A Review on the Terpenes from Genus Vitex
Abstract
:1. Introduction
2. Chemical Constituents
2.1. Monoterpenoids and Sesquiterpenoids
2.1.1. Monoterpenoids
2.1.2. Sesquiterpenoids
2.2. Diterpenoids
2.3. Triterpenoids
3. Pharmacological Effects
3.1. Anti-Inflammatory Activity
3.2. Anti-Tumor Activity
3.3. Antibacterial and Antifungal Activities
3.4. Antioxidant Activity
3.5. Other Pharmacological Activities
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Pei, J.; Chen, S.L. Flora of China; Science Press: Beijing, China, 1982. [Google Scholar]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China; Beijing Chemical Industry Press: Beijing, China, 2015. [Google Scholar]
- Zheng, C.J.; Li, H.Q.; Ren, S.C.; Xu, C.L.; Rahman, K.; Qin, L.P.; Sun, Y.H. Phytochemical and pharmacological profile of Vitex negundo. Phytother. Res. 2015, 29, 633–647. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Z.; Su, Y.F.; Jin, X.J. Advances in studies on chemical constituents from plants of Vitex L. and their bioactivities. Chin. Tradit. Herb. Drugs 2005, 36, 930–938. [Google Scholar]
- Wu, C.; Zhang, J.; Yin, Z.Q. Chemical constituents from the fruits of Vitex rotundifolia. Pharm. Biotechnol. 2010, 17, 504–507. [Google Scholar]
- Wu, J.; Zhou, T.; Zhang, S.W.; Zhang, X.H.; Xuan, L.J. Cytotoxic terpenoids from the fruits of Vitex trifolia L. Planta Med. 2009, 75, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.L.; Prabhakar, A.; Dhar, K.L.; Sachar, A. A new iridoid glycoside from Vitex negundo Linn (Verbenacea). Nat. Prod. Res. 2009, 23, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Iwagawa, T.; Nakahara, A.; Nakatani, M. Iridoids from Vitex cannabifolia. Phytochemistry 1993, 32, 453–454. [Google Scholar] [CrossRef]
- Yamasaki, T.; Kawabata, T.; Masuoka, C.; Kinjo, J.; Ikeda, T.; Nohara, T.; Ono, M. Two new lignan glucosides from the fruit of Vitex cannabifolia. J. Nat. Med. 2008, 62, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Suksamrarn, S.; Kumcharoen, S.; Suksamrarn, A. Iridoids of Vitex limonifolia. Planta. Med. 1999, 65. [Google Scholar] [CrossRef] [PubMed]
- Kuruüzüm-Uz, A.; Ströch, K.; Demirezer, L.Ö.; Zeeck, A. Glucosides from Vitex agnus-castus. Phytochemistry 2003, 63, 959–964. [Google Scholar]
- Kouno, I.; Inoue, M.; Onizuka, Y.; Fujisaki, T.; Kawano, N. Iridoid and phenolic glucoside from Vitex rotundifolia. Phytochemistry 1988, 27, 611–612. [Google Scholar] [CrossRef]
- Sridhar, C.; Subbaraju, G.V.; Venkateswarlu, Y.; Venugopal, R.T. New acylated iridoid glucosides from Vitex altissima. J. Nat. Prod. 2004, 67, 2012–2016. [Google Scholar] [CrossRef] [PubMed]
- One, M.; Ito, Y.; Kubo, S.; Nohara, T. Two new iridoids from Viticis trifoliae fructus (Fruit of Vitex rotundifolia L.). Chem. Pharm. Bull. 1997, 45, 1094–1096. [Google Scholar] [CrossRef]
- Okuyama, E.; Fujimori, S.; Yamazaki, M.; Deyama, T. Pharmacologically active components of viticis fructus (Vitex rotundifolia). II. The components having analgesic effects. Chem. Pharm. Bull. 1998, 46, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Suksamrarn, A.; Kumpun, S.; Kirtikara, K.; Yingyongnarongkul, B.; Suksamrarn, S. Iridoids with anti-inflammatory activity from Vitex peduncularis. Planta Med. 2002, 68, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Gu, Q.; Zhang, X.M.; Jiang, Z.Y.; Chen, J.J.; Zhou, J. Chemical constituents from fruits of Vitex trifolia. Chin. Tradit. Herb. Drugs 2007, 38, 656–659. [Google Scholar]
- Li, S.H.; Qiu, S.X.; Yao, P.; Sun, H.D.; Fong, H.H.S.; Zhang, H.J. Compounds from the fruits of the popular European medicinal plant Vitex agnus-castus in chemoprevention via NADP(H): Quinone oxidoreductase type 1 induction. Evid. Based Complement. Altern. Med. 2013, 2013, 77–133. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.A.; Fujimatsu, T.; Uchida, K.; Sadhu, S.K.; Ahmed, F.; Ishibashi, M. Hh signaling inhibitors from Vitex negundo; naturally occurring inhibitors of the GLI1–DNA complex. Mol. BioSyst. 2013, 9, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Bani, S.; Satti, N.K.; Gupta, B.D.; Suri, K.A. Anti-arthritic activity of agnuside mediated through the down-regulation of inflammatory mediators and cytokines. Inflamm. Res. 2012, 61, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Luqman, S.; Masood, N.; Gupta, M.M. Validated high performance thin layer chromatographic method for simultaneous quantification of major iridoids in Vitex trifolia and their antioxidant studies. J. Pharm. Biomed. Anal. 2012, 61, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Ramazanov, N.S. Ecdysteroids and iridoidal glycosides from Vitex agnus-castus. Chem. Nat. Compd. 2004, 40, 299–300. [Google Scholar] [CrossRef]
- Huang, J.; Wang, G.C.; Wang, C.H.; Huang, X.J.; Ye, W.C. Two new glycosides from Vitex negundo. Nat. Prod. Res. 2013, 27, 1837–1841. [Google Scholar] [CrossRef] [PubMed]
- Tasduq, S.A.; Kaiser, P.J.; Gupta, B.D.; Gupta, V.K.; Johri, R.K. Negundoside, an iridiod glycoside from leaves of Vitex negundo, protects human liver cells against calcium-mediated toxicity induced by carbon tetrachloride. World J. Gastroenterol. 2008, 14, 3693–3709. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, R.; Thiagarajan, R.; Beulaja, S.; Sivakumar, M.R.; Meiyalagan, V.; Sundaram, R.; Arumugam, M. 1,2 di-substituted idopyranose from Vitex negundo L. protects against streptozotocin-induced diabetes by inhibiting nuclear factor-kappa B and inducible nitric oxide synthase expression. Microsc. Res. Tech. 2011, 74, 301–307. [Google Scholar] [PubMed]
- Sundaram, R.; Naresh, R.; Shanthi, P.; Sachdanandam, P. Antihyperglycemic effect of iridoid glucoside, isolated from the leaves of Vitex negundo in streptozotocin-induced diabetic rats with special reference to glycoprotein components. Phytomedicine 2012, 19, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, R.; Naresh, R.; Ranadevan, R.; Shanthi, P.; Sachdanandam, P. Effect of iridoid glucoside on streptozotocin induced diabetic rats and its role in regulating carbohydrate metabolic enzymes. Eur. J. Pharmacol. 2012, 674, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Pu, J.; Zhang, H.; Han, T.; Rahman, K.; Qin, L.P. Sesquiterpenoids and norterpenoids from Vitex negundo. Fitoterapia 2012, 83, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Yadav, A.K.; Vasudev, P.G.; Gupta, M.M. Isolation and structure determination of furanoeremophilanes from Vitex negundo. Tetrahedron. Lett. 2013, 54, 2428–2430. [Google Scholar] [CrossRef]
- Barbosa, L.C.A.; Demuner, A.J.; Howarth, O.W.; Pereira, N.S.; Veloso, D.P. Chemical study of the leaves of Vitex poligama. Fitoterapia 1995, LXVI, 279–280. [Google Scholar]
- Hajdú, Z.; Hohmann, J.; Forgo, P.; Martinek, T.; Dervarics, M.; Zupkó, I.; Falkay, G.; Cossuta, D.; Máthé, I. Diterpenoids and flavonoids from the fruits of Vitex agnus-castus and antioxidant activity of the fruit extracts and their constituents. Phytother. Res. 2007, 21, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Nagasawa, Y.; Ikeda, T.; Tsuchihashi, R.; Okawa, M.; Kinjo, J.; Yoshimitsu, H.; Nohara, T. Three new diterpenoids from the fruit of Vitex agnus-castus. Chem. Pharm. Bull. 2009, 57, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Eguchi, K.; Konoshita, M.; Furusawa, C.; Sakamoto, J.; Yasuda, S.; Ikeda, T.; Okawa, M.; Kinjo, J.; Yoshimitsu, H.; et al. A new diterpenoid glucoside and two new diterpenoids from the fruit of Vitex agnus-castus. Chem. Pharm. Bull. 2011, 59, 392–396. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Ito, Y.; Nohara, T. A labdane diterpene glycoside from fruit of Vitex rotundifolia. Phytochemistry 1998, 48, 207–209. [Google Scholar] [CrossRef]
- Pal, M.; Li, S.H.; Tewari, S.K.; Sun, H.D. Diterpenoid compounds from Vitex agnus-castus. Chem. Nat. Compd. 2013, 49, 635–638. [Google Scholar] [CrossRef]
- Wang, X.Q.; Zhang, T.; Zheng, B.; Xie, W.D.; Shen, T. Labdane-type diterpenoids from the fruits of Vitex rotundifolia. Bull. Korean. Chem. Soc. 2014, 35, 672–674. [Google Scholar] [CrossRef]
- Zheng, C.J.; Huang, B.K.; Wang, Y.; Ye, Q.; Han, T.; Zhang, Q.Y.; Zhang, H.; Qin, L.P. Anti-inflammatory diterpenes from the seeds of Vitex negundo. Bioorg. Med. Chem. 2010, 18, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Zhu, J.Y.; Yu, W.; Ma, X.Q.; Rahman, K.; Qin, L.P. Labdane-type diterpenoids from the fruits of Vitex trifolia. J. Nat. Prod. 2013, 76, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Sawamura, H.; Ito, Y.; Mizuki, K.; Nohara, T. Diterpenoids from the fruits of Vitex trifolia. Phytochemistry 2000, 55, 873–877. [Google Scholar] [CrossRef]
- Hoberg, E.; Orjala, J.; Meier, B.; Sticher, O. Diterpenoids from the fruits of Vitex agnus-castus. Phytochemistry 1999, 52, 1555–1558. [Google Scholar] [CrossRef]
- Jarry, H.; Spengler, B.; Wuttke, W.; Christoffel, V. In vitro assays for bioactivity-guided isolation of endocrine active compounds in Vitex agnus-castus. Maturitas 2006, 55S, S26–S36. [Google Scholar] [CrossRef]
- Asaka, Y.; Kamikawa, T.; Kubota, T. Constituents of Vitex rotundifolia L. fil. Chem. Lett. 1973, 937–940. [Google Scholar] [CrossRef]
- Huang, D.N.; Qing, S.; Zeng, G.Y.; Wang, Y.J.; Guo, H.; Tan, J.B.; Zhou, Y.J. Lipophilic components from fructus Viticis negundo and their anti-tumor activities. Fitoterapia 2013, 86, 144–148. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.J.; Li, C.M.; Ling, W.W.; Wan, X.C.; Xia, T.; Du, X.F.; Zhang, Z.Z.; Ling, T.J. A rearranged labdane-type diterpenoid and other constituents from Vitex negundo var. cannabifolia. Biochem. Syst. Ecol. 2012, 40, 98–102. [Google Scholar] [CrossRef]
- Lee, C.; Lee, J.W.; Jin, Q.H.; Lee, H.J.; Lee, S.J.; Lee, D.; Lee, M.K.; Lee, C.K.; Hong, J.T.; Lee, M.K.; et al. Anti-inflammatory constituents from the fruits of Vitex rotundifolia. Bioorg. Med. Chem. Lett. 2013, 23, 6010–6014. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.A.; Kim, D.S.; Oh, K.; Seo, Y. Isolation of a new labdane-type diterpene from Vitex rotundifolia. Bull. Korean Chem. Soc. 2013, 34, 3840–3842. [Google Scholar] [CrossRef]
- Kondo, Y.; Sugiyama, K.; Nozoe, S. Studies on the constituents of Vitex rotundifolia L. fil. Chem. Pharm. Bull. 1986, 34, 4829–4832. [Google Scholar] [CrossRef] [PubMed]
- Kiuchi, F.; Matsuo, K.; Ito, M.; Qui, T.K.; Honda, G. New norditerpenoids with trypanocidal activity from Vitex trifolia. Chem. Pharm. Bull. 2004, 52, 1492–1494. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.H.; Zhang, Q.W.; Wang, Z.M.; Xu, L.Z.; Yang, S.L. Chemical constituents in Vitex trifolia L. (II). Chin. Tradit. Herb. Drugs 2010, 41, 1622–1624. [Google Scholar]
- Ono, M.; Yamamoto, M.; Yanaka, T.; Ito, Y.; Nohara, T. Ten new labdane-type diterpenes from the fruit of Vitex rotundifolia. Chem. Pharm. Bull. 2001, 49, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.N.; Friesen, J.B.; Webster, D.; Nikolic, D.; van Breemen, R.B.; Wang, Z.J.; Fong, H.H.S.; Farnsworth, N.R.; Pauli, G.F. Phytoconstituents from Vitex agnus-castus fruits. Fitoterapia 2011, 82, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Högner, C.; Sturm, S.; Seger, C.; Stuppner, H. Development and validation of a rapid ultra-high performance liquid chromatography diode array detector method for Vitex agnus-castus. J. Chromatogr. B. 2013, 927, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Thakur, J.; Saikia, D.; Gupta, M.M. Antitubercular diterpenoids from Vitex trifolia. Phytomedicine 2013, 20, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Huang, B.K.; Wu, Y.B.; Han, T.; Zhang, Q.Y.; Zhang, H.; Qin, L.P. Terpenoids from Vitex negundo seeds. Biochem. Syst. Ecol. 2010, 38, 247–249. [Google Scholar] [CrossRef]
- Corlay, N.; Lecsö-Bornet, M.; Leborgne, E.; Blanchard, F.; Cachet, X.; Bignon, J.; Roussi, F.; Butel, M.J.; Awang, K.; Litaudon, M. Antibacterial labdane diterpenoids from Vitex vestita. J. Nat. Prod. 2015, 78, 1348–1356. [Google Scholar] [CrossRef] [PubMed]
- Nyiligira, E.; Viljoen, A.M.; van Heerden, F.R.; van Zyl, R.L.; van Vuuren, S.F.; Steenkamp, P.A. Phytochemistry and in vitro pharmacological activities of South African Vitex (Verbenaceae) species. J. Ethnopharmacol. 2008, 119, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Yamasaki, T.; Konoshita, M.; Ikeda, K.; Okawa, M.; Kinjo, J.; Yoshimitsu, H.; Nohara, T. Five new diterpenoids, viteagnusins A–E, from the fruit of Vitex agnus-castus. Chem. Pharm. Bull. 2008, 56, 1621–1624. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Yamamoto, M.; Masuoka, C.; Ito, Y.; Yamashita, M.; Nohara, T. Diterpenes from the fruits of Vitex rotundifolia. J. Nat. Prod. 1999, 62, 1532–1537. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.J.; Lan, X.P.; Wang, Y.; Huang, B.K.; Han, T.; Zhang, Q.Y.; Qin, L.P. A new labdane diterpene from Vitex negundo. Pharm. Biol. 2012, 50, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Aphaijitt, S.; Nimgirawath, K.; Suksamrarn, A.; Tooptakong, U. Isolation and crystal structure of Limonidilactone—A labdane diterpene from Vitex limonifolia. Aust. J. Chem. 1995, 48, 133–137. [Google Scholar] [CrossRef]
- Ono, M.; Yanaka, T.; Yamamoto, M.; Ito, Y.; Nohara, T. New diterpenes and norditerpenes from the fruits of Vitex rotundifolia. J. Nat. Prod. 2002, 65, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, C.X.; Xie, W.D.; Row, K.H. Vitrifolin A: A norlabdane diterpenoid from the fruits of Vitex trifolia Linn. var. simplicifolia Cham. J. Chin. Chem. Soc. 2013, 60, 542–545. [Google Scholar] [CrossRef]
- Ono, M.; Nishida, Y.; Masuoka, C.; Li, J.C.; Okawa, M.; Ikeda, T.; Nohara, T. Lignan derivatives and a norditerpene from the seeds of Vitex negundo. J. Nat. Prod. 2004, 67, 2073–2075. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Ito, Y.; Nohara, T. Four new halimane-type diterpenes, vitetrifolins D–G, from the fruit of Vitex trifolia. Chem. Pharm. Bull. 2001, 49, 1220–1222. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.S.; Sharma, A.K.; Handa, S.S.; Dhar, K.L.J. Chemical investigation and anti-inflammatory activity of Vitex negundo seeds, Part I. Indian. J. Chem. 1991, 30B, 773–776. [Google Scholar]
- Sakurai, A.; Okamoto, Y.; Kokubo, S.; Chida, A. Abietane-type diterpenoids from the fruit of Vitex rotundifolia L. fil. J. Chem. Soc. Jpn. Chem. Ind. Chem. 1999, 3, 207–211. [Google Scholar] [CrossRef]
- Chen, J.; Fan, C.L.; Wang, Y.; Ye, W.C. A new triterpenoid glycoside from Vitex negundo. Chin. J. Nat. Med. 2014, 12, 0218–0221. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Abdou, A.M.; Hamed, M.M.; Saad, A.M. Characterization of bioactive phytochemical from the leaves of Vitex trifolia. Int. J. Pharm. Appl. 2012, 3, 419–428. [Google Scholar]
- Li, M.M.; Su, X.Q.; Sun, J.; Gu, Y.F.; Huang, Z.; Zeng, K.W.; Zhang, Q.; Zhao, Y.F.; Ferreira, D.; Zjawiony, J.K.; et al. Anti-inflammatory ursane- and oleanane-type triterpenoids from Vitex negundo var. cannabifolia. J. Nat. Prod. 2014, 77, 2248–2254. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Lin, X.Y.; Zhong, L.J.; Xie, J.M.; Zhang, Y.H. Chemical constituents of Vitex trifolia L. Nat. Prod. Res. Dev. 2011, 23, 1011–1013. [Google Scholar]
- Noel, M.G.; Dayrit, F.M. Triterpenes in the callus culture of Vitex negundo L. Philipp. J. Sci. 2005, 134, 5–19. [Google Scholar]
- Wang, Y.J.; He, X.; Zeng, G.Y.; Tan, J.B.; Li, X.; Zhou, Y.J. Constituents of triterpenes in the seeds of Vitex negundo L. Cent. South Pharm. 2012, 10, 409–412. [Google Scholar]
- Sridhar, C.; Rao, K.V.; Subbaraju, G.V. Flavonoids, triterpenoids and a lignan from Vitex altissima. Phytochemistry 2005, 66, 1707–1712. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.Y.; Chen, Y.S.; Wang, F.; Chen, S.W.; Zhang, Y.H. Chemical of Vitex trifolia. Zhongguo Zhongyao Zazhi. 2014, 39, 2024–2028. [Google Scholar] [PubMed]
- Mishra, S.; Pani, S.R.; Rout, K.K.; Nayak, S.K.; Sahoo, S. Bioassay guided fractionation and hepatoprotective activity of oleanolic acid acetate isolated from Vitex negundo Linn. J. Biol. Act. Prod. Nat. 2014, 4, 89–100. [Google Scholar]
- Verma, V.K.; Siddiqui, N.U.; Aslam, M. Phytochemical constituents from the bark of Vitex negundo Linn. Int. J. Pharm. Sci. Rev. Res. 2011, 7, 93–95. [Google Scholar]
- Chawla, A.S.; Sharma, A.K.; Handa, S.S. Chemical investigation and anti-inflammatory activity of Vitex negundo seeds. J. Nat. Prod. 1992, 55, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.S.; Xie, J.M.; Yao, H.; Lin, X.Y.; Zhang, Y.H. Studies on the triterpenoids of Vitex trifolia. J. Chin. Med. Mater. 2010, 33, 908–910. [Google Scholar]
- Huang, M.Y.; Zhong, L.J.; Xie, J.M.; Wang, F.; Zhang, Y.H. A new taraxastane-type triterpene from Vitex trifolia var. simplicifolia. Helv. Chim. Acta 2013, 96, 2040–2045. [Google Scholar] [CrossRef]
- Sahu, N.P.; Roy, S.K.; Mahato, S.B. Triterpenoids and flavonoids of Vitex peduncularis. Planta Med. 1984, 50, 527. [Google Scholar] [CrossRef] [PubMed]
- Chandramu, C.; Manohar, R.D.; Krupadanam, D.G.L.; Dashavantha, R.V. Isolation, characterization and biological activity of betulinic acid and ursolic acid from Vitex negundo L. Phytother. Res. 2003, 17, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Jangwan, J.S.; Aquino, R.P.; Mencherini, T.; Picerno, P.; Singh, R. Chemical constituents of ethanol extract of leaves and molluscicidal activity of crude extracts from Vitex trifolia Linn. Herba Pol. 2013, 59, 19–32. [Google Scholar] [CrossRef]
- Rudrapaul, P.; Sarma, I.S.; Das, N.; De, U.C.; Bhattacharjee, S.; Dinda, B. New flavonol methyl ether from the leaves of Vitex peduncularis exhibits potential inhibitory activity against Leishmania donovani through activation of iNOS expression. Eur. J. Med. Chem. 2014, 87, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Kannathasan, K.; Senthilkumar, A.; Venkatesalu, V. Crystal structure and antibacterial evaluation of epifriedelinol isolated from Vitex peduncularis Wall. ex Schauer. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Arumanyagam, S.; Arunmani, M.; Zariya, J.S.; Geetha, S. Hepatoprotective effect of Vitex negundo Linn. against CCl4. World J. Pharm. Res. 2015, 4, 1313–1325. [Google Scholar]
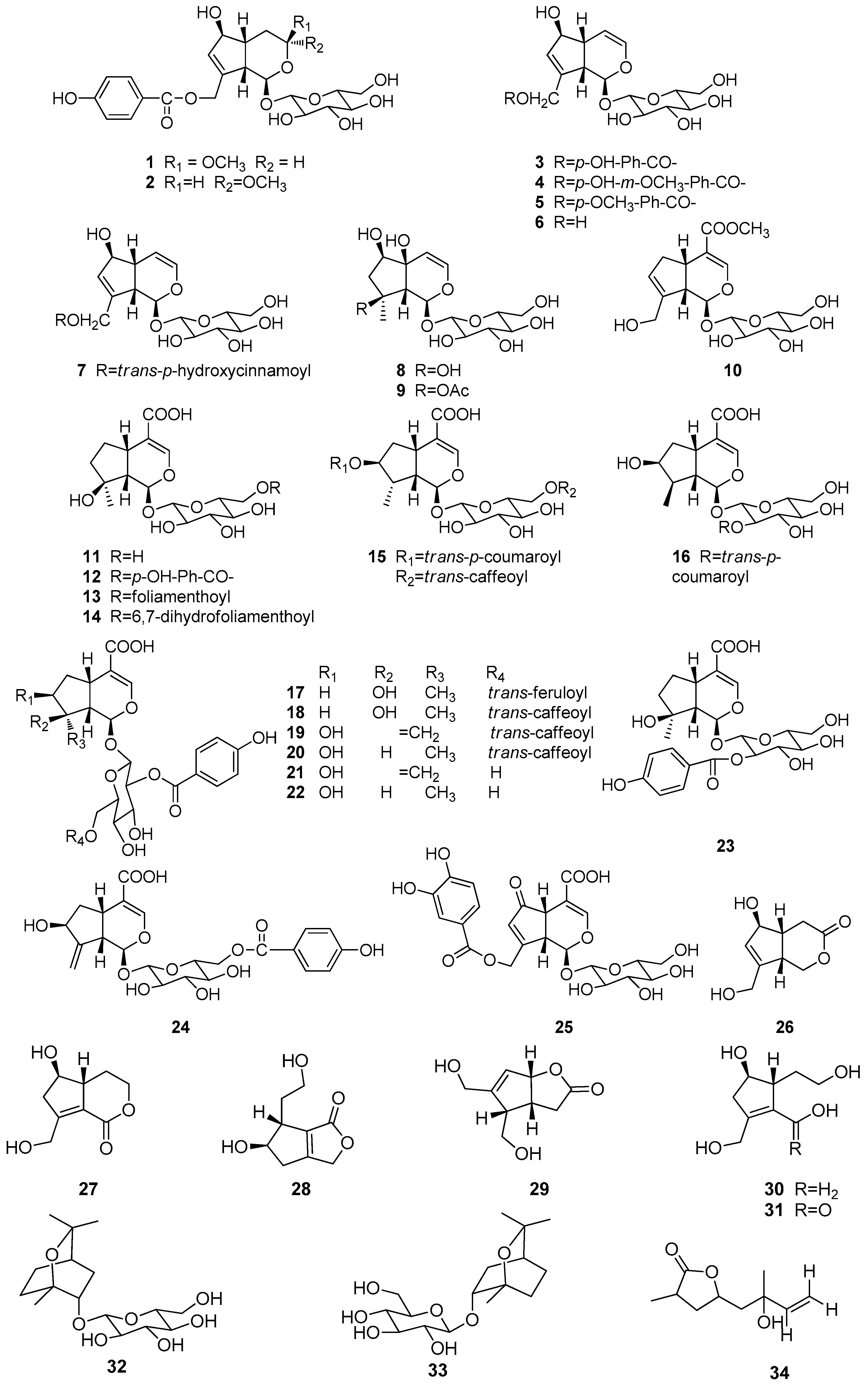
| No. | Compound Name | Source | Reference | |
|---|---|---|---|---|
| 1 | Nishindaside | a, h | [7,8,9] | |
| 2 | Isonishindaside | h | [8] | |
| 3 | Agnuside | a–h | [9,10,11,12,13,14,15,16,17,18,19,20,21] | |
| 4 | 10-O-Vanilloyl aucubin | d, e, h | [9,14,15,16] | |
| 5 | Limoniside | g | [10] | |
| 6 | Aucubin | f | [11,18] | |
| 7 | Eurostoside | d | [12] | |
| 8 | Harpagide | f | [22] | |
| 9 | 8-O-Acetylharpagide | f | [22] | |
| 10 | Geniposide | h | [9] | |
| 11 | Mussaenosidic acid | f | [11] | |
| 12 | 6′-O-p-Hydroxybenzoylmussaenosidic acid | a, b, f | [11,21,23] | |
| 13 | Agnucastoside A | f | [11] | |
| 14 | Agnucastoside B | f | [11] | |
| 15 | Agnucastoside C | f | [11] | |
| 16 | 2′-O-trans-p-Coumaroylloganic acid | a | [23] | |
| 17 | 6′-O-trans-Feruloylnegundoside | c | [13] | |
| 18 | 6′-O-trans-Caffeoylnegundoside | c | [13] | |
| 19 | 2′-O-p-Hydroxybenzoyl-6′-O-trans-caffeoylgardoside | c | [13] | |
| 20 | 2′-O-p-Hydroxybenzoyl-6′-O-trans-caffeoyl-8-epiloganic acid | c | [13] | |
| 21 | 2′-O-p-Hydroxybenzoyl gardoside | c | [13] | |
| 22 | 2′-O-p-Hydroxybenzoyl-8-epiloganic acid | c | [13] | |
| 23 | Negundoside | a, c | [7,13,23,24,25,26,27] | |
| 24 | 6′-O-p-Hydroxybenzoyl-gardoside | a | [23] | |
| 25 | 1,4a,5,7a-Tetrahydro-1-β-d-glucosyl-7-(3′,4′-dihydroxybenzoyloxymethyl)-5-ketocyclopenta[c]pyran-4-carboxylic acid | a | [7] | |
| 26 | Iridolactone | d | [14] | |
| 27 | Viteoid II | d | [14] | |
| 28 | Viteoid I | d | [14] | |
| 29 | Pedicularis lactone | d | [14] | |
| 30 | Eucommiol | d | [14] | |
| 31 | 1-Oxoeucommiol | d | [14] | |
| 32 | (1S,2S,4R)-2-endo-Hydroxy-1,8-cineole-β-d-glucopyranoside | d | [5] | |
| 33 | (1R,2R,4S)-2-endo-Hydroxy-1,8-cineole-β-d-glucopyranoside | d | [5] | |
| 34 | Vitexoid | b | [6] |
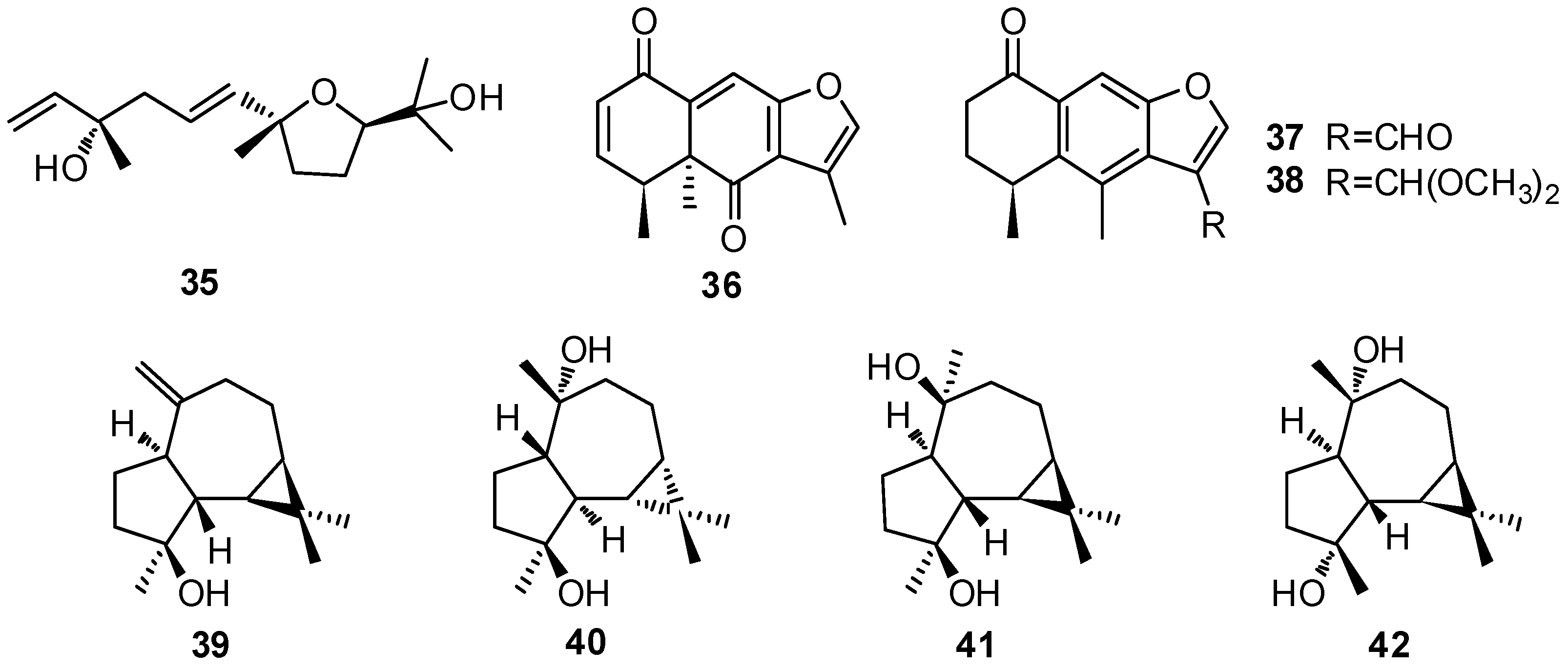
| No. | Compound Name | Source | Reference |
|---|---|---|---|
| 35 | Negunfurol | a | [28] |
| 36 | 1,6-Dioxo-2(3),9(10)-dehydrofuranoeremophilane | a | [29] |
| 37 | 4,6-Dimethyl-11-formyl-1-oxo-4H,2,3-dihydronaphthofuran | a | [28,29] |
| 38 | 4,6-Dimethyl-11-dimethoxymethyl-1-oxo-4H,2,3-dihydronaphthofuran | a | [29] |
| 39 | Spathulenol | b, f, i | [17,18,30,31] |
| 40 | ent-4α,10β-Dihydroxyaromadendrane | b | [17] |
| 41 | 4β,10β-Dihydroxyaromadendrane | f | [32] |
| 42 | 4α,10α-Dihydroxyaromadendrane | f | [33] |
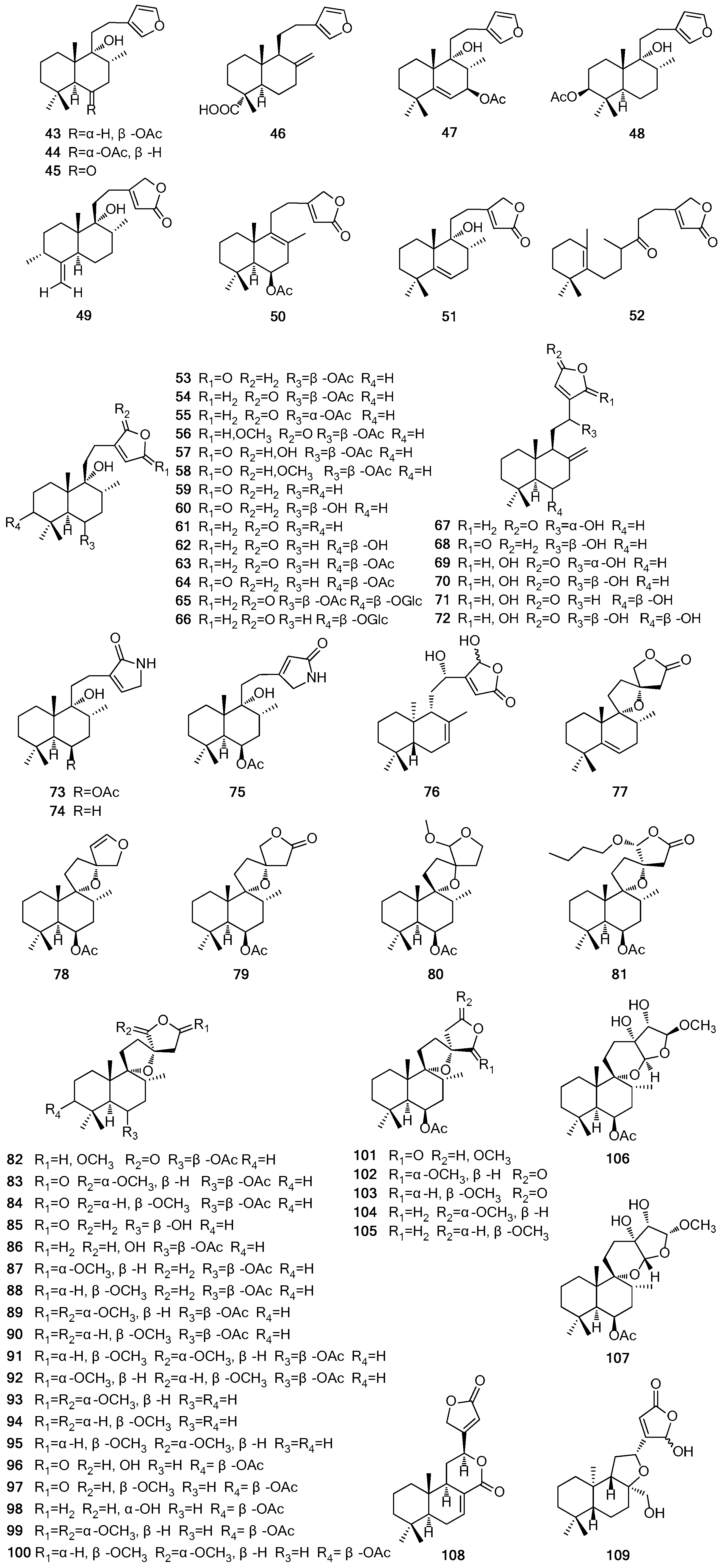
| Type | No. | Compound Name | Source | Reference |
|---|---|---|---|---|
| Labdane | 43 | Rotundifuran | b, d, f | [31,35,38,39,40,41,42] |
| 44 | Vitetrifolin B | b, f | [31,39] | |
| 45 | Dihydrosolidagenone | b | [39] | |
| 46 | (+)-Polyalthic acid | a | [43] | |
| 47 | Vitetrifolin C | b, f | [31,39] | |
| 48 | Vitetrifolin H | b | [6,38] | |
| 49 | Vitexilactone C | h | [44] | |
| 50 | Vitextrifolin C | b | [38] | |
| 51 | Vitextrifolin D | b | [38] | |
| 52 | Vitextrifolin E | b | [38] | |
| 53 | Vitexilactone | b, d, f | [6,31,33,35,36,40,45,46,47,48,49,50] | |
| 54 | (rel 5S,6R,8R,9R,10S)-6-Acetoxy-9-hydroxy-13(14)-labden-16,15-olide | b,d | [6,48,50] | |
| 55 | (rel 5S,6S,8R,9R,10S)-6-Acetoxy-9-hydroxy-13(14)-labden-16,15-olide | d | [50] | |
| 56 | (rel 5S,6R,8R,9R,10S)-6-Acetoxy-9-hydroxy-15-methoxy-13(14)-labden-16,15-olide | d | [46,50] | |
| 57 | Viteagnusin I | b, d, f | [38,45,51] | |
| 58 | Viteagnusin H (methoxy-vitexilactone) | f | [32,52] | |
| 59 | 9-Hydroxy-13(14)-labden-15,16-olide | b | [53] | |
| 60 | Deacetylvitexilactone | b | [38] | |
| 61 | Viterotulin A | d | [45] | |
| 62 | (rel 3S,5S,8R,9R,10S)-3,9-Dihydroxy-13(14)-labden-16,15-olide | d | [45] | |
| 63 | Viterotulin B | d | [45] | |
| 64 | Vitexilactone B | a, b | [38,54] | |
| 65 | Viteoside A | d | [34] | |
| 66 | Viteagnuside A | f | [33] | |
| 67 | Vitexolide E | j | [55] | |
| 68 | Vitexolide D | j | [55] | |
| 69 | Vitexolide A | j | [55] | |
| 70 | 12-Epivitexolide A | j | [55] | |
| 71 | Vitexolide B | j | [55] | |
| 72 | Vitexolide C | j | [55] | |
| 73 | Vitexlactam A | d,f | [18,36] | |
| 74 | Vitexlactam B | f | [18,35] | |
| 75 | Vitexlactam C | f | [18,35] | |
| 76 | 12S,16S/R-Dihydroxy-ent-labda-7,13-dien-15,16-olide | k | [56] | |
| 77 | Vitextrifolin G | b | [38] | |
| 78 | Prerotundifuran | d | [42] | |
| 79 | Previtexilactone | b, d | [6,38,47,48,49] | |
| 80 | 6-Acetoxy-9,13,15,16-diepoxy-15-methoxylabdane | b | [6] | |
| 81 | Viteagnusin E | f | [57] | |
| 82 | (rel 5S,6R,8R,9R,10S,13S)-6-Acetoxy-9,13-epoxy-15-methoxylabdan-16,15-olide | d, f | [33,50] | |
| 83 | Viteagnusin I | f | [33] | |
| 84 | (rel 5S,6R,8R,9R,10S,13S,16S)-6-Acetoxy-9,13-epoxy-16-methoxy-labdan-15,16-olide | d, f | [33,50] | |
| 85 | Vitextrifolin F | b | [38] | |
| 86 | Nishindanol | a | [19] | |
| 87 | (rel 5S,6R,8R,9R,10S,13S,15S)-6-Acetoxy-9,13,15,16-diepoxy-15-methoxylabdane | d, f | [32,58] | |
| 88 | (rel 5S,6R,8R,9R,10S,13S,15R)-6-Acetoxy-9,13;15,16-diepoxy-15-methoxylabdane | d, f | [32,58] | |
| 89 | (rel 5S,6R,8R,9R,10S,13S,15S,16R)-6-Acetoxy-9,13,15,16-diepoxy-15,16-dimethoxylabdane | d | [58] | |
| 90 | (rel 5S,6R,8R,9R,10S,13S,15R,16S)-6-Acetoxy-9,13,15,16-diepoxy-15,16-dimethoxylabdane | d | [58] | |
| 91 | (rel 5S,6R,8R,9R,10S,13S,15R,16R)-6-Acetoxy-9,13,15,16-diepoxy-15,16-dimethoxylabdane | d | [58] | |
| 92 | (rel 5S,6R,8R,9R,10S,13S,15S,16S)-6-Acetoxy-9,13,15,16-diepoxy-15,16-dimethoxylabdane | d | [58] | |
| 93 | (rel 5S,8R,9R,10S,13S,15S,16R)-9,13;15,16-Diepoxy-15,16-dimethoxylabdane | d | [50] | |
| 94 | (rel 5S,8R,9R,10S,13S,15R,16S)-9,13;15,16-Diepoxy-15,16-dimethoxylabdane | d | [50] | |
| 95 | (rel 5S,8R,9R,10S,13S,15R,16R)-9,13;15,16-Diepoxy-15,16-dimethoxylabdane | d | [50] | |
| 96 | Negundol | a, b | [38,59] | |
| 97 | Negundoin D | a | [37] | |
| 98 | Negundoin E | a | [37] | |
| 99 | Vitextrifolin A | b | [38] | |
| 100 | Vitextrifolin B | b | [38] | |
| 101 | (rel 5S,6R,8R,9R,10S,13R)-6-Acetoxy-9,13-epoxy-15-methoxylabdan-16,15-olide | d,f | [33,50,57] | |
| 102 | (rel 5S,6R,8R,9R,10S,13R,16S)-6-Acetoxy-9,13-epoxy-16-methoxylabdan-15,16-olide | d,f | [33,50] | |
| 103 | Viteagnusin J | f | [33] | |
| 104 | (rel 5S,6R,8R,9R,10S,13R,15R)-6-Acetoxy-9,13,15,16-diepoxy-15-methoxylabdane | d, f | [32,58] | |
| 105 | (rel 5S,6R,8R,9R,10S,13R,15S)-6-Acetoxy-9,13,15,16-diepoxy-15-methoxylabdane | d, f | [32,58] | |
| 106 | Viteagnusin F | d, f | [32,45] | |
| 107 | Viteagnusin G | d, f | [32,45] | |
| 108 | Limonidilactone | g | [60] | |
| 109 | Acuminolide | j | [55] | |
| 110 | Vitexolin A | j | [55] | |
| 111 | Vitexolin B | j | [55] | |
| 112 | 6α,7α-Diacetoxy-13-hydroxy-8(9),14-labdadiene | b | [53] | |
| 113 | 6β,7β-Diacetoxy-13-hydroxylabda-8,14-diene | f | [40,41] | |
| 114 | Viteagnusin D | f | [57] | |
| 115 | Vitexifolin A | d | [61] | |
| 116 | Viteagnusin C | a, f | [33,54,57] | |
| 117 | 8,13-Dihydroxy-14-labdene | f | [41] | |
| 118 | 8-epi-Sclareol | a, f | [19,33,54,57] | |
| 119 | Vitrifolin B | d | [36] | |
| 120 | 8-Epimanoyl oxide | f | [18,35,51] | |
| Norlabdane | 121 | Vitrifolin A | l | [62] |
| 122 | Negundoal | a | [28] | |
| 123 | Negundoin A | a | [37] | |
| 124 | Negundoin B | a | [37] | |
| 125 | Negundoin C | a | [37] | |
| 126 | 9,13-Epoxy-16-norlabda-13E-en-15-al (norditerpene aldehyde 1) | b, d | [45,48] | |
| 127 | Norditerpene aldehyde 2 | b | [48] | |
| 128 | Vitexifolin D | d | [61] | |
| 129 | Trisnor-γ-lactone | d | [61] | |
| 130 | Isoambreinolide | b, d | [53,61] | |
| 131 | Vitedoin B | a, d | [37,45,63] | |
| 132 | Vitexifolin E | d | [61] | |
| Halimane | 133 | Vitetrifolin G | b | [64] |
| 134 | 13-Hydroxy-5(10),14-halimadien-6-one | b | [53] | |
| 135 | Viteagnusin A | f | [57] | |
| 136 | Viteagnusin B | f | [57] | |
| 137 | Vitetrifolin I | b | [6] | |
| 138 | Vitetrifolin D | a, b, d, f | [6,19,33,35,45,46,52,54,61,64] | |
| 139 | Vitetrifolin E | b, d | [6,45,46,64] | |
| 140 | Vitetrifolin F | b, d | [6,45,46,64] | |
| 141 | Vitetrifolin H | d | [45] | |
| Abietane | 142 | Ferruginol | d | [58] |
| 143 | Abietatrien-3β-ol | b, d | [39,58] | |
| 144 | 5β-Hydro-8,11,13-abietatrien-6α-ol | a | [65] | |
| 145 | 3β-Hydroxyabieta-8,11,13-trien-7-one | a | [37] | |
| 146 | Isolophanthin A | d | [45] | |
| 147 | Abieta-9(11),12-diene | d | [66] | |
| 148 | Vitetrifolin A | b | [39] | |
| 149 | Abietane 9(11):12(13)-di-α-epoxide | d | [66] | |
| 150 | Vitexifolin C | d | [61] | |
| 151 | Negundoin F | a | [37] | |
| Clerodane | 152 | Vitexifolin B | d | [61] |
| 153 | Cleroda-7,14-dien-13-ol | f | [41] | |
| 154 | Cleroda-1,3,14-trien-13-ol | f | [41] | |
| 155 | 13-epi-2-Oxokolavelool | d | [45] | |
| Isopimarane | 156 | Negundoin G | a | [37] |
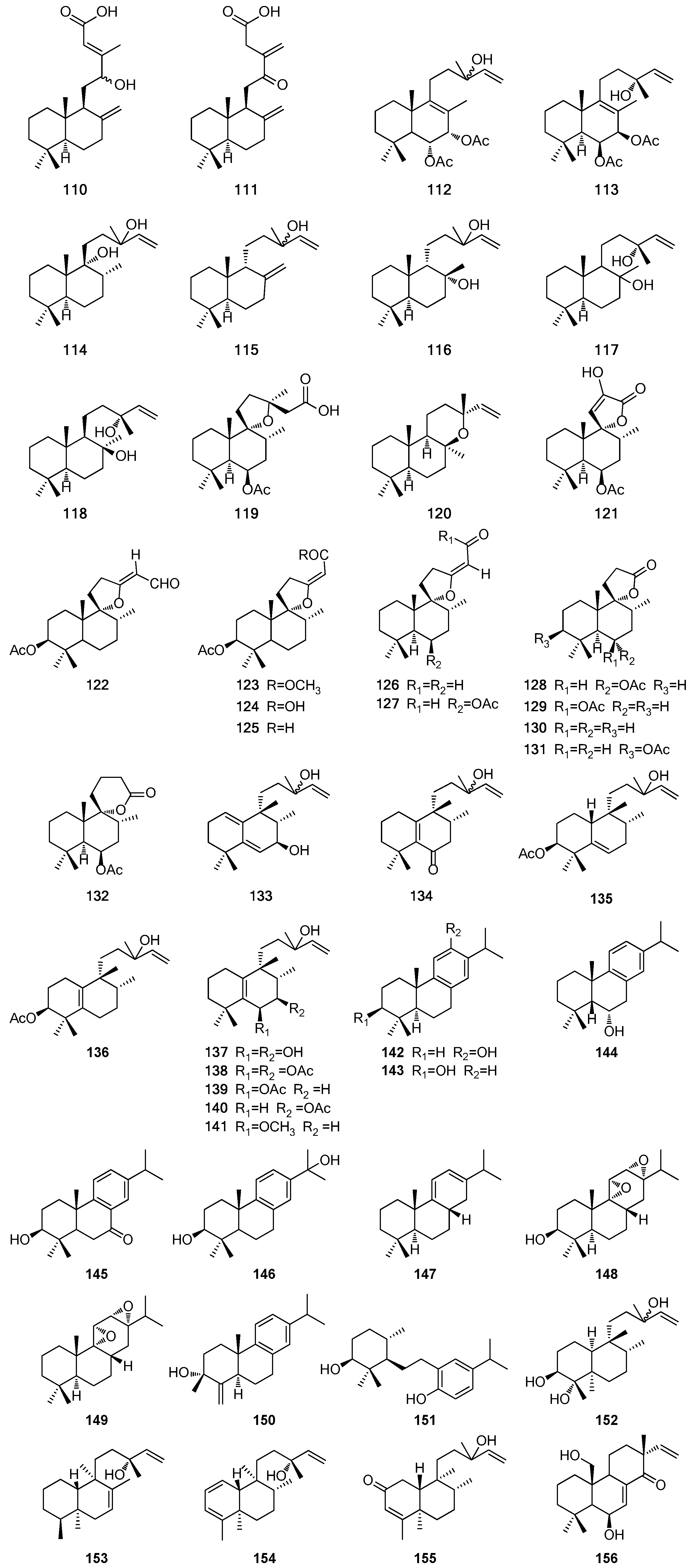
| Type | No. | Compound Name | Source | Reference |
|---|---|---|---|---|
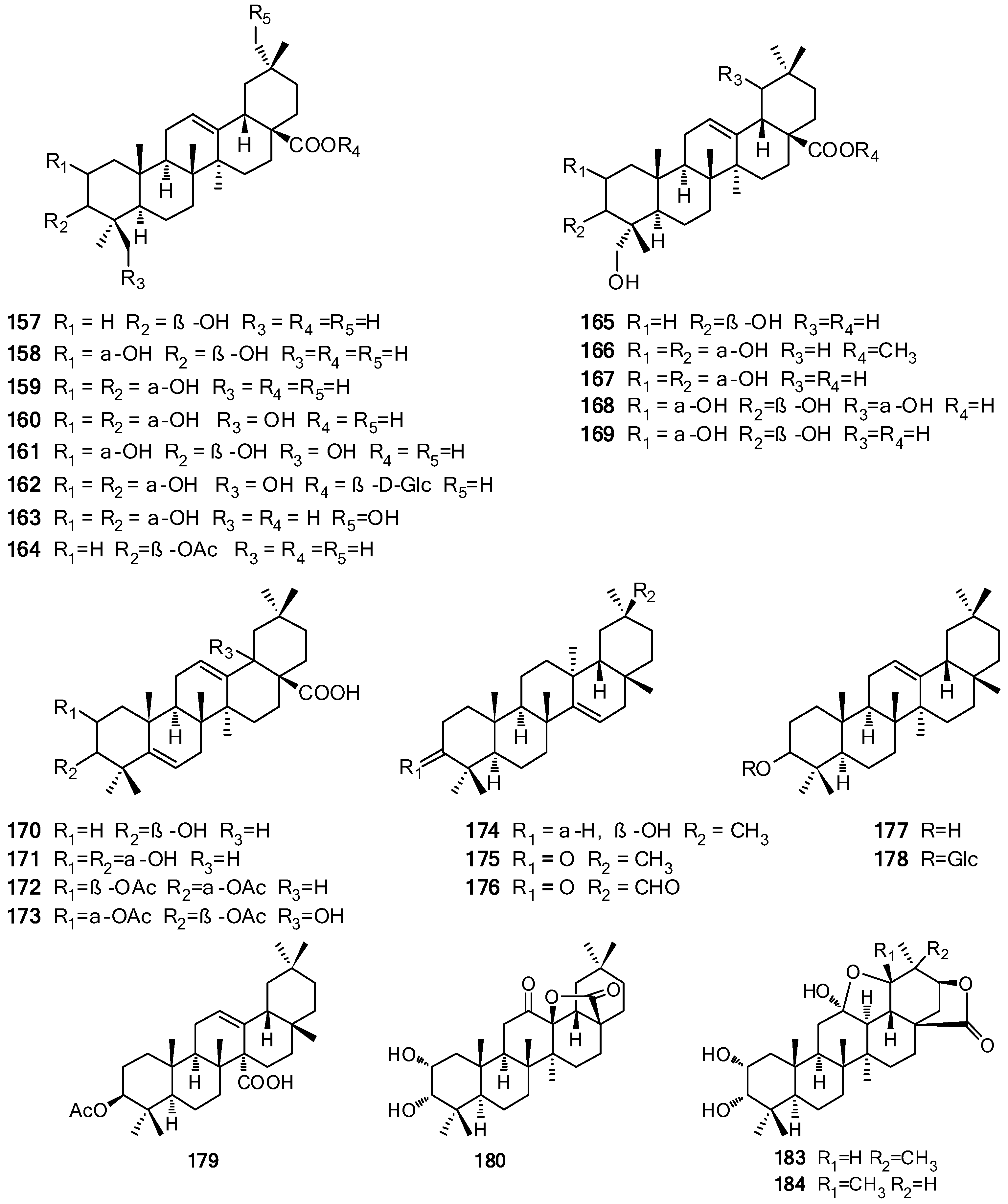
| Oleanane | 157 | Oleanolic acid | a, b | [54,68,70,71] |
| 158 | Maslinic acid | a, c, f | [33,54,72,73] | |
| 159 | 3-Epimaslinic acid | a, c, f, h | [33,51,69,71,73] | |
| 160 | 2α,3α,24-Trihydroxyolean-12-en-28-oic acid | a, b, h | [69,72,74] | |
| 161 | 2α,3β,24-Trihydroxyolean-12-en-28-oic acid | b | [74] | |
| 162 | Vulgarsaponin A | a | [67] | |
| 163 | Cannabifolin F | h | [69] | |
| 164 | Oleanolic acid acetate | a | [75] | |
| 165 | Hederagenin | b | [68] | |
| 166 | 2α,3α,23-Trihydroxyolean-12-en-28-oic acid methyl ester | a | [71] | |
| 167 | 2α,3α,23-Trihydroxyolean-12-en-28-oic acid | a | [71] | |
| 168 | 2α,3β,19α,23-Tetrahydroxyolean-12-en-28-oic acid | a | [72] | |
| 169 | 2α,3β,23-Trihydroxyolean-12-en-28-oic acid | a | [72] | |
| 170 | 3β-Hydroxyolean-5,12-dien-28-oic acid | a | [76] | |
| 171 | 2α,3α-Dihydroxyoleana-5,12-dien-28-oic acid | a | [77] | |
| 172 | 2β,3α-Diacetoxyoleana-5,12-dien-28-oic acid | a | [77] | |
| 173 | 2α,3β-Diacetoxy-18-hydroxyoleana-5,12-dien-28-oic acid | a | [77] | |
| 174 | Taraxerol | b | [78] | |
| 175 | Taraxerone | l | [79] | |
| 176 | 3-Oxotaraxer-14-en-30-al | l | [79] | |
| 177 | β-Amyrin | a, b | [68,71] | |
| 178 | β-Amyrin-3-O-β-d-glucopyranoside | b | [68] | |
| 179 | 3β-Acetoxyolean-12-en-27-oic acid | a | [76,77] | |
| 180 | Cannabifolin E | h | [69] | |
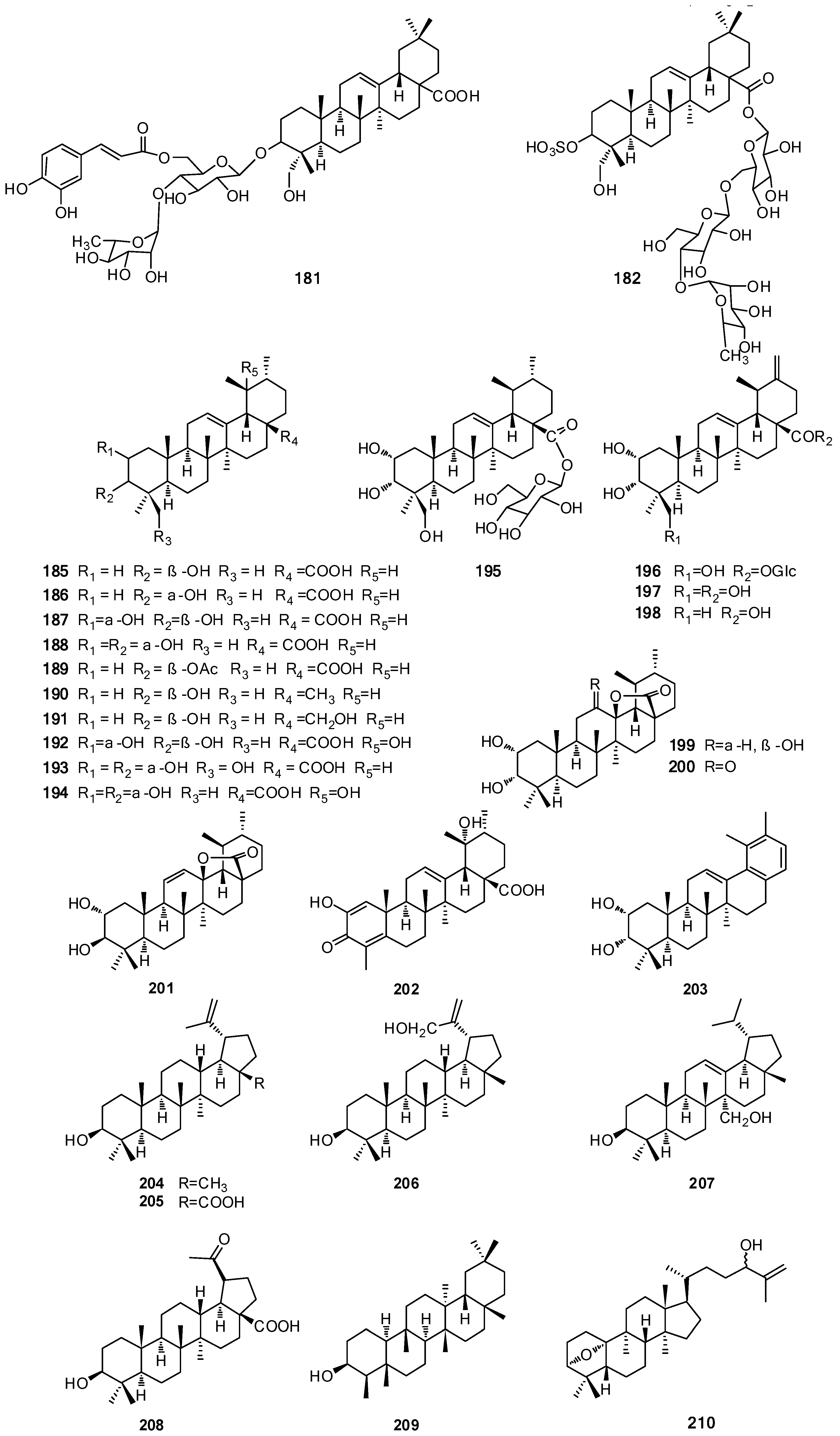
| 181 | 23-Hydroxy-3α-[O-α-l-rhamnopyranosyl-(1′′′→4″)-O-[β-d-(E-6″-O-caffeoyl)-glucopyranosyl]-oxy]-olean-12-en-28-oic acid | b | [68] | |
| 182 | 23-hydroxy-3α-(O-sulfonyloxy)-olean-12-en-28-oic acid-28-O-[α-l-rhamnopyranosyl-(1′′′→4″)-O-β-d-glucopyranosyl-(1″→6′)-O-β-d-glucopyranosyl] ester | b | [68] | |
| 183 | Cannabifolin B | h | [69] | |
| Ursane | 184 | Cannabifolin A | h | [69] |
| 185 | Ursolic acid | a–c, e, h | [49,53,54,69,72,73,78,80,81] | |
| 186 | 3-Epiursolic acid | b, l | [74,79] | |
| 187 | Corosolic acid | a–c, e, f, h, l | [33,53,67,69,73,79,80] | |
| 188 | 3-Epicorosolic acid | a–c, f, h | [28,33,51,69,73,78] | |
| 189 | 3β-Acetoxyurs-12-en-28-oic acid | b | [49,82] | |
| 190 | α-Amyrin | b | [53] | |
| 191 | Uvaol | b | [74] | |
| 192 | Tormentic acid | a, b, e, h | [69,72,78,83] | |
| 193 | 2α,3α,24-Trihydroxyurs-12-en-28-oic acid | b, c | [73,74] | |
| 194 | Euscaphic acid | c, h | [69,73] | |
| 195 | 2α,3α,24-Trihydroxyurs-12-en-28-oic acid-28-O-β-d-glucopyranosyl ester | a | [67] | |
| 196 | 2α,3α,24-Trihydroxyurs-12,20(30)-dien-28-oic acid-28-O-β-d-glucopyranosyl ester | a | [67] | |
| 197 | 2α,3α,24-Trihydroxyurs-12,20(30)-dien-28-oic acid | c | [73] | |
| 198 | 2α,3α-Dihydroxyurs-12,20(30)-dien-28-oic acid | h | [69] | |
| 199 | Cannabifolin C | h | [69] | |
| 200 | Cannabifolin D | h | [69] | |
| 201 | Ilelatifol D | f | [51] | |
| Norursane | 202 | Negundonorin A | a | [28] |
| 203 | Negundonorin B | a | [28] | |
| Lupane | 204 | Lupeol | l | [79] |
| 205 | Betulinic acid | a, b, l | [49,54,78,79,81] | |
| 206 | Lup-20(29)-en-3β,30-diol | a | [54] | |
| 207 | Obtusalin | a | [54] | |
| 208 | Platanic acid | b | [82] | |
| Friedelane | 209 | Epifriedelinol | e | [84] |
| 9-epi-Cucurbitane | 210 | (24R/S)-24-Hydroxy-3α,10α-epoxy-9-epi-cucurbita-25-ene | a | [43] |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, J.-L.; Fang, S.-M.; Liu, R.; Oppong, M.B.; Liu, E.-W.; Fan, G.-W.; Zhang, H. A Review on the Terpenes from Genus Vitex. Molecules 2016, 21, 1179. https://doi.org/10.3390/molecules21091179
Yao J-L, Fang S-M, Liu R, Oppong MB, Liu E-W, Fan G-W, Zhang H. A Review on the Terpenes from Genus Vitex. Molecules. 2016; 21(9):1179. https://doi.org/10.3390/molecules21091179
Chicago/Turabian StyleYao, Jin-Long, Shi-Ming Fang, Rui Liu, Mahmood Brobbey Oppong, Er-Wei Liu, Guan-Wei Fan, and Han Zhang. 2016. "A Review on the Terpenes from Genus Vitex" Molecules 21, no. 9: 1179. https://doi.org/10.3390/molecules21091179
APA StyleYao, J.-L., Fang, S.-M., Liu, R., Oppong, M. B., Liu, E.-W., Fan, G.-W., & Zhang, H. (2016). A Review on the Terpenes from Genus Vitex. Molecules, 21(9), 1179. https://doi.org/10.3390/molecules21091179





