Evaluation of the Effect of Two Volatile Organic Compounds on Barley Pathogens
Abstract
:1. Introduction
2. Results
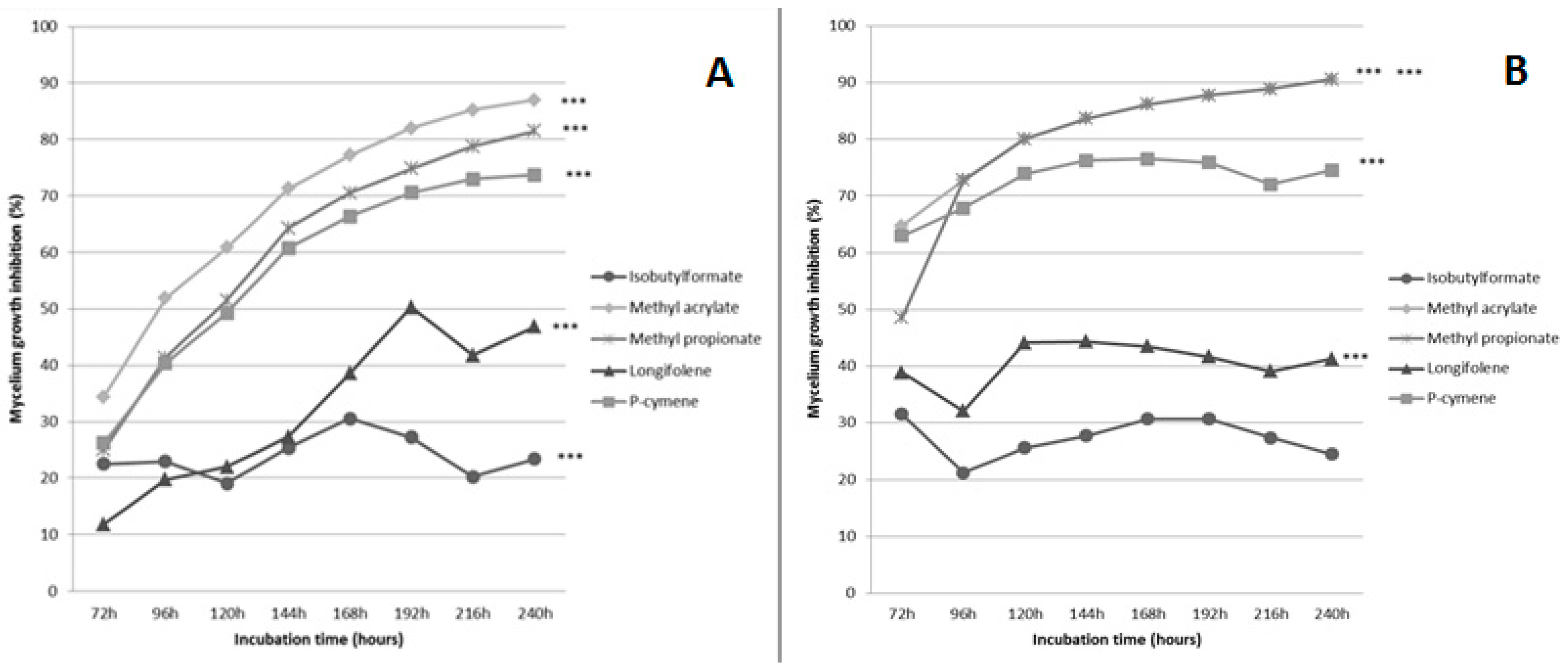
2.1. Evaluation of the Effect of Five Volatiles Organic Compounds on the Growth of Fusarium culmorum and Cochliobolus sativus
2.2. Effect of Methyl Prop-2-Enoate and Methyl Propanoate on Spore Germination
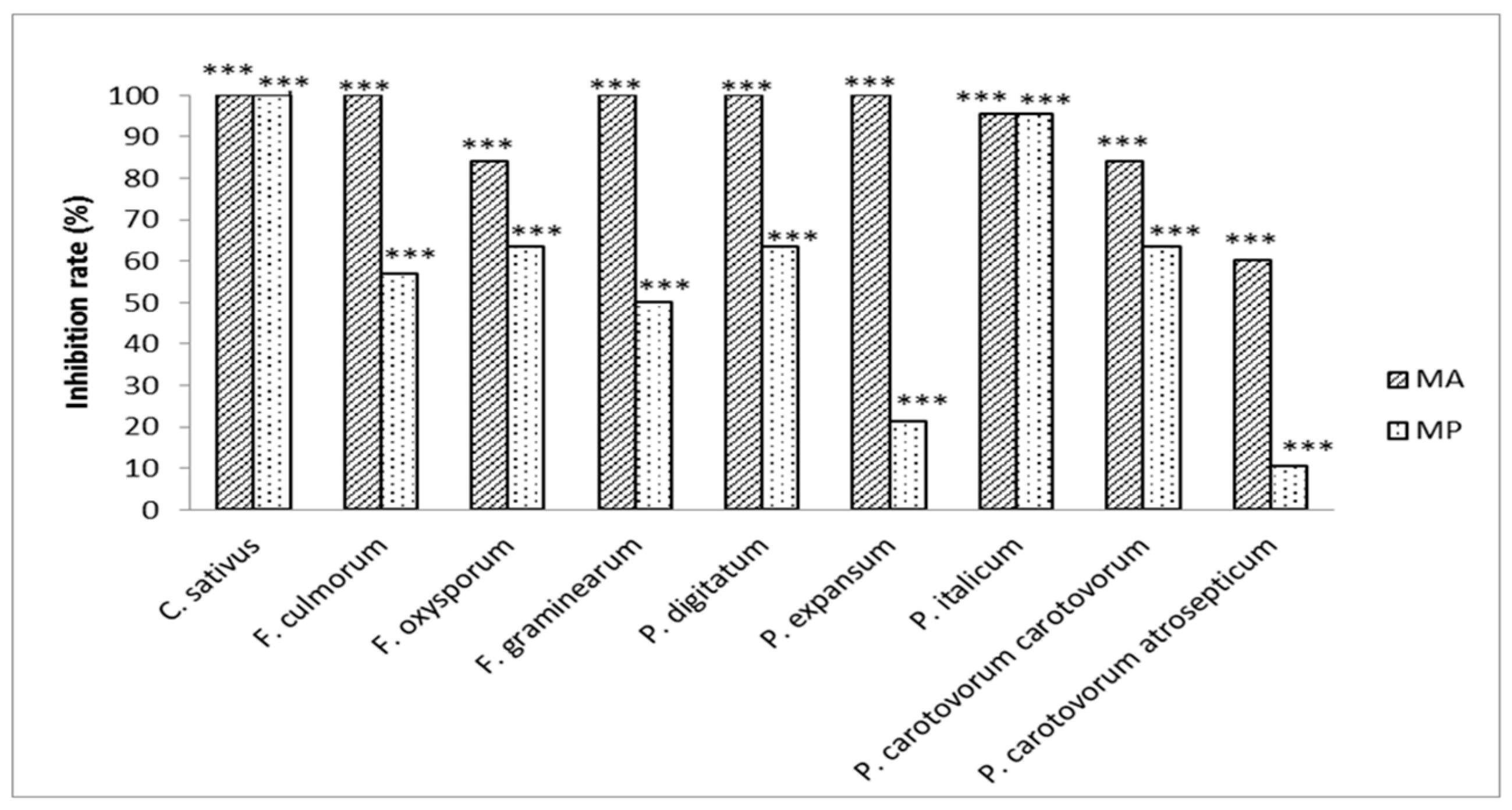
2.3. Evaluation of the Antifungal and/or Antibacterial Activity of Methyl Propanoate and Methyl Prop-2-Enoate on Several Pathogens
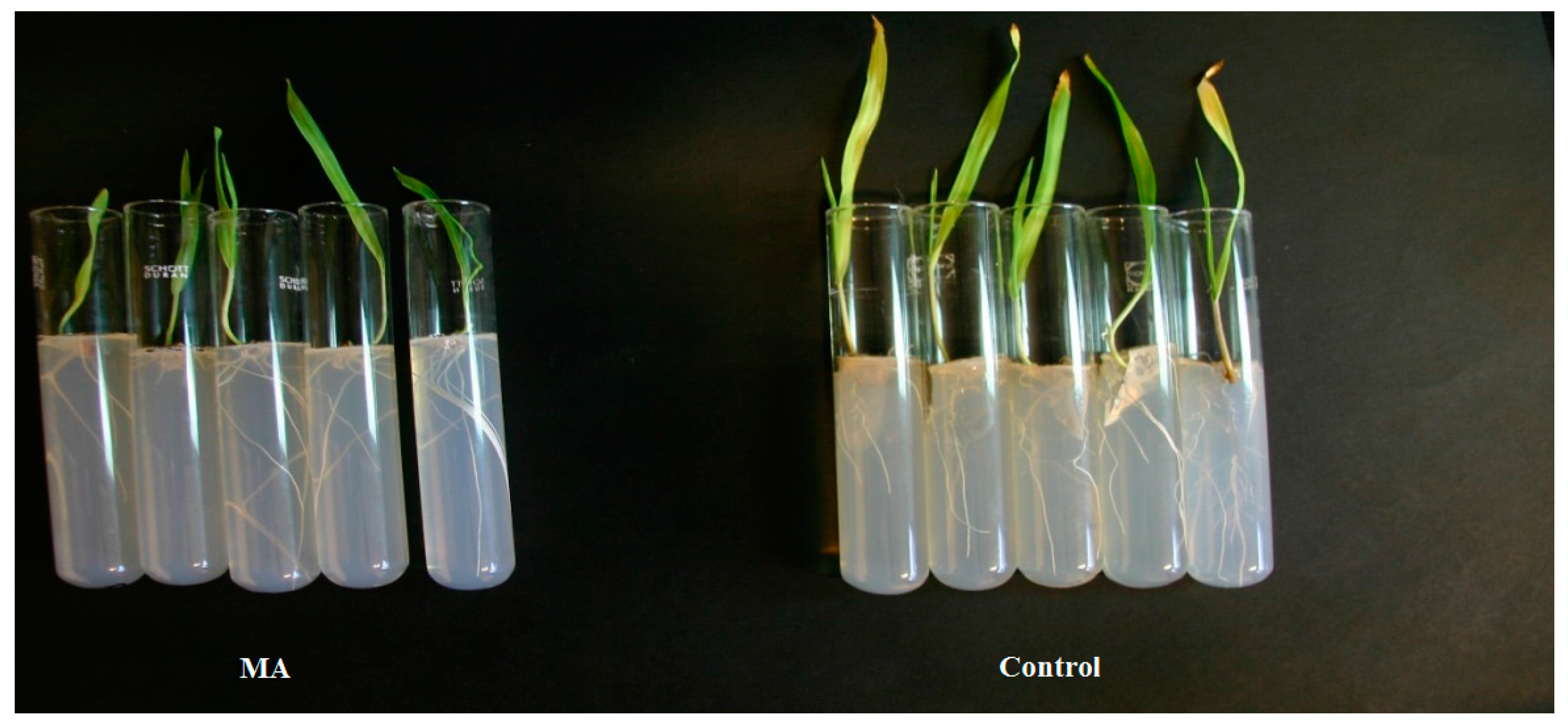
2.4. Evaluation of the Protective Activity of Methyl Propanoate and Methyl Prop-2-Enoate on Infected Barley Seeds
3. Discussion
4. Materials and Methods
4.1. Biological Material
4.2. Evaluation of the Effect of Five Volatiles Organic Compounds on the Growth of Fusarium culmorum and Cochliobolus sativus
4.3. Effect of Methyl Prop-2-Enoate and Methyl Propanoate on Spore Germination
4.4. Evaluation of the Antifungal and/or Antibacterial Activity of Methyl Propanoate and Methyl Prop-2-Enoate on Other Pathogens
4.5. Evaluation of the Protective Activity of Methyl Propanoate and Methyl Prop-2-Enoate on Infected Barley Seeds
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; von Dahl, C.C.; Preston, C.A. Volatile signaling in plant-plant interactions: “Talking trees” in the genomics era. Science 2006, 311, 812–815. [Google Scholar] [CrossRef] [PubMed]
- Kessler, A.; Baldwin, I.T. Defensive function of herbivore-induced plant volatile emissions in nature. Science 2001, 291, 2141–2144. [Google Scholar] [CrossRef] [PubMed]
- Fiers, M.; Lognay, G.; Fauconnier, M.L.; Jijakli, M.H. Volatile compound-mediated interactions between barley and pathogenic fungi in the soil. PLoS ONE 2013, 8, e66805. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Fu, X.; Watanabe, N.; Su, X.; Yang, Z. Recent advances in the emission and functions of plant vegetative volatiles. Molecules 2016, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, I.T. Plant volatiles. Curr. Biol. 2010, 20, R392–R397. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Wright, G.A.; Lutmerding, A.; Dudareva, N.; Smith, B.H. Intensity and the ratios of compounds in the scent of snapdragon flowers affect scent discrimination by honeybees (Apis mellifera). J. Comp. Physiol. A 2005, 191, 105–114. [Google Scholar] [CrossRef]
- Delory, B.M.; Delaplace, P.; Fauconnier, M.L.; Jardin, P. Root-emitted volatile organic compounds: Can they mediate belowground plant-plant interactions? Plant Soil 2016, 402, 1–26. [Google Scholar] [CrossRef]
- Arimura, G.I.; Muroi, A.; Nishihara, M. Plant-plant-plant communications, mediated by (E)-β-ocimene emitted from transgenic tobacco plants, prime indirect defense responses of lima beans. J. Plant Interact. 2012, 7, 193–196. [Google Scholar] [CrossRef]
- Chaves-Lopez, C.; Serio, A.; Gianotti, A.; Sacchetti, G.; Ndagijimana, M.; Ciccarone, C.; Stellarini, A.; Corsetti, A.; Paparella, A. Diversity of food-borne bacillus volatile compounds and influence on fungal growth. J. Appl. Microbiol. 2015, 119, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, R.; Ryu, C.M. Are bacterial volatile compounds poisonous odors to a fungal pathogen Botrytis cinerea, alarm signals to Arabidopsis seedlings for eliciting induced resistance, or both? Front. Microbiol. 2016, 7, 196. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wu, L.; Hao, J.; Luo, L.; Cao, Y.; Li, J. Biofumigation on post-harvest diseases of fruits using a new volatile-producing fungus of Ceratocystis fimbriata. PLoS ONE 2015, 10, e0132009. [Google Scholar]
- Zhang, Q.; Yang, L.; Zhang, J.; Wu, M.; Chen, W.; Jiang, D.; Li, G. Production of anti-fungal volatiles by non-pathogenic Fusarium oxysporum and its efficacy in suppression of verticillium wilt of cotton. Plant Soil 2015, 392, 101–114. [Google Scholar] [CrossRef]
- Schalchli, H.; Tortella, G.R.; Rubilar, O.; Parra, L.; Hormazabal, E.; Quiroz, A. Fungal volatiles: An environmentally friendly tool to control pathogenic microorganisms in plants. Crit. Rev. Biotechnol. 2016, 36, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Schulz-Bohm, K.; Zweers, H.; de Boer, W.; Garbeva, P. A fragrant neighborhood: Volatile mediated bacterial interactions in soil. Front. Microbiol. 2015, 6, 1212. [Google Scholar] [CrossRef] [PubMed]
- Schenkel, D.; Lemfack, M.C.; Piechulla, B.; Splivallo, R. A meta-analysis approach for assessing the diversity and specificity of belowground root and microbial volatiles. Front. Plant Sci. 2015, 6, 707. [Google Scholar] [CrossRef] [PubMed]
- Hiltpold, I.; Erb, M.; Robert, C.A.; Turlings, T.C. Systemic root signalling in a belowground, volatile-mediated tritrophic interaction. Plant Cell Environ. 2011, 34, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.O.; Kim, H.Y.; Choi, G.J.; Lee, H.B.; Jang, K.S.; Choi, Y.H.; Kim, J.C. Mycofumigation with Oxyporus latemarginatus EF069 for control of postharvest apple decay and rhizoctonia root rot on moth orchid. J. Appl. Microbiol. 2009, 106, 1213–1219. [Google Scholar] [CrossRef] [PubMed]
- Delory, B.M.; Delaplace, P.; du Jardin, P.; Fauconnier, M.L. Barley (Hordeum distichon L.) roots synthesise volatile aldehydes with a strong age-dependent pattern and release (E)-non-2-enal and (E,Z)-nona-2,6-dienal after mechanical injury. Plant Physiol. Bioch. 2016, 104, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Kunova, A.; Pizzatti, C.; Cortesi, P. Impact of tricyclazole and azoxystrobin on growth, sporulation and secondary infection of the rice blast fungus, magnaporthe oryzae. Pest Manag. Sci. 2013, 69, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.W.; Clough, J.M.; Godwin, J.R.; Hall, A.A.; Hamer, M.; Parr-Dobrzanski, B. Review: The strobilurin fungicides. Pest Manag. Sci. 2004, 60, 309. [Google Scholar] [CrossRef]
- Edwards, S.G. Influence of agricultural practices on fusarium infection of cereals and subsequent contamination of grain by trichothecene mycotoxins. Toxicol. Lett. 2004, 153, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Haque, M.; Chowdhury, R.; Islam, K.; Akbar, M. Propionic acid is an alternative to antibiotics in poultry diet. Bangl. J. Anim. Sci. 2009, 38, 115–122. [Google Scholar] [CrossRef]
- Conkova, E.; Para, L.; Kocisova, A. Inhibition of growth of microscopic fungi with organic acids. Vet. Med. 1992, 38, 723–727. [Google Scholar]
- Hassan, R.; El-Kadi, S.; Sand, M. Effect of some organic acids on some fungal growth and their toxins production. Int. J. Adv. Biol. 2015, 2. [Google Scholar] [CrossRef]
- Molina, M.; Giannuzzi, L. Modelling of aflatoxin production by Aspergillus parasiticus in a solid medium at different temperatures, pH and propionic acid concentrations. Food Res. Int. 2002, 35, 585–594. [Google Scholar] [CrossRef]
- Strobel, G.A.; Spang, S.; Kluck, K.; Hess, W.M.; Sears, J.; Livinghouse, T. Synergism among volatile organic compounds resulting in increased antibiosis in Oidium sp. FEMS Microbiol. Lett. 2008, 283, 140–145. [Google Scholar] [CrossRef]
- Chitarra, G.S.; Abee, T.; Rombouts, F.M.; Dijksterhuis, J. 1-Octen-3-ol inhibits conidia germination of Penicillium paneum despite of mild effects on membrane permeability, respiration, intracellular pH, and changes the protein composition. FEMS Microbiol. Ecol. 2005, 54, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.; Kalkhove, S.; Lugones, L.; Baars, J.; Wösten, H.; Bakker, P. Effects of the mushroom-volatile 1-octen-3-ol on dry bubble disease. Appl. Microbiol. Biotechnol. 2013, 97, 5535–5543. [Google Scholar] [CrossRef] [PubMed]
- Pirgozliev, S.R.; Edwards, S.G.; Hare, M.C.; Jenkinson, P. Effect of dose rate of azoxystrobin and metconazole on the development of fusarium head blight and the accumulation of deoxynivalenol (DON) in wheat grain. Eur. J. Plant Pathol. 2002, 108, 469–478. [Google Scholar] [CrossRef]
- Singh, S.K.; Strobel, G.A.; Knighton, B.; Geary, B.; Sears, J.; Ezra, D. An endophytic Phomopsis sp. possessing bioactivity and fuel potential with its volatile organic compounds. Microb. Ecol. 2011, 61, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Quintana-Rodriguez, E.; Morales-Vargas, A.T.; Molina-Torres, J.; Ádame-Alvarez, R.M.; Acosta-Gallegos, J.A.; Heil, M. Plant volatiles cause direct, induced and associational resistance in common bean to the fungal pathogen Colletotrichum lindemuthianum. J. Ecol. 2015, 103, 250–260. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, X.; Kim, M.S.; Kornyeyev, D.A.; Holaday, S.; Paré, P.W. Soil bacteria augment Arabidopsis photosynthesis by decreasing glucose sensing and abscisic acid levels in planta. Plant J. 2008, 56, 264–273. [Google Scholar] [CrossRef] [PubMed]
- De Cremer, K.; Mathys, J.; Vos, C.; Froenicke, L.; Michelmore, R.W.; Cammue, B.; de Coninck, B. Rnaseq-based transcriptome analysis of lactuca sativa infected by the fungal necrotroph Botrytis cinerea. Plant Cell Environ. 2013, 36, 1992–2007. [Google Scholar] [PubMed]
- Wedge, D.E.; Galindo, J.C.G.; Macı́as, F.A. Fungicidal activity of natural and synthetic sesquiterpene lactone analogs. Phytochemistry 2000, 53, 747–757. [Google Scholar] [CrossRef]
- Bryman, A.; Cramer, D. Quantitative Data Analysis with Minitab: A Guide for Social Scientists; Routledge: London, UK, 1996. [Google Scholar]
- Gemeda, N.; Woldeamanuel, Y.; Asrat, D.; Debella, A. Effect of essential oils on Aspergillus spore germination, growth and mycotoxin production: A potential source of botanical food preservative. Asian Pac. J. Trop. Biomed. 2014, 4, S373–S381. [Google Scholar] [CrossRef] [PubMed]
- Kouassi, K.H.S.; Bajji, M.; Brostaux, Y.; Zhiri, A.; Samb, A.; Lepoivre, P.; Jijakli, H. Development and application of a microplate method to evaluate the efficacy of essential oils against Penicillium italicum Wehmer, Penicillium digitatum Sacc. and Colletotrichum musea (Berk. M.A. Curtis) Arx, three postharvest fungal pathogens of fruits. BASE 2012, 16, 325–336. [Google Scholar]
- Heuskin, S.; Lorge, S.; Lognay, G.; Wathelet, J.P.; Béra, F.; Leroy, P.; Haubruge, E.; Brostaux, Y. A Semiochemical slow-release formulation in a biological control approach to attract hoverflies. J. Environ. Ecol. 2012, 3, 72–85. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.

| Pathogen | VOC | Inhibition Rate of Spore Germination (%) at Given Time (h) | ||
|---|---|---|---|---|
| 2 | 4 | 6 | ||
| Fusarium culmorum | MA | 55, 1 *** | 65, 7 *** | 53, 3 *** |
| MP | 61, 2 *** | 62, 2 *** | 41, 9 *** | |
| Cochliobolus sativus | MA | 69, 4 *** | 69, 1 *** | 65, 1 *** |
| MP | 57, 6 *** | 59, 0 *** | 41, 6 *** | |
| Effect | G1 | L1 | L2 | S1 | S2 | SNGM | |
|---|---|---|---|---|---|---|---|
| Seed infected by F. culmorum | 6 | 6 | 3 | 5 | 3 | 9 | |
| Seed infected by C. sativus | 7 | 7 | 7 | 7 | 7 | 8 | |
| Seed infected by F. culmorum + MA | 15 | 13 | 12 | 0 | 0 | 0 | * |
| Seed infected by C. sativus + MA | 14 | 12 | 11 | 0 | 0 | 1 | * |
| Seed infected by F. culmorum + MP | 15 | 13 | 12 | 0 | 0 | 0 | * |
| Seed infected by C. sativus + MP | 14 | 11 | 11 | 0 | 0 | 1 | * |
| Type of Organism | Species | Reference | Origin | Medium | Temperature | Concentration |
|---|---|---|---|---|---|---|
| Fungus | Fusarium culmorum | MUCL28166 | Finland | PDA | 20 °C | 106 sp/mL |
| Fungus | Fusarium oxysporum | MUCL38936 | Belgium | PDA | 20 °C | 106 sp/mL |
| Fungus | Cochliobolus sativus | MUCL46854 | Georgia | PDA | 20 °C | 106 sp/mL |
| Fungus | Fusarium graminearum | Pers. collection | Belgium | PDA | 25 °C | 106 sp/mL |
| Fungus | Penicillium italicum | MUCL15608 | United States | PDA | 20 °C | 106 sp/mL |
| Fungus | Penicillium digitatum | CBS319.48 | Baarn/Netherlands | PDA | 20 °C | 106 sp/mL |
| Bacterium | Pectobacterium carotovorum atrosepticum | PCA 332 | France | V8 | 28 °C | 107 cfu/mL |
| Bacterium | Pectobacterium carotovorum carotovorum | PCC 380 | France | V8 | 28 °C | 107 cfu/mL |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaddes, A.; Parisi, O.; Berhal, C.; Ben Kaab, S.; Fauconnier, M.-L.; Nasraoui, B.; Jijakli, M.H.; Massart, S.; De Clerck, C. Evaluation of the Effect of Two Volatile Organic Compounds on Barley Pathogens. Molecules 2016, 21, 1124. https://doi.org/10.3390/molecules21091124
Kaddes A, Parisi O, Berhal C, Ben Kaab S, Fauconnier M-L, Nasraoui B, Jijakli MH, Massart S, De Clerck C. Evaluation of the Effect of Two Volatile Organic Compounds on Barley Pathogens. Molecules. 2016; 21(9):1124. https://doi.org/10.3390/molecules21091124
Chicago/Turabian StyleKaddes, Amine, Olivier Parisi, Chadi Berhal, Sofiene Ben Kaab, Marie-Laure Fauconnier, Bouzid Nasraoui, M. Haissam Jijakli, Sébastien Massart, and Caroline De Clerck. 2016. "Evaluation of the Effect of Two Volatile Organic Compounds on Barley Pathogens" Molecules 21, no. 9: 1124. https://doi.org/10.3390/molecules21091124
APA StyleKaddes, A., Parisi, O., Berhal, C., Ben Kaab, S., Fauconnier, M.-L., Nasraoui, B., Jijakli, M. H., Massart, S., & De Clerck, C. (2016). Evaluation of the Effect of Two Volatile Organic Compounds on Barley Pathogens. Molecules, 21(9), 1124. https://doi.org/10.3390/molecules21091124






