Effects of Substitution on Solid-State Fluorescence in 9-Aryl-9-methyl-9H-9-silafluorenes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization
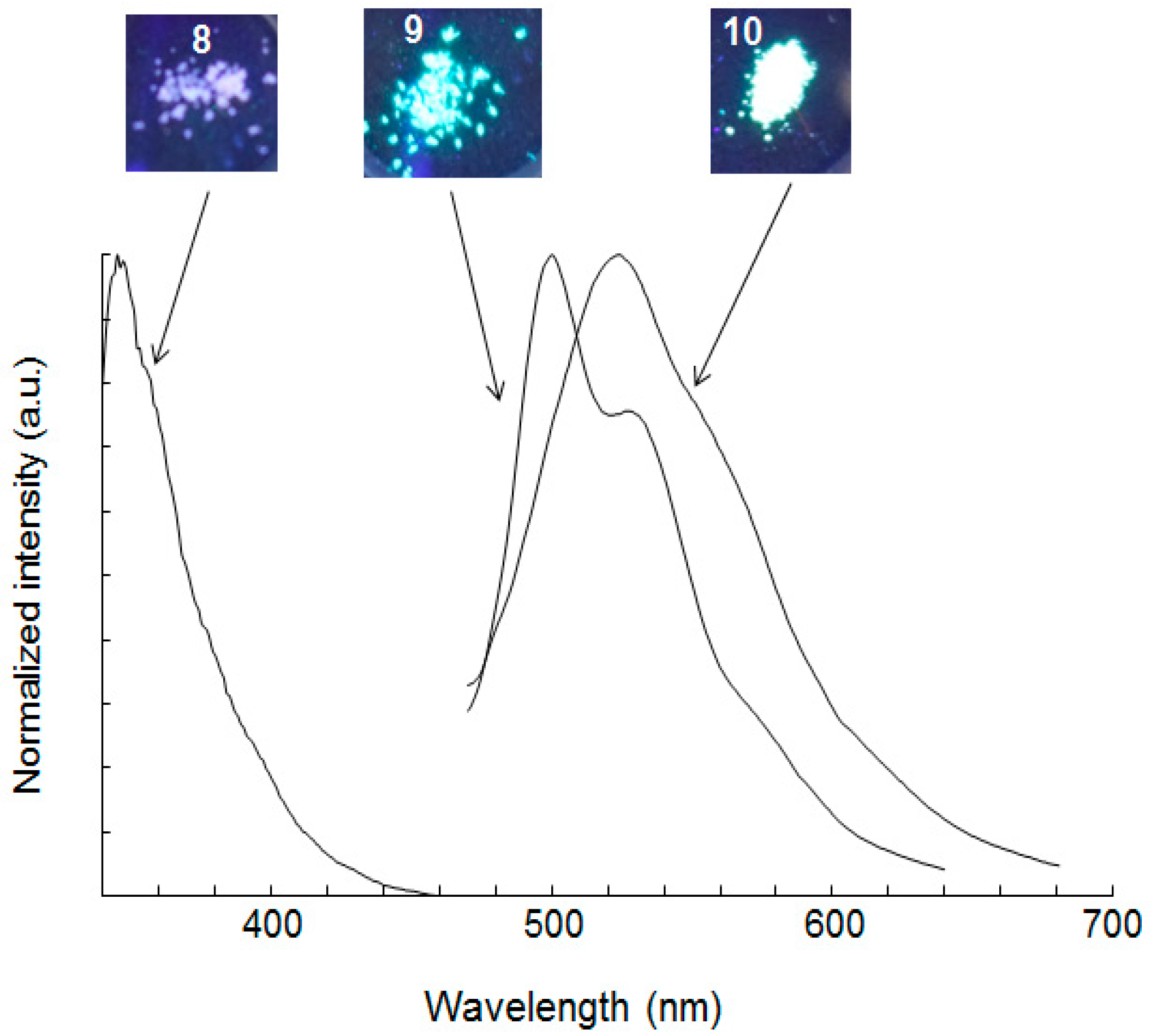
2.2. Absorption and Emission Spectra
2.3. Thermal Stability
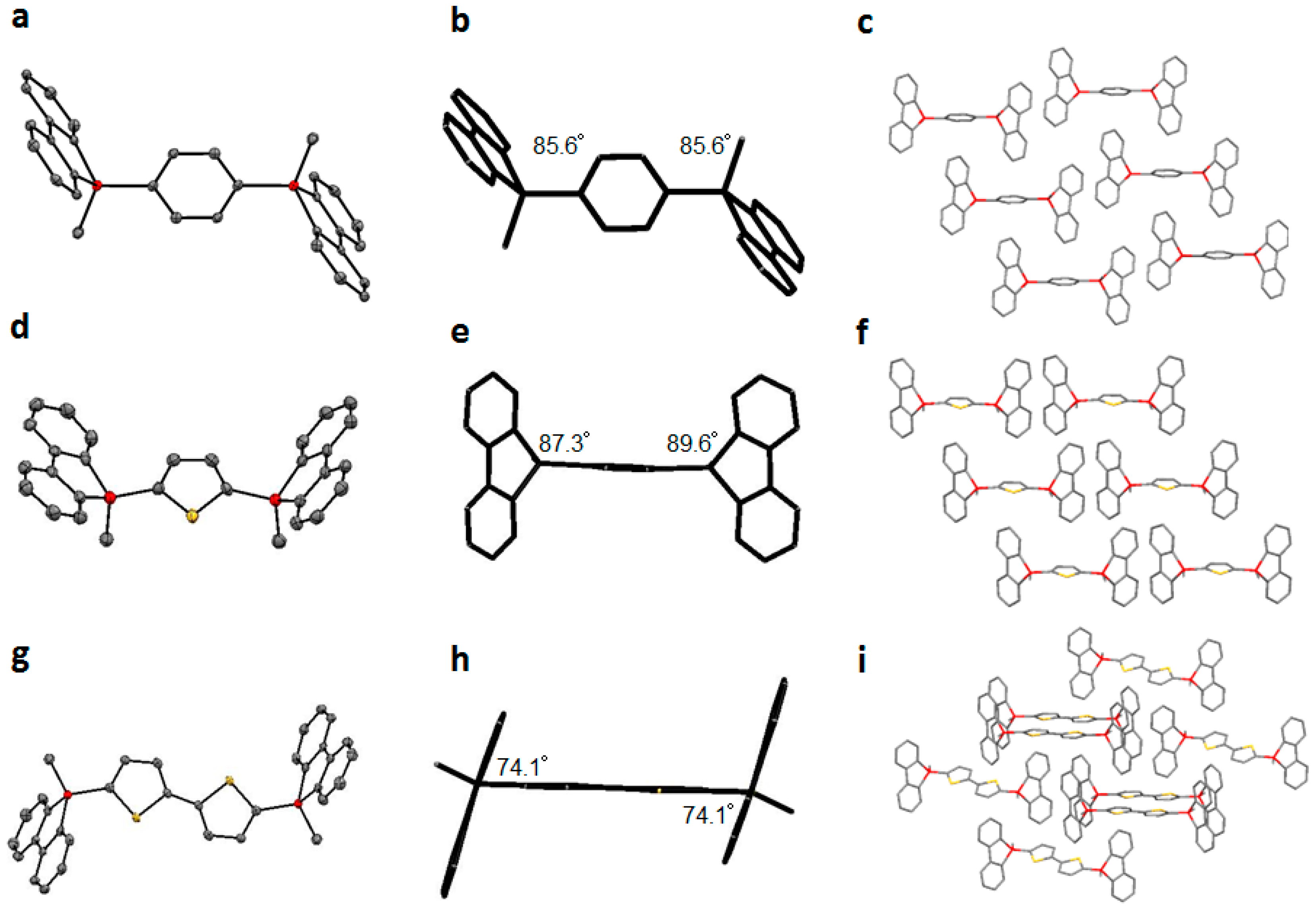
2.4. X-ray Analysis
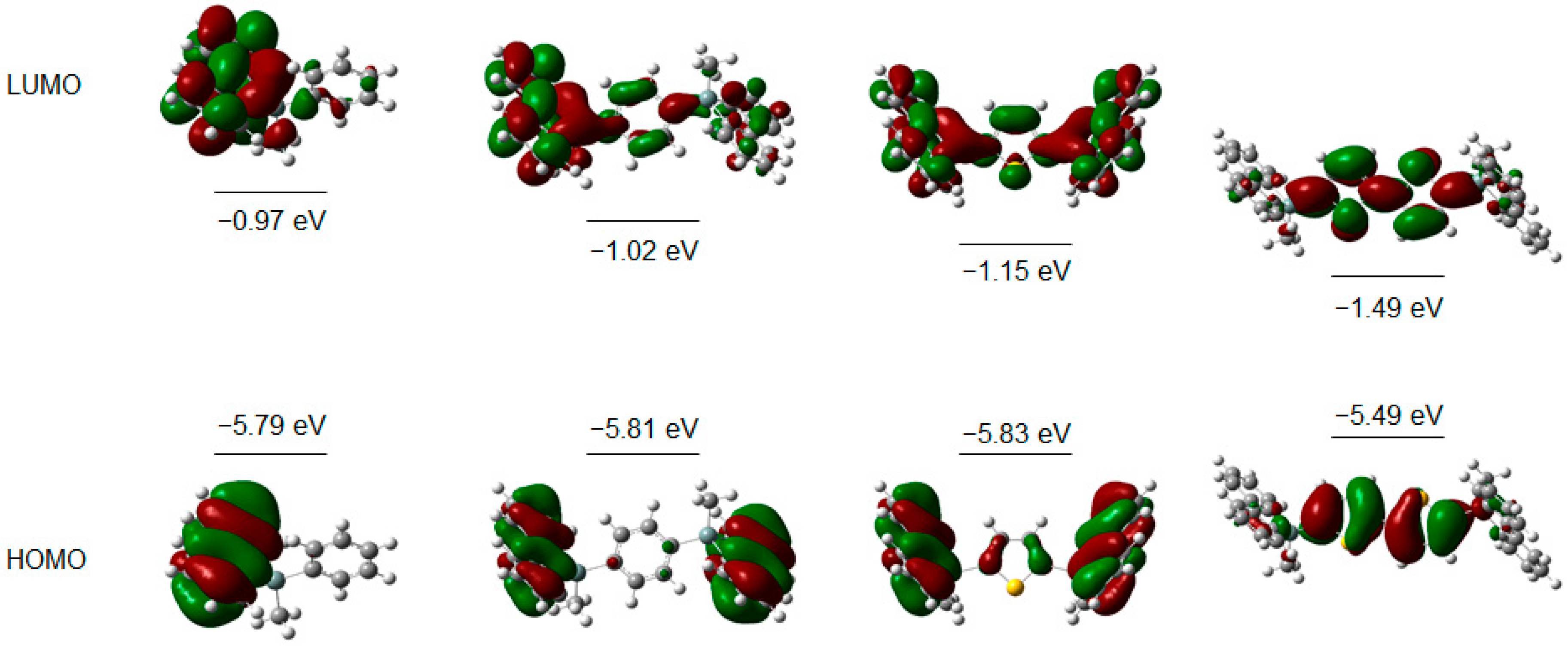
2.5. Theoretical Modeling
3. Experimental Section
3.1. General Information
3.2. Typical Experimental Procedure for the Preparation of 1–7
3.3. Typical Experimental Procedure for the Preparation of 8–10
3.4. X-ray Crystallography
3.5. Physical Data of 1–10
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Corey, J.Y. Siloles: Part 2: Silaindenes (Benzosiloles) and Silafluorenes (Dibenzosiloles): Synthesis, Characterization, and Applications. Adv. Organomet. Chem. 2011, 59, 181–328. [Google Scholar] [CrossRef]
- Shimizu, M.; Hiyama, T. Silicon-bridged biaryls: Molecular design, new synthesis, and luminescence control. Synlett 2012, 23, 973–989. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; An, K.; He, W. Catalytic Synthesis of π-Conjugated Silole through Si–C (sp3) Bond Activation. Synlett 2015, 26, 1145–1152. [Google Scholar]
- Shimizu, M. Novel synthesis and luminescent properties of silicon-bridged biaryls. J. Synth. Org. Chem. Jpn. 2013, 71, 307–318. [Google Scholar] [CrossRef]
- Fukazawa, A.; Yamaguchi, S. Ladder π-Conjugated Materials Containing Main-Group Elements. Chem. Asian J. 2009, 4, 1386–1400. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, S.; Xu, C.; Okamoto, T. Ladder π-Conjugated Materials with Main Group Elements. Pure Appl. Chem. 2006, 78, 721–730. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Xu, C. The Chemistry of Silicon-Containing Ladder π-Conjugated Systems. J. Synth. Org. Chem. Jpn. 2005, 63, 1115–1123. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Tamao, K. A Key Role of Orbital Interaction in the Main Group Element-Containing π-Electron Systems. Chem. Lett. 2005, 34, 2–7. [Google Scholar] [CrossRef]
- Zhan, X.; Barlow, S.; Marder, S.R. Substituent effects on the electronic structure of siloles. Chem. Commun. 2009, 1948–1955. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, Q.; Yu, Y.; Wu, Y.; Zhang, X.; Dong, H.; Ma, L.; Zhou, G.; Jiao, B.; Wu, Z.; et al. Silafluorene moieties as promising building blocks for constructing wide-energy-gap host materials of blue phosphorescent organic light-emitting devices. Sci. Chin. Chem. 2015, 58, 993–998. [Google Scholar] [CrossRef]
- Yamanoi, Y. Palladium-Catalyzed Silylations of Hydrosilanes with Aryl Halides Using Bulky Alkyl Phosphine. J. Org. Chem. 2005, 70, 9607–9609. [Google Scholar] [CrossRef] [PubMed]
- Yamanoi, Y.; Taira, T.; Sato, J.-I.; Nakamula, I.; Nishihara, H. Efficient Preparation of Monohydrosilanes Using Palladium-Catalyzed Si–C Bond Formation. Org. Lett. 2007, 9, 4543–4546. [Google Scholar] [CrossRef] [PubMed]
- Yamanoi, Y.; Nishihara, H. Efficient Synthesis of Arylsilanes by Cross-coupling of Aromatic Compounds with Hydrosilanes as Silicon Sources. J. Synth. Org. Chem. Jpn. 2009, 67, 778–786. [Google Scholar] [CrossRef]
- Lesbani, A.; Kondo, H.; Yabusaki, Y.; Nakai, M.; Yamanoi, Y.; Nishihara, H. Integrated Palladium-Catalyzed Arylation of Heavier Group 14 Hydrides. Chem. Eur. J. 2010, 16, 13519–13527. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Yamanoi, Y.; Nishihara, H. Unusual Reactivity of Group 14 Hydrides toward Organic Halides: Synthetic Studies and Application to Functional Materials. J. Synth. Org. Chem. Jpn. 2016, 74. in press. [Google Scholar]
- Yabusaki, Y.; Ohshima, N.; Kondo, H.; Kusamoto, T.; Yamanoi, Y.; Nishihara, H. Versatile Synthesis of Blue Luminescent Siloles and Germoles and Hydrogen-Bond-Assisted Color Alteration. Chem. Eur. J. 2010, 16, 5581–5585. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T.; Shimada, M.; Kurihara, Y.; Tsuchiya, M.; Yamanoi, Y.; Nishibori, E.; Sugimoto, K.; Nishihara, H. Fluorescence and phosphorescence of a series of silicon-containing six-membered-ring molecules. J. Organomet. Chem. 2016, 805, 27–33. [Google Scholar] [CrossRef]
- Chang, L.S.; Corey, J.Y. Dehydrogenative Coupling of Diarylsilanes. Organometallics 1989, 8, 1885–1893. [Google Scholar] [CrossRef]
- Becker, B.; Corriu, R.J.P.; Henner, B.J.L.; Wojnowski, W.; Peters, K.; von Schnering, H.G. Contributions to the chemistry of silicon-sulphur compounds: XLII. Bis(1-methyldibenzosilole)sulphide: Its synthesis, crystal structure, and reaction with chromium hexacarbonyl. J. Organomet. Chem. 1986, 312, 305–311. [Google Scholar] [CrossRef]
- Cai, Y.; Samedov, K.; Dolinar, B.S.; Albright, H.; Guzei, I.A.; Hu, R.; Zhang, C.; West, R. Ring-Shaped Silafluorene Derivatives as Efficient Solid-State UV-Fluorophores: Synthesis, Characterization, and Photoluminescent Properties. Chem. Eur. J. 2014, 20, 14040–14050. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, J.C.; Trogler, W.C. Efficient blue-emitting silafluorene–fluorene-conjugated copolymers: Selective turn-off/turn-on detection of explosives. J. Mater. Chem. 2008, 18, 3143–3156. [Google Scholar] [CrossRef]
- Pusztai, E.; Toulokhonova, I.S.; Temple, N.; Albright, H.; Zakai, U.I.; Guo, S.; Guzei, I.A.; Hu, R.R.; West, R. Synthesis and Photophysical Properties of Asymmetric Substituted Silafluorenes. Organometallics 2013, 32, 2529–2535. [Google Scholar] [CrossRef]
- Maiti, N.C.; Mazumdar, S.; Periasamy, N. J- and H-Aggregates of Porphyrin-Surfactant Complexes: Time-Resolved Fluorescence and Other Spectroscopic Studies. J. Phys. Chem. B 1998, 102, 1528–1538. [Google Scholar] [CrossRef]
- Venkatramaiah, N.; Ramakrishna, B.; Venkatesan, R.; Pazb, F.A. A.; Tomé, J.P.C. Facile synthesis of highly stable BF3-induced meso-tetrakis (4-sulfonato phenyl) porphyrin (TPPS4)-J-aggregates: Structure, photophysical and electrochemical properties. New J. Chem. 2013, 37, 3745–3754. [Google Scholar] [CrossRef]
- CCDC 1458152 (8), 1447977 (9) and 1447978 (10) contain the supplementary crystallographic data for this paper. These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44-1223-336033; E-mail: deposit@ccdc.cam.ac.uk).
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09W; Revision A.02 and Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Kasha, M.; Rawls, H.R.; Ashraf El-Bayoumi, M. The exciton model in molecular spectroscopy. Pure Appl. Chem. 1965, 11, 371–392. [Google Scholar] [CrossRef]
- Altomare, A.; Cascarano, G.; Giacovazzo, C.; Guagliardi, A.; Burla, M.C.; Polidori, G.; Camalli, M. SIR92—A program for automatic solution of crystal structures by direct methods. J. Appl. Cryst. 1994, 27, 435. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M.; Schneider, T.R. [16] SHELXL: High-Resolution Refinement. Methods Enzymol. 1997, 277, 319–343. [Google Scholar] [PubMed]
- Sample Availability: Samples of the compounds 1–10 are available from the authors.

| Compound | In n-Hexane | In the Solid State | |||||||
|---|---|---|---|---|---|---|---|---|---|
| λabs (nm) b | ε (104 M−1·cm−1) | λem (nm) c | Φ d | τ (ns) e | λex (nm) | λem (nm) c | Φ d | τ (ns) | |
| 1 | 277, 287 | 1.10 | 342 | 0.08 | 4.0 | 320 | 346 | 0.05 | 7.2 e |
| 2 | 277, 288 | 1.10 | 340 | 0.10 | 3.9 | - f | - f | - f | - f |
| 3 | 278, 289 | 1.73 | 341 | 0.07 | 3.7 | 324 | 345 | 0.18 | 7.2e |
| 4 | 278, 289 | 1.39 | 341 | 0.14 | 4.0 | - f | - f | - f | - f |
| 5 | 280, 288 | 1.66 | 340 | 0.04 | 3.8 | 321 | 344 | 0.18 | 7.8 e |
| 6 | 277, 288 | 1.24 | 343 | 0.09 | 3.9 | 326 | 348 | 0.18 | 7.7 e |
| 7 | 277, 288 | 1.16 | 344 | 0.07 | 3.6 | 323 | 347, 385 | 0.11 | 3.3, 0.9 e |
| 8 | 277, 289 | 2.25 | 342 | 0.12 | 3.8 | 321 | 345 | 0.11 | 4.4 e |
| 9 | 264, 277, 289 | 3.31 | 348, 490 g | 0.07 | 2.3 | 444 | 500 | 0.20 | 0.5 h |
| 10 | 279, 323 | 2.54 | 390, 505 i | 0.04 | 0.6 | 446 | 524 | 0.06 | 0.6 h |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamanoi, Y.; Nakashima, T.; Shimada, M.; Maeda, H.; Nishihara, H. Effects of Substitution on Solid-State Fluorescence in 9-Aryl-9-methyl-9H-9-silafluorenes. Molecules 2016, 21, 1173. https://doi.org/10.3390/molecules21091173
Yamanoi Y, Nakashima T, Shimada M, Maeda H, Nishihara H. Effects of Substitution on Solid-State Fluorescence in 9-Aryl-9-methyl-9H-9-silafluorenes. Molecules. 2016; 21(9):1173. https://doi.org/10.3390/molecules21091173
Chicago/Turabian StyleYamanoi, Yoshinori, Takayuki Nakashima, Masaki Shimada, Hiroaki Maeda, and Hiroshi Nishihara. 2016. "Effects of Substitution on Solid-State Fluorescence in 9-Aryl-9-methyl-9H-9-silafluorenes" Molecules 21, no. 9: 1173. https://doi.org/10.3390/molecules21091173
APA StyleYamanoi, Y., Nakashima, T., Shimada, M., Maeda, H., & Nishihara, H. (2016). Effects of Substitution on Solid-State Fluorescence in 9-Aryl-9-methyl-9H-9-silafluorenes. Molecules, 21(9), 1173. https://doi.org/10.3390/molecules21091173






