Induction of heme oxygenase-1 expression in murine macrophages is essential for the anti-inflammatory effect of low dose 15-deoxy-delta 12,14-prostaglandin J2. J. Biol. Chem. 2003, 278, 19325–19330. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Lu, H.T.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [PubMed]
- Wiesel, P.; Foster, L.C.; Pellacani, A.; Layne, M.D.; Hsieh, C.M.; Huggins, G.S.; Strauss, P.; Yet, S.F.; Perrella, M.A. Thioredoxin facilitates the induction of heme oxygenase-1 in response to inflammatory mediators. J. Biol. Chem. 2000, 275, 24840–24846. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.M.; Pae, H.O.; Park, J.E.; Lee, Y.C.; Woo, J.M.; Kim, N.H.; Choi, Y.K.; Lee, B.S.; Kim, S.R.; Chung, H.T. Heme oxygenase in the regulation of vascular biology: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2011, 14, 137–167. [Google Scholar] [CrossRef] [PubMed]
- Graziose, R.; Lila, M.A.; Raskin, I. Merging traditional Chinese medicine with modern drug discovery technologies to find novel drugs and functional foods. Curr. Drug. Discov. Technol. 2010, 7, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Kiken, D.A.; Cohen, D.E. Contact dermatitis to botanical extracts. Am. J. Contact. Dermat. 2002, 13, 148–152. [Google Scholar] [PubMed]
- Fan, J.P.; He, C.H. Simultaneous quantification of three major bioactive triterpene acids in the leaves of Diospyros kaki by high-performance liquid chromatography method. J. Pharm. Biomed. Anal. 2006, 41, 950–956. [Google Scholar] [CrossRef] [PubMed]
- Kotani, M.; Matsumoto, M.; Fujita, A.; Higa, S.; Wang, W.; Suemura, M.; Kishimoto, T.; Tanaka, T. Persimmon leaf extract and astragalin inhibit development of dermatitis and IgE elevation in NC/Nga mice. J. Allergy Clin. Immunol. 2000, 106, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Kameda, K.; Takaku, T.; Okuda, H.; Kimura, Y.; Okuda, T.; Hatano, T.; Agata, I.; Arichi, S. Inhibitory effects of various flavonoids isolated from leaves of persimmon on angiotensin-converting enzyme activity. J. Nat. Prod. 1987, 50, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhang, J.; Lu, X.; Zhang, L.; Zhang, Y. Evaluation to the antioxidant activity of total flavonoids extract from persimmon (Diospyros kaki L.) leaves. Food Chem. Toxicol. 2011, 49, 2689–2696. [Google Scholar] [CrossRef] [PubMed]
- Mallavadhani, U.V.; Panda, A.K.; Rao, Y.R. Pharmacology and chemotaxonomy of Diospyros. Phytochemistry 1998, 49, 901–951. [Google Scholar] [CrossRef]
- Duan, J.; Zheng, Y.; Dong, Q.; Fang, J. Structural analysis of a pectic polysaccharide from the leaves of Diospyros kaki. Phytochemistry 2004, 65, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Lu, H.; Wang, C.; Yamashita, K.; Manabe, M.; Xu, S.; Kodama, H. Effect of five triterpenoid compounds isolated from leaves of Diospyros kaki on stimulus-induced superoxide generation and tyrosyl phosphorylation in human polymorphonuclear leukocytes. Clin. Chim. Acta 2002, 320, 11–16. [Google Scholar] [CrossRef]
- Thuong, P.T.; Lee, C.H.; Dao, T.T.; Nguyen, P.H.; Kim, W.G.; Lee, S.J.; Oh, W.K. Triterpenoids from the leaves of Diospyros kaki (persimmon) and their inhibitory effects on protein tyrosine phosphatase 1B. J. Nat. Prod. 2008, 71, 1775–1778. [Google Scholar] [CrossRef] [PubMed]
- Khanal, P.; Oh, W.K.; Thuong, P.T.; Cho, S.D.; Choi, H.S. 24-hydroxyursolic acid from the leaves of the Diospyros kaki (Persimmon) induces apoptosis by activation of AMP-activated protein kinase. Planta Med. 2010, 76, 689–693. [Google Scholar] [CrossRef] [PubMed]
- Pisha, E.; Chai, H.; Lee, I.S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.; Fong, H.H.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of BA as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Saha, K.; Das, J.; Pal, M.; Saha, B.P. Studies on the anti-inflammatory activity of rhizomes of Nelumbo nucifera. Planta Med. 1997, 63, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Recio, M.C.; Giner, R.M.; Máñez, S.; Gueho, J.; Julien, H.R.; Hostettmann, K.; Ríos, J.L. Investigations on the steroidal anti-inflammatory activity of triterpenoids from Diospyros leucomelas. Planta Med. 1995, 61, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Bringmann, G.; Saeb, W.; Assi, L.A.; François, G.; Narayanan, A.S.; Peters, K.; Peters, E.M. Betulinic acid: isolation from Triphyophyllum peltatum and Ancistrocladus heyneanus, antimalarial activity, and crystal structure of the benzyl ester. Planta Med. 1997, 63, 255–257. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Geoffrey, B.; Shengxiang, Q.; Harry, H.S.F.; Norman, R.F.; Shengang, Y.; Chongzhi, Z. Computer-assisted structure elucidation: Application of CISOC-SES to the resonance assignment and structure generation of betulinic acid. Magn. Reson. Chem. 1998, 36, 267–278. [Google Scholar]
- Su, B.N.; Kang, Y.H.; Pinos, R.E.; Santarsiero, B.D.; Mesecar, A.D.; Soejarto, D.D.; Fong, H.H.; Pezzuto, J.M.; Kinghorn, A.D. Isolation and absolute stereochemistry of coussaric acid, a new bioactive triterpenoid from the stems of Coussarea brevicaulis. Phytochemistry 2003, 64, 293–302. [Google Scholar] [CrossRef]
- Amoussa, A.M.; Lagnika, L.; Bourjot, M.; Vonthron-Senecheau, C.; Sanni, A. Triterpenoids from Acacia ataxacantha DC: Antimicrobial and antioxidant activities. BMC Complement. Altern. Med. 2016, 16, 284. [Google Scholar] [CrossRef] [PubMed]
- Bildziukevich, U.; Kaletová, E.; Šaman, D.; Sievänen, E.; Kolehmainen, E.T.; Šlouf, M.; Wimmer, Z. Spectral and microscopic study of self-assembly of novel cationic spermine amides of betulinic acid. Steroids. 2016. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.K.; Chattopadhyay, S.; Ghosh, T.; Dash, S.S.; Tripathy, S.; Das, B.; Bag, B.G.; Das, D.; Roy, S. Self-assembled betulinic acid protects doxorubicin induced apoptosis followed by reduction of ROS-TNF-α-caspase-3 activity. Biomed. Pharmacother. 2015, 72, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.M.; Alam, J. Heme oxygenase-1: Function, regulation, and implication of a novel stress-inducible protein in oxidant-induced lung injury. Am. J. Respir. Cell Mol. Biol. 1996, 15, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.H.; Kim, D.S.; Kim, S.W.; Lim, S.H.; Kim, D.K.; Shin, T.Y.; Kim, S.H. Inhibitory effects of Diospyros kaki in a model of allergic inflammation: Role of cAMP, calcium and nuclear factor-κB. Int. J. Mol. Med. 2013, 32, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, A.; Khan, S.; Shah, S.M.H. Larvicidal, insecticidal, brine shrimp cytotoxicity and anti-oxidant activities of Diospyros kaki (L.) reported from Pakistan. Pak. J. Pharm. Sci. 2015, 28, 1239–1243. [Google Scholar]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, S.; Suk, K.; Bark, H.; Jun, C.D.; Kim, D.K.; Choi, C.H.; Yoshimura, T. Discoidin domain receptor 1 mediates collagen-induced nitric oxide production in J774A.1 murine macrophages. Free Radic. Biol. Med. 2007, 42, 343–352. [Google Scholar] [CrossRef] [PubMed]
- McCartney-Francis, N.; Allen, J.B.; Mizel, D.E.; Albina, J.E.; Xie, Q.W.; Nathan, C.F.; Wahl, S.M. Suppression of arthritis by an inhibitor of nitric oxide synthase. J. Exp. Med. 1993, 178, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C.; Thiemermann, C.; Wu, C.C.; Perretti, M.; Vane, J.R. Attenuation of the induction of nitric oxide synthase by endogenous glucocorticoids accounts for endotoxin tolerance in vivo. Proc. Natl. Acad. Sci. USA 1994, 91, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Galli, S.J.; Tsai, M.; Piliponsky, A.M. The development of allergic inflammation. Nature 2008, 454, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Hayden, M.S. New regulators of NF-kappaB in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Ben-Neriah, Y. Phosphorylation meets ubiquitination: The control of NF-kappaB activity. Annu. Rev. Immunol. 2000, 18, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Lappas, M.; Permezel, M.; Georgiou, H.M.; Rice, G.E. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol. Reprod. 2002, 67, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kauppinen, A.; Kaarniranta, K. Phytochemicals suppress nuclear factor-κB signaling: Impact on health span and the aging process. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.T.; Pae, H.O.; Cha, Y.N. Role of heme oxygenase-1 in vascular disease. Curr. Pharm. Des. 2008, 14, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, A.K. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic. Biol. Med. 2004, 36, 1199–1207. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the coussaric acid (CA) and betulinic acid (BA) are available from the authors.
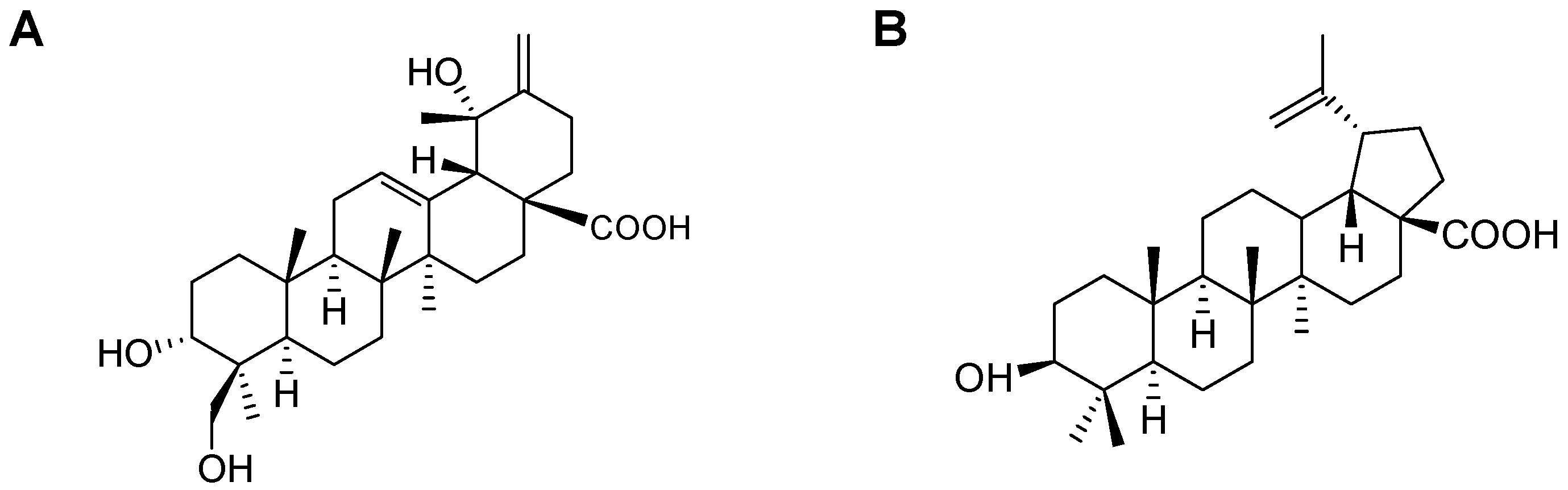
| Carbon No. | δ 13C (ppm) | δ 1H (ppm) | Carbon No. | δ 13C (ppm) | δ 1H (ppm) |
|---|---|---|---|---|---|
| 1 | 34.0 | 1.39–1.46 (m), 1.90 (m) | 16 | 26.8 | 2.10–2.16 (m), 3.20–3.27 (m) |
| 2 | 26.5 | 1.86 (m), 2.10–2.16 (m) | 17 | 48.4 | |
| 3 | 70.0 | 4.45 (br s) | 18 | 55.4 | 3.23 (br s) |
| 4 | 44.0 | 19 | 73.0 | 5.74 (s) | |
| 5 | 50.2 | 1.95 (br s) | 20 | 156.7 | |
| 6 | 19.2 | 1.56 (m), 1.72–1.77 (m) | 21 | 29.0 | 2.45 (m), 3.12–3.27 (m), 3.16 (m) |
| 7 | 34.3 | 1.41–1.48 (m), 1.72–1.77 (m) | 22 | 39.5 | 2.10–2.16 (m), 2.30–2.34 (m) |
| 8 | 40.4 | 23 | 23.6 | 1.62 (s) | |
| 9 | 47.8 | 2.10–2.16 (m) | 24 | 65.7 | 2.45 (m), 3.16 (m) |
| 10 | 37.5 | 25 | 16.1 | 0.99 (s) | |
| 11 | 24.3 | 2.07 (m), 2.10–2.16 (m) | 26 | 17.2 | 1.10 (s) |
| 12 | 128.3 | 5.63 (br s) | 27 | 24.0 | 1.69 (s) |
| 13 | 139.6 | 28 | 180.2 | ||
| 14 | 42.2 | 29 | 27.6 | 1.64 (s) | |
| 15 | 29.2 | 1.34 (m), 2.30–2.34 (m) | 30 | 105.3 | 4.80 (s), 5.00 (s) |
| Carbon No. | δ 13C (ppm) | δ 1H (ppm) | Carbon No. | δ 13C (ppm) | δ 1H (ppm) |
|---|---|---|---|---|---|
| 1 | 39.1 | 1.01 (m), 1.68 (br s) | 16 | 32.7 | 1.56 (m), 2.65 (m) |
| 2 | 28.1 | 1.87 (m) | 17 | 56.3 | |
| 3 | 78.1 | 3.47 (t, J = 7.2 Hz) | 18 | 47.6 | 1.77 (br s) |
| 4 | 39.4 | 19 | 49.5 | 3.55 (m) | |
| 5 | 55.7 | 0.82 (m) | 20 | 150.7 | |
| 6 | 18.6 | 1.57 (m), 1.39 (m) | 21 | 30.1 | 1.54 (m), 2.25 (m) |
| 7 | 34.7 | 1.46 (m), 1.39 (m) | 22 | 37.5 | 1.58 (m), 2.26 (m) |
| 8 | 40.9 | 23 | 28.5 | 1.24 (s) | |
| 9 | 50.8 | 1.38 (m) | 24 | 16.3 | 1.02 (s) |
| 10 | 37.3 | 25 | 16.3 | 0.83 (s) | |
| 11 | 21.1 | 1.44 (m), 1.21 (m) | 26 | 16.2 | 1.07 (s) |
| 12 | 25.9 | 1.21 (m), 1.95 (m) | 27 | 14.8 | 1.08 (s) |
| 13 | 38.4 | 2.74 (m) | 28 | 178.7 | |
| 14 | 42.4 | 29 | 110.3 | 4.96 (br s), 4.78 (s) | |
| 15 | 31.1 | 1.26 (m), 1.88 (m) | 30 | 19.4 | 1.8 (s) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-S.; Lee, D.-S.; Kim, D.-C.; Yoon, C.-S.; Ko, W.; Oh, H.; Kim, Y.-C. Anti-Inflammatory Effects and Mechanisms of Action of Coussaric and Betulinic Acids Isolated from Diospyros kaki in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Molecules 2016, 21, 1206. https://doi.org/10.3390/molecules21091206
Kim K-S, Lee D-S, Kim D-C, Yoon C-S, Ko W, Oh H, Kim Y-C. Anti-Inflammatory Effects and Mechanisms of Action of Coussaric and Betulinic Acids Isolated from Diospyros kaki in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Molecules. 2016; 21(9):1206. https://doi.org/10.3390/molecules21091206
Chicago/Turabian StyleKim, Kyoung-Su, Dong-Sung Lee, Dong-Cheol Kim, Chi-Su Yoon, Wonmin Ko, Hyuncheol Oh, and Youn-Chul Kim. 2016. "Anti-Inflammatory Effects and Mechanisms of Action of Coussaric and Betulinic Acids Isolated from Diospyros kaki in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages" Molecules 21, no. 9: 1206. https://doi.org/10.3390/molecules21091206
APA StyleKim, K.-S., Lee, D.-S., Kim, D.-C., Yoon, C.-S., Ko, W., Oh, H., & Kim, Y.-C. (2016). Anti-Inflammatory Effects and Mechanisms of Action of Coussaric and Betulinic Acids Isolated from Diospyros kaki in Lipopolysaccharide-Stimulated RAW 264.7 Macrophages. Molecules, 21(9), 1206. https://doi.org/10.3390/molecules21091206





