Improvement in Flavonoids and Phenolic Acids Production and Pharmaceutical Quality of Sweet Basil (Ocimum basilicum L.) by Ultraviolet-B Irradiation
Abstract
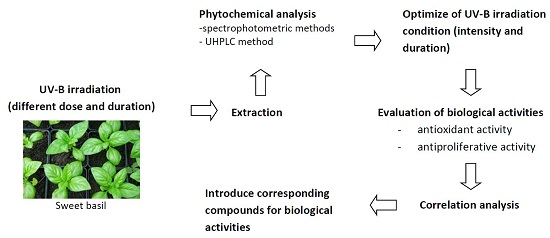
:1. Introduction
2. Results and Discussion
2.1. Changes in TFC and TPC under Different UV-B Irradiation Intensities
2.2. Changes of TFC and TPC under Different UV-B Irradiation Durations
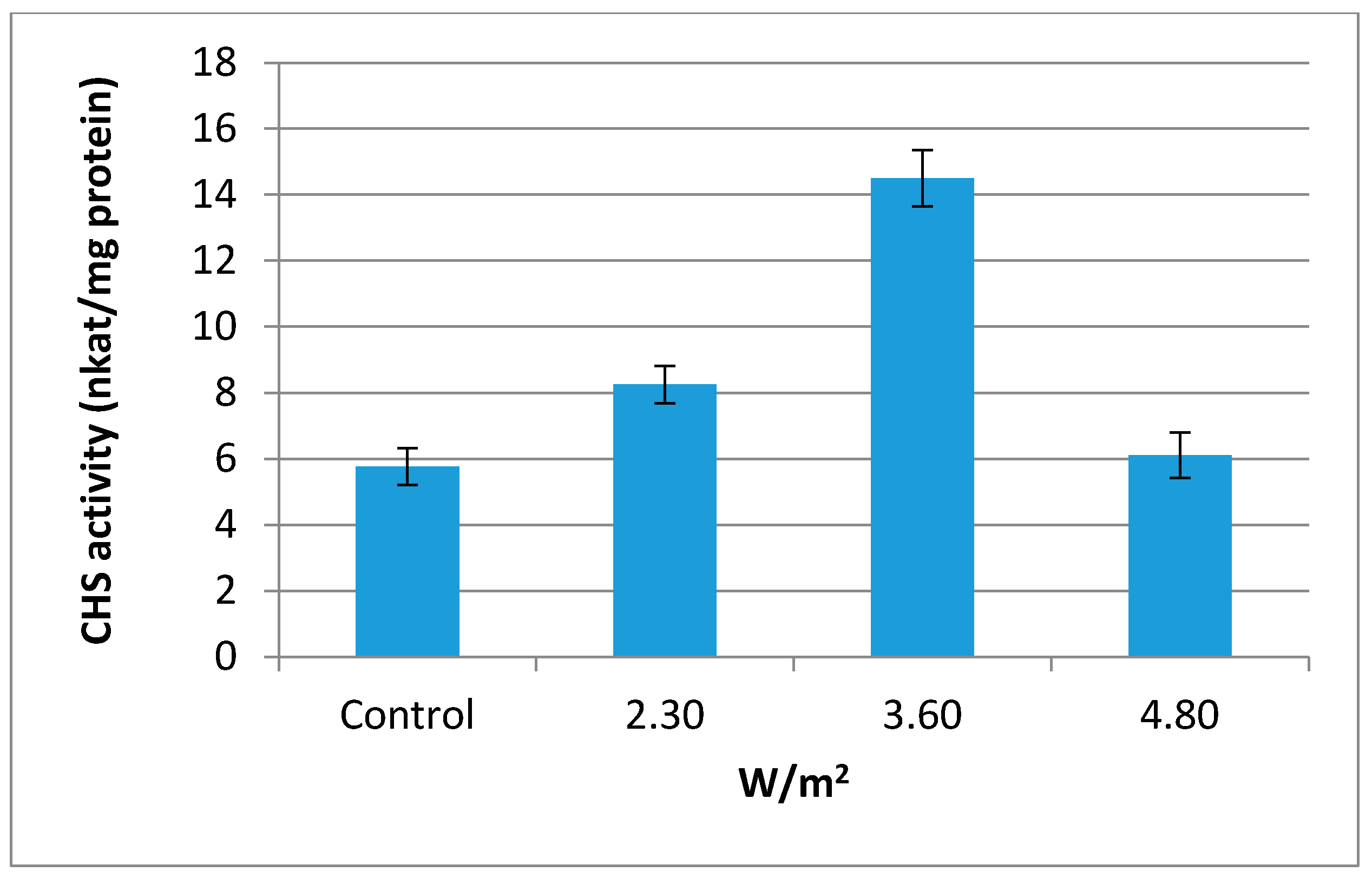
2.3. Chalcone Synthase (CHS, EC 2.3.1.74) Activity
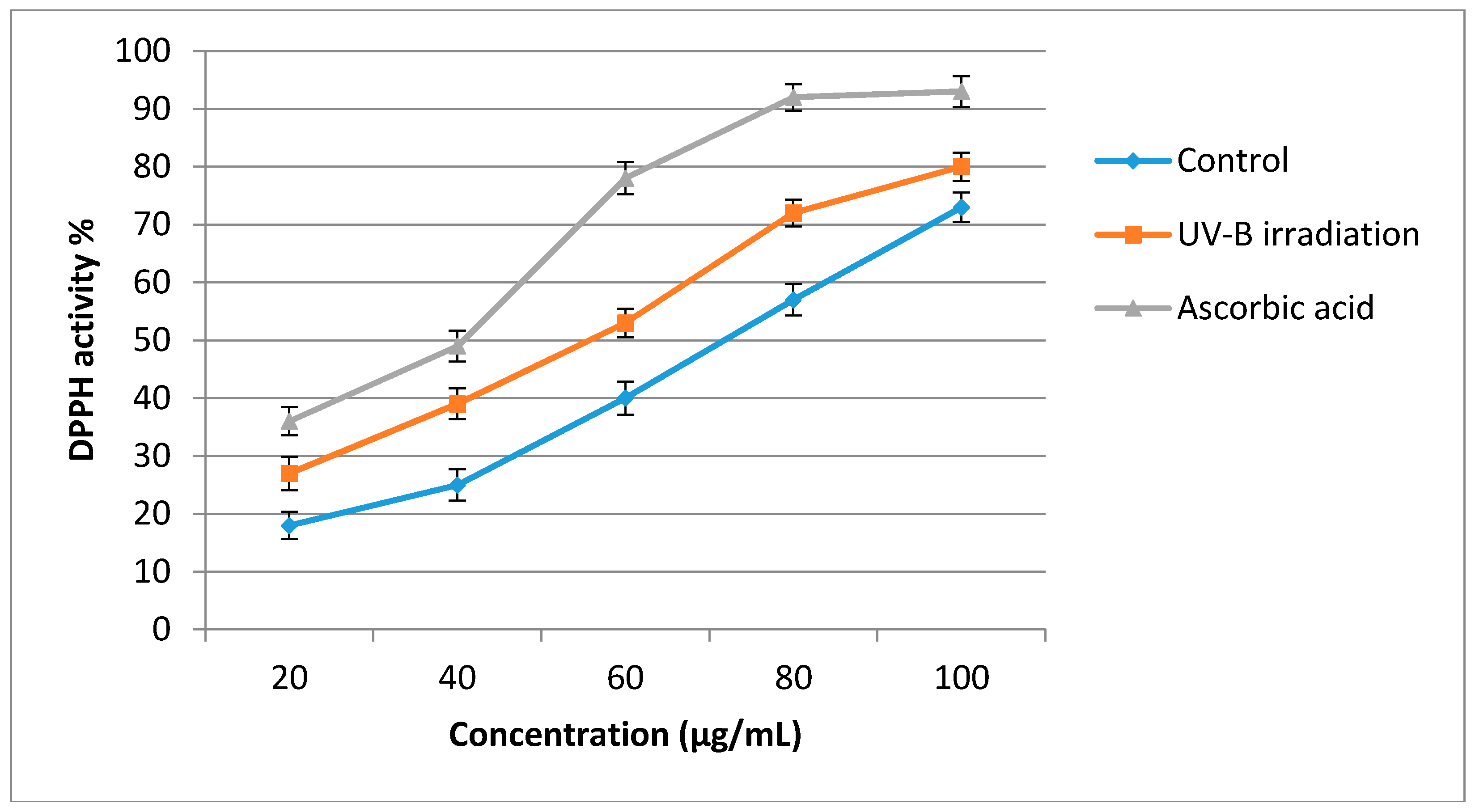
2.4. Antioxidant Activity
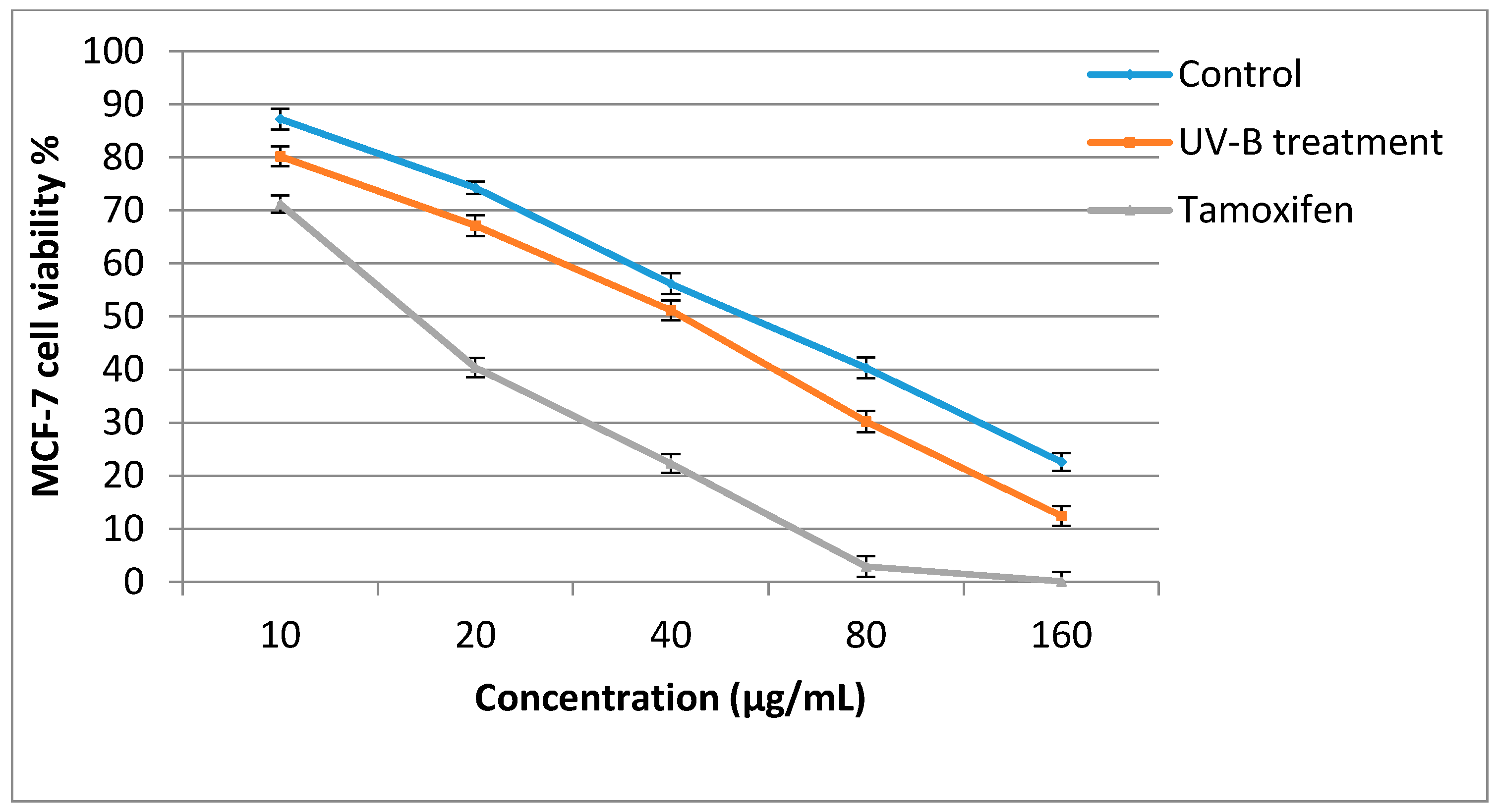
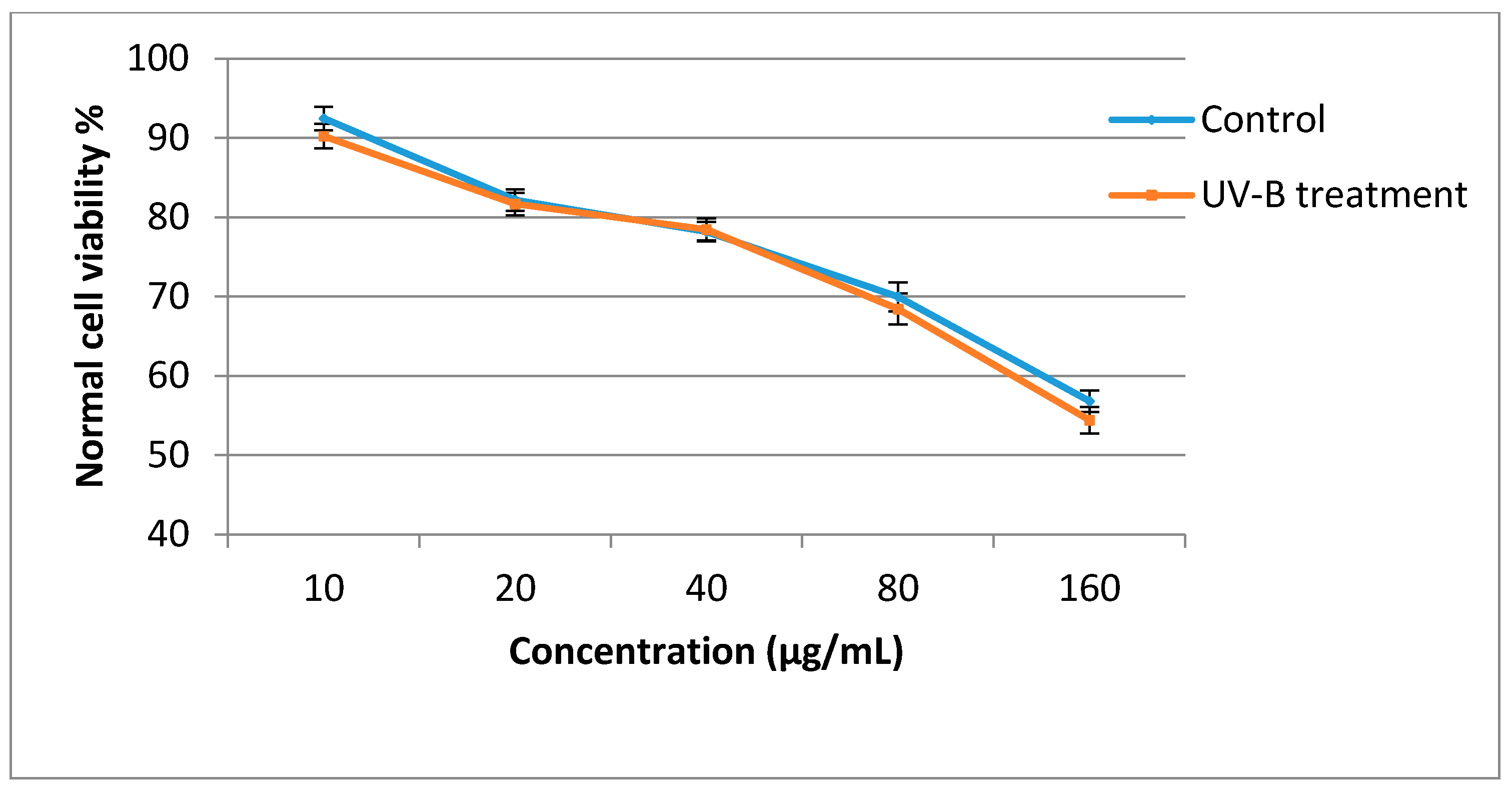
2.5. Antiproliferative Activity
2.6. Correlation Analysis
3. Materials and Methods
3.1. Plant Samples
3.2. UV-B Radiation
3.3. Preparation of Plant Extracts
3.4. Estimation of Total Phenolic Content (TPC)
3.5. Estimation of Total Flavonoid Contents (TFC)
3.6. Identification of Flavonoids and Phenolic Acids
3.7. Chalcone Synthase (CHS) Assay
3.8. In Vitro Evaluation of Antioxidant Activity
1,1-Diphenyl-2-picrylhydrazyl (DPPH) Assay
3.9. Determination of AntiProliferative Activity
3.9.1. Cell Culture and Treatment
3.9.2. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) Assay
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curre. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Koes, R.; Verweij, W.; Quattrocchio, F. Flavonoids: A colorful model for the regulation and evolution of biochemical pathways. Trends Plant Sci. 2005, 10, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.A.; Achnine, L.; Kota, P.; Liu, C.J.; Reddy, M.; Wang, L. The phenylpropanoid pathway and plant defence—A genomics perspective. Mol. Plant Pathol. 2002, 3, 371–390. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Nasiri, A.; Jaafar, H.Z.; Baghdadi, A.; Ahmad, I. Changes in phytochemical synthesis, chalcone synthase activity and pharmaceutical qualities of Sabah snake grass (Clinacanthus nutans L.) in relation to plant age. Molecules 2014, 19, 17632–17648. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.Z.; Karimi, E.; Ibrahim, M.H. Combined effect of CO 2 enrichment and foliar application of salicylic acid on the production and antioxidant activities of anthocyanin, flavonoids and isoflavonoids from ginger. BMC Complement. Altern. Med. 2012, 12, 1. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Sugaya, S.; Gemma, H. Decreased anthocyanin biosynthesis in grape berries grown under elevated night temperature condition. Sci. Hortic. 2005, 105, 319–330. [Google Scholar] [CrossRef]
- Dos Santos Nascimento, L.B.; Leal-Costa, M.V.; Menezes, E.A.; Lopes, V.R.; Muzitano, M.F.; Costa, S.S.; Tavares, E.S. Ultraviolet-B radiation effects on phenolic profile and flavonoid content of Kalanchoe pinnata. J. Photochem. Photobiol. B Biol. 2015, 148, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, M.; Mewis, I.; Neugart, S.; Zrenner, R.; Glaab, J.; Wiesner, M.; Jansen, M.A. UV-B Elicitation of Secondary Plant Metabolites. In III-Nitride Ultraviolet Emitters; Kneissl, M., Rass, J., Eds.; Springer: Cham, Switzerland, 2016; pp. 387–414. [Google Scholar]
- Eichholz, I.; Rohn, S.; Gamm, A.; Beesk, N.; Herppich, W.B.; Kroh, L.W.; Ulrichs, C.; Huyskens-Keil, S. UV-B-mediated flavonoid synthesis in white asparagus (Asparagus officinalis L.). Food Res. Int. 2012, 48, 196–201. [Google Scholar] [CrossRef]
- Schreiner, M.; Krumbein, A.; Mewis, I.; Ulrichs, C.; Huyskens-Keil, S. Short-term and moderate UV-B radiation effects on secondary plant metabolism in different organs of nasturtium (Tropaeolum majus L.). Innov. Food Sci. Emerg. Technol. 2009, 10, 93–96. [Google Scholar] [CrossRef]
- Ries, G.; Heller, W.; Puchta, H.; Sandermann, H.; Seidlitz, H.K.; Hohn, B. Elevated UV-B radiation reduces genome stability in plants. Nature 2000, 406, 98–101. [Google Scholar] [CrossRef] [PubMed]
- Costa, H.; Gallego, S.M.; Tomaro, M.L. Effect of UV-B radiation on antioxidant defense system in sunflower cotyledons. Plant Sci. 2002, 162, 939–945. [Google Scholar] [CrossRef]
- Heo, S.-J.; Ko, S.-C.; Cha, S.-H.; Kang, D.-H.; Park, H.-S.; Choi, Y.-U.; Kim, D.; Jung, W.-K.; Jeon, Y.-J. Effect of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo-oxidative stress induced by UV-B radiation. Toxicol. In Vitro 2009, 23, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Duell-Pfaff, N.; Wellmann, E. Involvement of phytochrome and a blue light photoreceptor in UV-B induced flavonoid synthesis in parsley (Petroselinum hortense Hoffm.) cell suspension cultures. Planta 1982, 156, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Gu, X.; Fu, H.; Zhang, L.; Chen, R.; Cui, L.; Zheng, L.; Zhang, D.; Tian, J. Change of secondary metabolites in leaves of Ginkgo biloba L. in response to UV-B induction. Innov. Food Sci. Emerg. Technol. 2010, 11, 672–676. [Google Scholar] [CrossRef]
- Harbaum-Piayda, B.; Walter, B.; Bengtsson, G.B.; Hubbermann, E.M.; Bilger, W.; Schwarz, K. Influence of pre-harvest UV-B irradiation and normal or controlled atmosphere storage on flavonoid and hydroxycinnamic acid contents of pak choi (Brassica campestris L. ssp. chinensis var. communis). Postharvest Biol. Technol. 2010, 56, 202–208. [Google Scholar] [CrossRef]
- Flora of China. Ocimum basilicum Linnaeus, Vol.17. Available online: http://efloras.org/florataxon.aspx?flora_id = 2&taxon_id = 200019914 (accessed on 5 September 2016).
- Jayasinghe, C.; Gotoh, N.; Aoki, T.; Wada, S. Phenolics composition and antioxidant activity of sweet basil (Ocimum basilicum L.). J. Agric. Food Chem. 2003, 51, 4442–4449. [Google Scholar] [CrossRef] [PubMed]
- Arranz, E.; Jaime, L.; de las Hazas, M.L.; Reglero, G.; Santoyo, S. Supercritical fluid extraction as an alternative process to obtain essential oils with anti-inflammatory properties from marjoram and sweet basil. Ind. Crops Prod. 2015, 67, 121–129. [Google Scholar] [CrossRef]
- Kathirvel, P.; Ravi, S. Chemical composition of the essential oil from basil (Ocimum basilicum Linn.) and its in vitro cytotoxicity against HeLa and HEp-2 human cancer cell lines and NIH 3T3 mouse embryonic fibroblasts. Nat. Prod. Res. 2012, 26, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Viyoch, J.; Pisutthanan, N.; Faikreua, A.; Nupangta, K.; Wangtorpol, K.; Ngokkuen, J. Evaluation of in vitro antimicrobial activity of Thai basil oils and their micro-emulsion formulas against Propionibacterium acnes. Inte. J. Cosmet. Sci. 2006, 28, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Salmah, I.; Mahmood, A.; Sidik, K. Synergistic effects of alcoholic extract of sweet basil (Ocimum basilicum L.) leaves and honey on cutaneous wound healing in rats. Int. J. Mol. Med. Adv. Sci 2005, 1, 220–224. [Google Scholar]
- Marvat, S.K.; Rehman, F.U.; Khan, M.S.; Ghulam, S.; Anwar, N.; Mustafa, G.; Usman, K.H. Phytochemical constituents and pharmacological activities of sweet basil-Ocimum basilicum L. (Lamiaceae). Asian J. Chem. 2011, 23, 3773–3782. [Google Scholar]
- Jenkins, G.I. Signal transduction in responses to UV-B radiation. Annu. Rev. Plant Biol. 2009, 60, 407–431. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Boggia, R.; Zunin, P.; Cerutti, A.K.; Guido, M.; Mellano, M.G.; Prgomet, Z.; Beccaro, G.L. Phytochemical fingerprint and chemometrics for natural food preparation pattern recognition: An innovative technique in food supplement quality control. J. Food Sci. Technol. 2016, 53, 1071–1083. [Google Scholar] [CrossRef] [PubMed]
- Donno, D.; Cavanna, M.; Beccaro, G.L.; Mellano, M.G.; Torello-Marinoni, D.; Cerutti, A.K.; Bounous, G. Currants and strawberries as bioactive compound sources: Determination of antioxidant profiles with hplc-dad/ms. J. Appl. Bot. Food Qual. 2013, 86, 1–10. [Google Scholar]
- Peev, C.I.; Vlase, L.; Antal, D.S.; Dehelean, C.A.; Szabadai, Z. Determination of some polyphenolic compounds in buds of alnus and corylus species by hplc. Chem. Nat. Compd. 2007, 43, 259–262. [Google Scholar] [CrossRef]
- Olsson, L.; Veit, M.; Weissenböck, G.; Bornman, J. Differential flavonoid response to enhanced UV-B radiation in Brassica napus. Phytochemistry 1998, 49, 1021–1028. [Google Scholar] [CrossRef]
- Hagen, S.F.; Borge, G.I.A.; Bengtsson, G.B.; Bilger, W.; Berge, A.; Haffner, K.; Solhaug, K.A. Phenolic contents and other health and sensory related properties of apple fruit (Malus domestica Borkh. cv. Aroma): Effect of postharvest UV-B irradiation. Postharvest Biol. Technol. 2007, 45, 1–10. [Google Scholar] [CrossRef]
- Antognoni, F.; Zheng, S.; Pagnucco, C.; Baraldi, R.; Poli, F.; Biondi, S. Induction of flavonoid production by UV-B radiation in Passiflora quadrangularis callus cultures. Fitoterapia 2007, 78, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Rozema, J.; Björn, L.O.; Bornman, J.; Gaberščik, A.; Häder, D.-P.; Trošt, T.; Germ, M.; Klisch, M.; Gröniger, A.; Sinha, R. The role of UV-B radiation in aquatic and terrestrial ecosystems—an experimental and functional analysis of the evolution of UV-absorbing compounds. J. Photochem. Photobiol. B Biol. 2002, 66, 2–12. [Google Scholar] [CrossRef]
- Searles, P.S.; Flint, S.D.; Caldwell, M.M. A meta-analysis of plant field studies simulating stratospheric ozone depletion. Oecologia 2001, 127, 1–10. [Google Scholar] [CrossRef]
- Berli, F.J.; Moreno, D.; Piccoli, P.; Hespanhol-viana, L.; Silva, M.F.; Bressan-smith, R.; Cavagnaro, J.B.; Bottini, R. Abscisic acid is involved in the response of grape (Vitis vinifera L.) cv. Malbec leaf tissues to ultraviolet-B radiation by enhancing ultraviolet-absorbing compounds, antioxidant enzymes and membrane sterols. Plant Cell Environ. 2010, 33, 1–10. [Google Scholar] [PubMed]
- Casati, P.; Walbot, V. Gene expression profiling in response to ultraviolet radiation in maize genotypes with varying flavonoid content. Plant Physiol. 2003, 132, 1739–1754. [Google Scholar] [CrossRef] [PubMed]
- Scattino, C.; Castagna, A.; Neugart, S.; Chan, H.M.; Schreiner, M.; Crisosto, C.H.; Tonutti, P.; Ranieri, A. Post-harvest UV-B irradiation induces changes of phenol contents and corresponding biosynthetic gene expression in peaches and nectarines. Food Chem. 2014, 163, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Dao, T.; Linthorst, H.; Verpoorte, R. Chalcone synthase and its functions in plant resistance. Phytochem. Rev. 2011, 10, 397–412. [Google Scholar] [CrossRef] [PubMed]
- Wade, H.K.; Bibikova, T.N.; Valentine, W.J.; Jenkins, G.I. Interactions within a network of phytochrome, cryptochrome and UV-B phototransduction pathways regulate chalcone synthase gene expression in Arabidopsis leaf tissue. Plant J. 2001, 25, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Loyall, L.; Uchida, K.; Braun, S.; Furuya, M.; Frohnmeyer, H. Glutathione and a UV light–induced glutathione S-transferase are involved in signaling to chalcone synthase in cell cultures. Plant Cell 2000, 12, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Choung, M.-G.; Kim, J.-B.; Hahn, B.-S.; Kim, J.-B.; Bae, S.-C.; Roh, K.-H.; Kim, Y.-H.; Cheon, C.-I.; Sung, M.-K. Genes up-regulated during red coloration in UV-B irradiated lettuce leaves. Plant Cell Rep. 2007, 26, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Kontogianni, V.G.; Tomic, G.; Nikolic, I.; Nerantzaki, A.A.; Sayyad, N.; Stosic-Grujicic, S.; Stojanovic, I.; Gerothanassis, I.P.; Tzakos, A.G. Phytochemical profile of Rosmarinus officinalis and Salvia officinalis extracts and correlation to their antioxidant and anti-proliferative activity. Food Chem. 2013, 136, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A.; Ashkani, S. Secondary metabolites constituents and antioxidant, anticancer and antibacterial activities of Etlingera elatior (Jack) RM Sm grown in different locations of Malaysia. BMC Complement. Altern. Med. 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Ghasemzadeh, A.; Omidvar, V.; Jaafar, H.Z. Polyphenolic content and their antioxidant activity in leaf extract of sweet potato (Ipomoea batatas). J. Med. Plants Res. 2012, 6, 2971–2976. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Talei, D.; Jaafar, H.Z.; Juraimi, A.S.; Mohamed, M.T.M.; Puteh, A.; Halim, M.R.A. Plant-growth regulators alter phytochemical constituents and pharmaceutical quality in Sweet potato (Ipomoea batatas L.). BMC Complement. Altern. Med. 2016, 16. [Google Scholar] [CrossRef] [PubMed]
- Castagna, A.; Dall’Asta, C.; Chiavaro, E.; Galaverna, G.; Ranieri, A. Effect of post-harvest UV-B irradiation on polyphenol profile and antioxidant activity in flesh and peel of tomato fruits. Food Bioprocess Technol. 2014, 7, 2241–2250. [Google Scholar] [CrossRef]
- Rybarczyk-Plonska, A.; Hansen, M.K.; Wold, A.-B.; Hagen, S.F.; Borge, G.I. A.; Bengtsson, G.B. Vitamin C in broccoli (Brassica oleracea L. var. italica) flower buds as affected by postharvest light, UV-B irradiation and temperature. Postharvest Biol. Technol. 2014, 98, 82–89. [Google Scholar] [CrossRef]
- Harbaum-Piayda, B.; Palani, K.; Schwarz, K. Influence of postharvest UV-B treatment and fermentation on secondary plant compounds in white cabbage leaves. Food Chem. 2016, 197, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Javanmardi, J.; Stushnoff, C.; Locke, E.; Vivanco, J. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003, 83, 547–550. [Google Scholar] [CrossRef]
- González-Vallinas, M.; González-Castejón, M.; Rodríguez-Casado, A.; de Molina, A.R. Dietary phytochemicals in cancer prevention and therapy: A complementary approach with promising perspectives. Nutr. Rev. 2013, 71, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Zarlaha, A.; Kourkoumelis, N.; Stanojkovic, T.; Kovala-Demertzi, D. Cytotoxic activity of essential oil and extracts of Ocimum basilicum against human carcinoma cells. Molecular docking study of isoeugenol as a potent cox and lox inhibitor. J. Nanomater. Biostruct. 2014, 9, 907–917. [Google Scholar]
- Arshad Qamar, K.; Dar, A.; Siddiqui, B.S.; Kabir, N.; Aslam, H.; Ahmed, S.; Erum, S.; Habib, S.; Begum, S. Anticancer activity of Ocimum basilicum and the effect of ursolic acid on the cytoskeleton of MCF-7 human breast cancer cells. Lett. Drug Des. Dis. 2010, 7, 726–736. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Sample Availability: Crude extracts of sweet basil leaves are available from the authors.
| UV-B (W/m2) | TFC (mg QE/g DM) | TPC (mg GAE/g DM) | Gallic Acid (mg/g DM) | Cinnamic Acid (mg/g DM) | Ferulic Acid (mg/g DM) | Quercetin (mg/g DM) | Catechin (mg/g DM) | Kaempferol (mg/g DM) | Rutin (mg/g DM) | Luteolin (mg/g DM) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 22.26 ± 1.57 d | 48.22 ± 2.12 b | 4.76 ± 0.344 c | ND | 3.29 ± 0.209 c | 2.73 ± 0.183 c | 2.16 ± 0.156 d | 1.75 ± 0.155 d | 1.16 ± 0.112 c | ND |
| 2.30 | 37.18 ± 1.19 b | 50.41 ± 3.21 b | 5.22 ± 0.275 b | 3.24 ± 0.215 b | 4.10 ± 0.224 b | 3.48 ± 0.325 b | 3.50 ± 0.176 b | 2.44 ± 0.162 b | 1.52 ± 0.106 b | 0.88 ± 0.053 a |
| 3.60 | 41.25 ± 1.88 a | 56.12 ± 3.45 a | 6.46 ± 0.311 a | 3.91 ± 0.264 a | 4.88 ± 0.218 a | 3.87 ± 0.266 a | 4.00 ± 0.325 a | 2.90 ± 0.255 a | 1.96 ± 0.124 a | 0.86 ± 0.070 a |
| 4.80 | 27.62 ± 1.27 c | 49.16 ± 2.16 b | 4.11 ± 0.282 d | 3.08 ± 0.372 b | ND | 3.10 ± 0.304 b | 3.10 ± 0.224 c | 2.12 ± 0.183 c | 0.93 ± 0.067 d | 0.64 ± 0.046 b |
| Time (h) | TFC (mg QE/g DM) | TPC (mg GAE/g DM) | Gallic Acid (mg/g DM) | Cinnamic Acid (mg/g DM) | Ferulic Acid (mg/g DM) | Quercetin (mg/g DM) | Catechin (mg/g DM) | Kaempferol (mg/g DM) | Rutin (mg/g DM) | Luteolin (mg/g DM) |
|---|---|---|---|---|---|---|---|---|---|---|
| Control | 20.88 ± 1.40 d | 44.52 ± 2.29 d | 4.32 ± 0.324 c | ND | 2.43 ± 0.144 d | 2.82 ± 0.142 c | 2.44 ± 0.165 e | 1.59 ± 0.140 e | 1.27 ± 0.138 b | ND |
| 4 | 37.76 ± 1.52 b | 47.53 ± 2.57 c | 4.59 ± 0.208 c | 3.41 ± 0.146 b | 3.16 ± 0.173 c | 3.24 ± 0.226 b | 3.86 ± 0.328 c | 2.74 ± 0.216 c | 1.50 ± 0.109 b | 0.70 ± 0.081 b |
| 6 | 40.11 ± 1.29 a | 51.12 ± 2.73 b | 5.12 ± 0.264 b | 3.96 ± 0.188 a | 4.55 ± 0.269 b | 3.45 ± 0.247 b | 4.24 ± 0.237 b | 3.18 ± 0.227 b | 1.53 ± 0.122 b | 0.93 ± 0.056 a |
| 8 | 41.19 ± 1.46 a | 55.79 ± 2.31 a | 6.07 ± 0.416 a | 3.24 ± 0.247 b | 5.10 ± 0.320 a | 3.85 ± 0.166 a | 4.67 ± 0.216 a | 3.80 ± 0.148 a | 2.12 ± 0.144 a | 0.96 ± 0.072 a |
| 10 | 28.11 ± 1.75 c | 43.20 ± 2.70 d | 3.72 ± 0.244 d | 3.11 ± 0.263 b | 2.10 ± 0.108 e | 2.43 ± 0.204 d | 2.80 ± 0.155 d | 2.26 ± 0.117 d | 1.00 ± 0.172 c | 0.65 ± 0.059 b |
| Phytochemicals | Antiproliferative Activity | Antioxidant Activity |
|---|---|---|
| TFC | −0.9031 ** | 0.9100 ** |
| TPC | −0.8940 ** | 0.8862 ** |
| Gallic acid | −0.8225 * | 0.8325 ** |
| Cinnamic acid | −0.7433 * | 0.8039 * |
| Ferulic acid | −0.8661 ** | 0.8590 ** |
| Quercetin | −0.9327 ** | 0.9168 ** |
| Catechin | −0.8266 * | 0.8892 ** |
| Kaempferol | −0.9240 ** | 0.9315 ** |
| Rutin | −0.8590 ** | 0.8872 ** |
| Luteolin | −0.8229 * | 0.8126 * |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghasemzadeh, A.; Ashkani, S.; Baghdadi, A.; Pazoki, A.; Jaafar, H.Z.E.; Rahmat, A. Improvement in Flavonoids and Phenolic Acids Production and Pharmaceutical Quality of Sweet Basil (Ocimum basilicum L.) by Ultraviolet-B Irradiation. Molecules 2016, 21, 1203. https://doi.org/10.3390/molecules21091203
Ghasemzadeh A, Ashkani S, Baghdadi A, Pazoki A, Jaafar HZE, Rahmat A. Improvement in Flavonoids and Phenolic Acids Production and Pharmaceutical Quality of Sweet Basil (Ocimum basilicum L.) by Ultraviolet-B Irradiation. Molecules. 2016; 21(9):1203. https://doi.org/10.3390/molecules21091203
Chicago/Turabian StyleGhasemzadeh, Ali, Sadegh Ashkani, Ali Baghdadi, Alireza Pazoki, Hawa Z. E. Jaafar, and Asmah Rahmat. 2016. "Improvement in Flavonoids and Phenolic Acids Production and Pharmaceutical Quality of Sweet Basil (Ocimum basilicum L.) by Ultraviolet-B Irradiation" Molecules 21, no. 9: 1203. https://doi.org/10.3390/molecules21091203
APA StyleGhasemzadeh, A., Ashkani, S., Baghdadi, A., Pazoki, A., Jaafar, H. Z. E., & Rahmat, A. (2016). Improvement in Flavonoids and Phenolic Acids Production and Pharmaceutical Quality of Sweet Basil (Ocimum basilicum L.) by Ultraviolet-B Irradiation. Molecules, 21(9), 1203. https://doi.org/10.3390/molecules21091203