Pro-Apoptotic Activity of New Honokiol/Triphenylmethane Analogues in B-Cell Lymphoid Malignancies
Abstract
:1. Introduction
2. Results
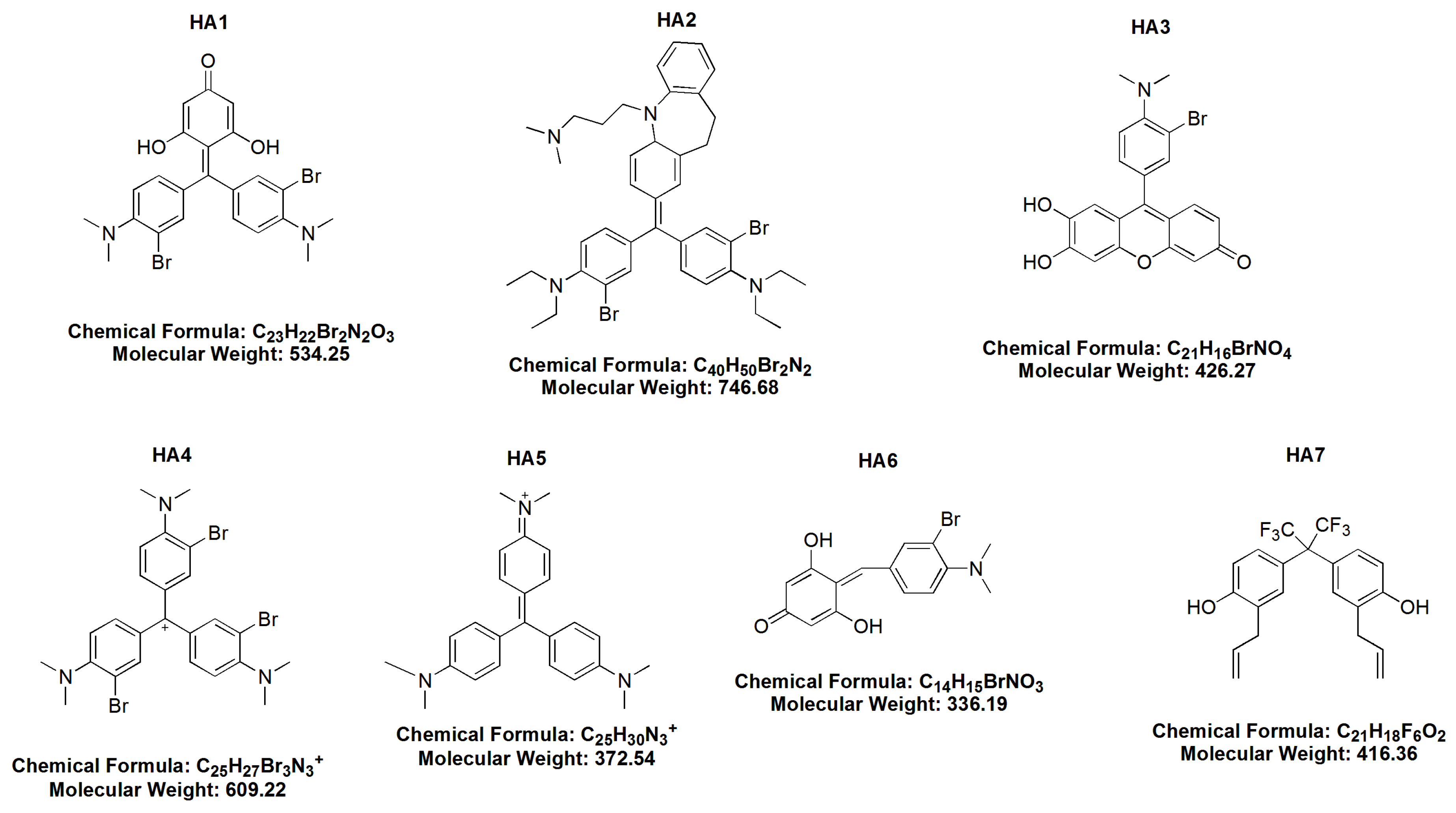
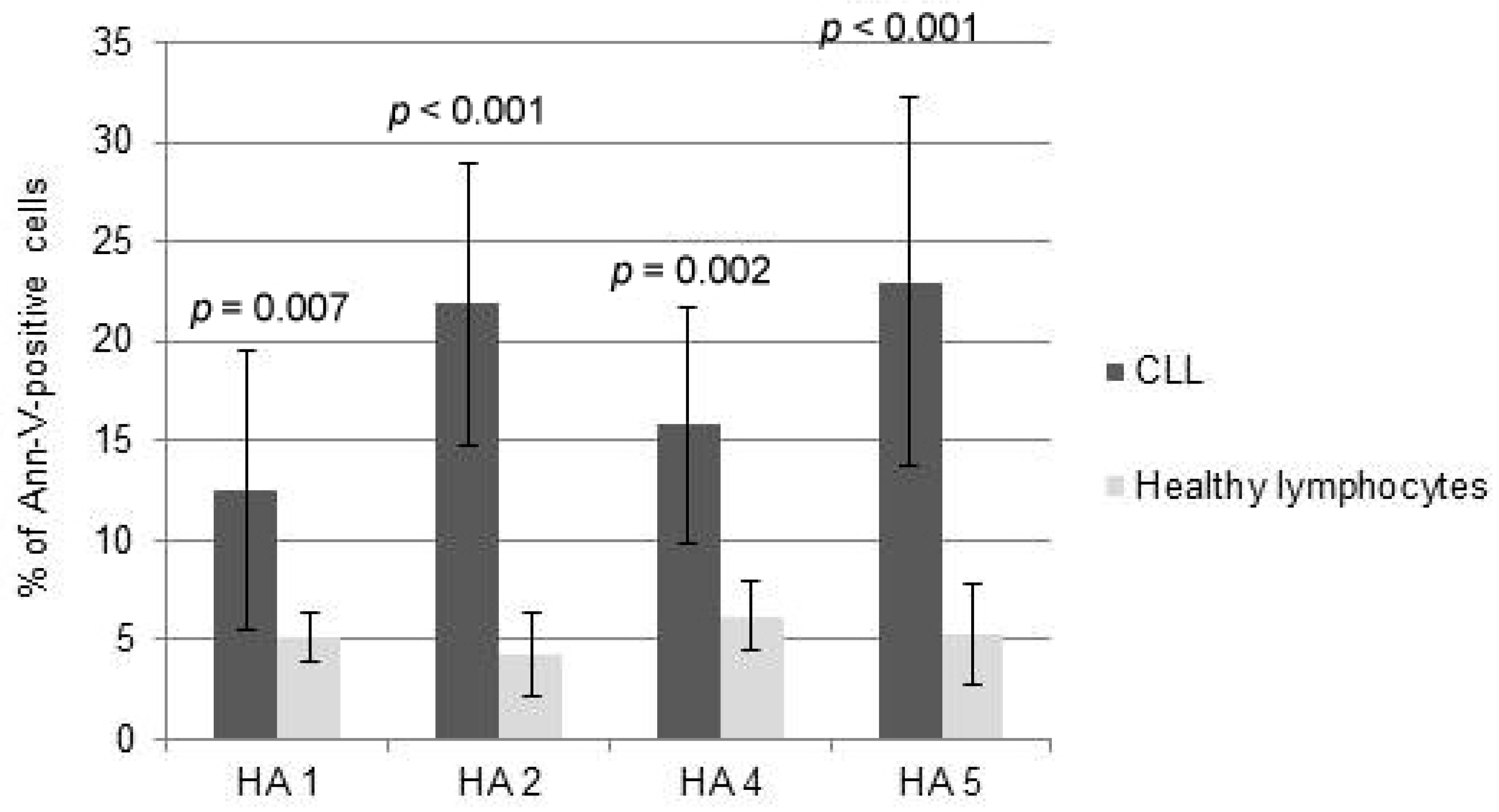
2.1. Cytotoxicity of HAs
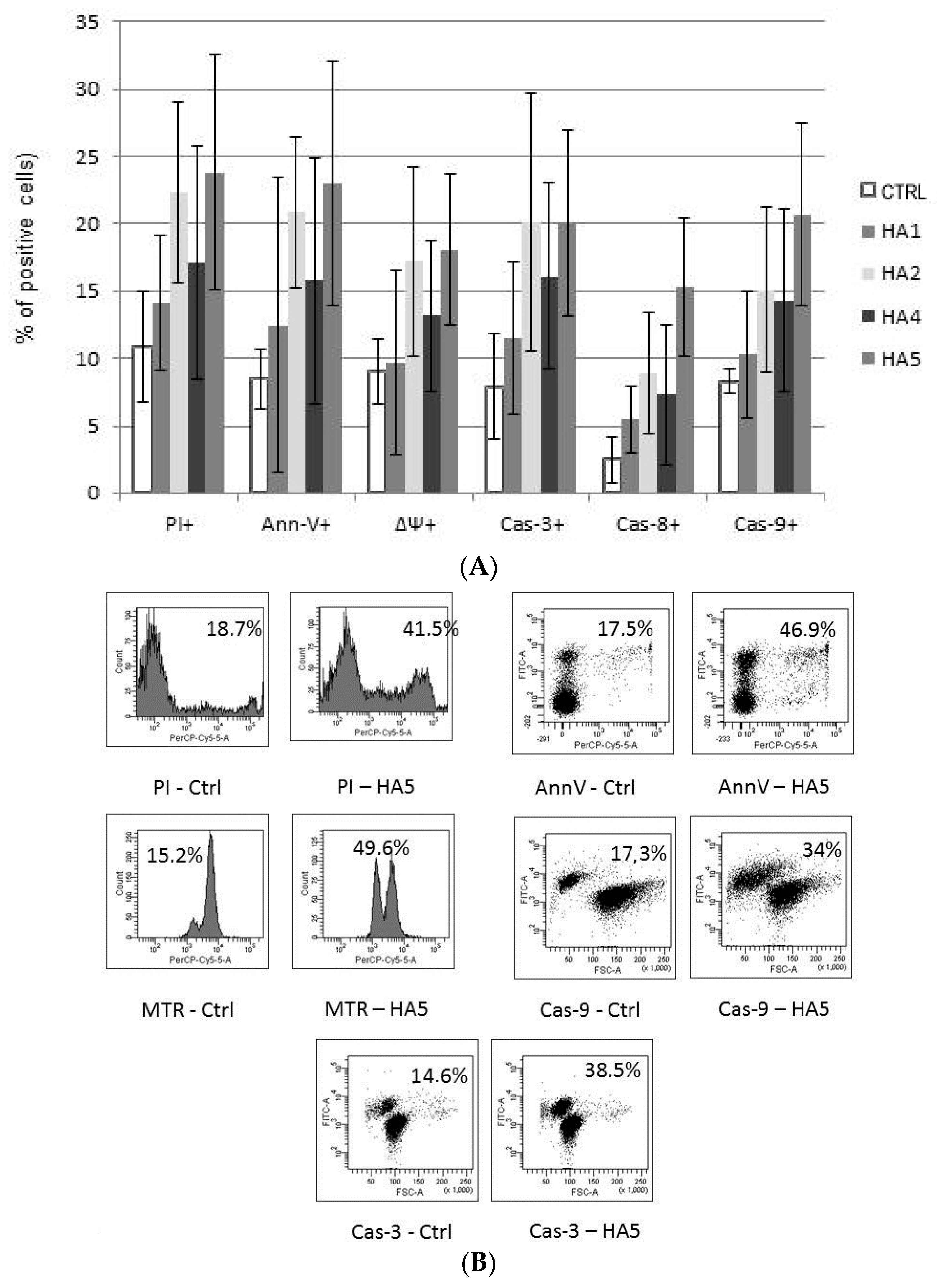
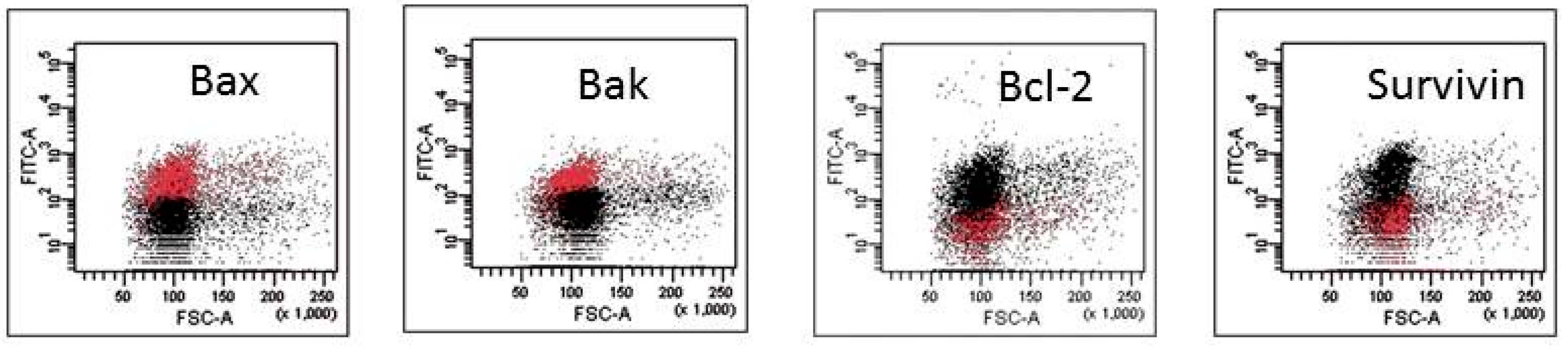
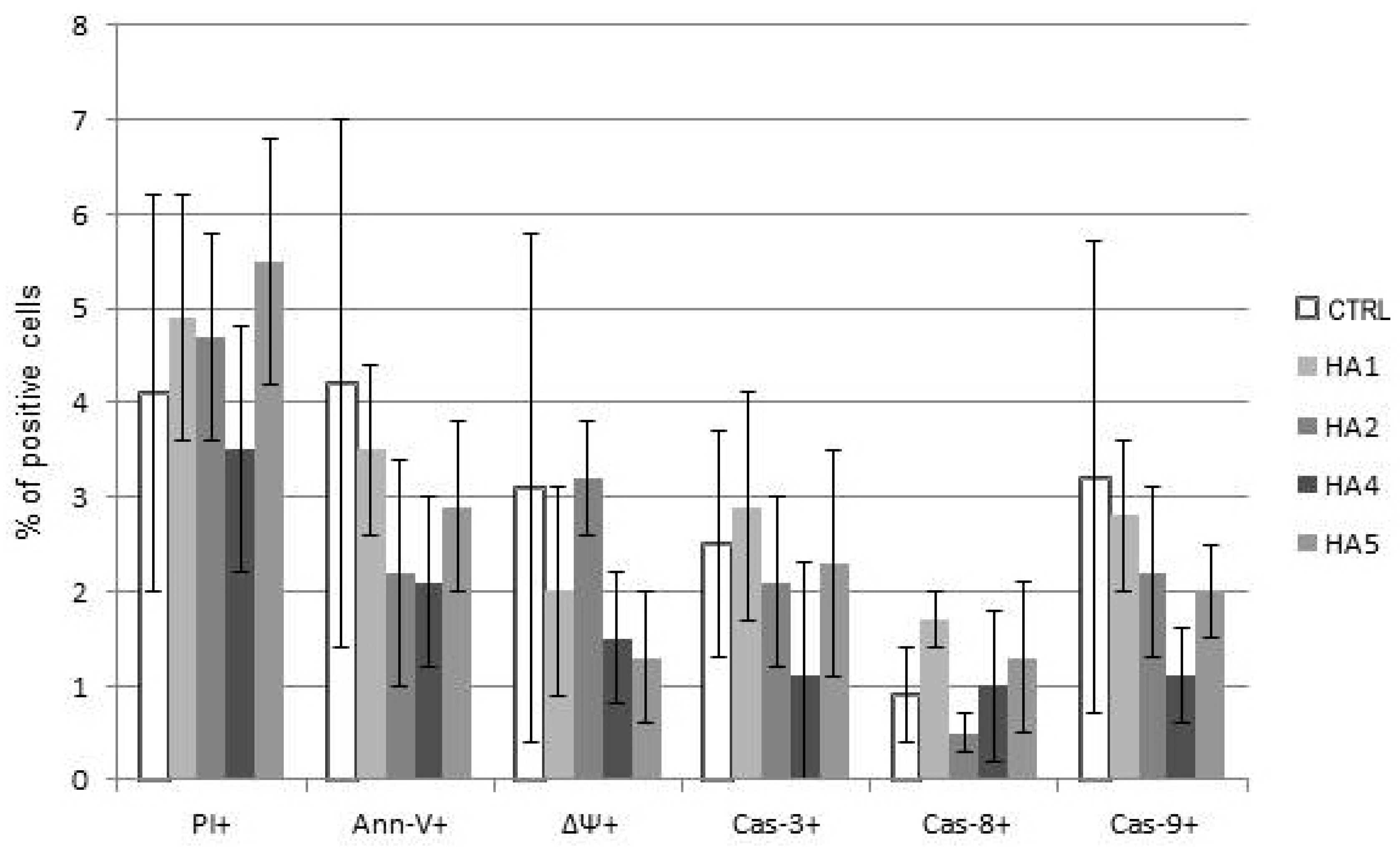
2.2. Mechanisms of HA Action
3. Discussion
4. Material and Methods
4.1. CLL and Healthy Cell Isolation
4.2. Cell Cultures
4.3. Drug Dosing and Administration
- (1)
- BR MMK + phloroG-3,3′ dibromo4,4′ bis (dimethylamino) benzylidenephloroglucinol (HA 1),
- (2)
- dibromoimipramine blue (HA 2),
- (3)
- 3 bromo-4 dimethylamino diuhydroxyphenoxane (HA 3),
- (4)
- bromo Gentian Violet-tribromogentian violet (HA 4),
- (5)
- Gentian Violet (HA 5),
- (6)
- BR dimethylaminobenzaldehyde + phloroG 3 bromo-4 dimethlyaminobenzylidenephloroglucinol (HA 6) and
- (7)
- hexafluoro-diallylhexaflurobisphenol (HA 7).
4.4. Cytotoxicity and Apoptosis Assays
4.5. Drop of Mitochondrial Potential (MTR, ΔΨ)
4.6. Caspases and Apoptosis-Regulating Protein Expression
4.7. Flow Cytometry Analysis
4.8. Statistics
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shen, C.C.; Ni, C.L.; Shen, Y.C.; Huang, Y.L.; Kuo, C.H.; Wu, T.S.; Chen, C.C. Phenolic constituents from the stem bark of Magnolia officinalis. J. Nat. Prod. 2009, 72, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, Y.; Kuribara, H.; Morita, M.; Yuzurihara, M.; Weintraub, S.T. Identification of magnolol and honokiol as anxiolytic agents in extracts of saiboku-to, an oriental herbal medicine. J. Nat. Prod. 1998, 61, 135–138. [Google Scholar] [CrossRef] [PubMed]
- Liou, K.T.; Shen, Y.C.; Chen, C.F.; Tsao, C.M.; Tsai, S.K. The anti-inflammatory effect of honokiol on neutrophils: Mechanisms in the inhibition of reactive oxygen species production. Eur. J. Pharmacol. 2003, 47, 19–27. [Google Scholar] [CrossRef]
- Park, J.; Lee, J.; Jung, E.; Park, Y.; Kim, K.; Park, B.; Jung, K.; Park, E.; Kim, J.; Park, D. In vitro antibacterial and anti-inflammatory effects of honokiol and magnolol against Propionibacterium sp. Eur. J. Pharmacol. 2004, 496, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Liou, K.T.; Lin, S.M.; Huang, S.S.; Chih, C.L.; Tsai, S.K. Honokiol ameliorates cerebral infarction from ischemia-reperfusion injury in rats. Planta Med. 2003, 69, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Chang, B.; Lee, Y.; Ku, Y.; Bae, K.; Chung, C. Antimicrobial activity of magnolol and honokiol against periodontopathic microorganisms. Planta Med. 1998, 64, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Cho, J.Y. Anti-inflammatory effect of honokiol is mediated by PI3K/Akt pathway suppression. Acta Pharmacol. Sin. 2008, 29, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.M.; El-Feraly, F.S.; Li, W.S. Antimicrobal activity of phenolic constituents of Magnolia grandiflora L. J. Pharm. Sci. 1981, 70, 951–952. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.L.; Tang, W.; Du, G.H.; Kokudo, N. Targeting apoptosis pathways in cancer with magnolol and honokiol, bioactive constituent of the bark of Magnolia officinalis. Drug Discov. Ther. 2011, 5, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Shigemura, K.; Arbiser, J.L.; Sun, S.Y.; Zayzafoon, M.; Johnstone, P.A.; Fujisawa, M.; Gotoh, A.; Weksler, B.; Zhau, H.E.; Chung, L.W. Honokiol, a natural plant product, inhibits the bone metastatic growth of human prostate cancer cells. Cancer 2007, 109, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Cerimele, F.; Ushio-Fukai, M.; Waqas, M.; Campbell, P.M.; Govindarajan, B.; Der, C.J.; Battle, T.; Frank, D.A.; Ye, K.; et al. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J. Biol. Chem. 2003, 278, 35501–35507. [Google Scholar] [CrossRef] [PubMed]
- Battle, T.E.; Abriser, J.; Frank, D.A. The natural product honokiol induces caspase-dependant apoptosis in B-cell chronic lymphocytic leukemia. Blood 2005, 106, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Ishitsuka, K.; Hideshima, T.; Hamasaki, M.; Raje, N.; Kumar, S.; Hideshima, H.; Shiraishi, N.; Yasui, H.; Roccaro, A.M.; Richardson, P.; et al. Honokiol overcomes conventional drug resistance in human multiple myeloma by induction of caspase-dependent and -independent apoptosis. Blood 2005, 106, 1794–1800. [Google Scholar] [CrossRef] [PubMed]
- Park, E.J.; Zhao, Y.Z.; Kim, Y.H.; Lee, B.H.; Sohn, D.H. Honokiol induces apoptosis via cytochrome c release and caspase activation in activated rat hepatic stellate cells in vitro. Planta Med. 2005, 71, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Bhardwaj, A.; Srivastava, S.K.; Singh, S.; McClellan, S.; Wang, B.; Singh, A.P. Honokiol arrest cell cycle, induces apoptosis and potentiates the cytotoxic effect of gemcitabine in human pancreatic cancer cells. PLoS ONE 2011, 6, e21573. [Google Scholar] [CrossRef] [PubMed]
- Kitada, S.; Andersen, J.; Akar, S.; Zapata, J.M.; Takayama, S.; Krajewski, S.; Wang, H.G.; Zhang, X.; Bullrich, F.; Croce, C.M.; et al. Expression of apoptosis-regulating proteins in chronic lymphocytic leukemia, correlations with in vitro and in vivo chemoresponses. Blood 1998, 91, 3379–3389. [Google Scholar] [PubMed]
- Yang, S.E.; Hsieh, M.T.; Tsai, T.H.; Hsu, S.L. Down-modulation of Bcl-XL, release of cytochrome c and sequential activation of caspases during honokiol-induced apoptosis in human squamous lung cancer CH27 cells. Biochem. Pharmacol. 2002, 63, 1641–1651. [Google Scholar] [CrossRef]
- Ahn, K.S.; Sethi, G.; Shishodia, S.; Sung, B.; Arbiser, J.L.; Aggarwal, B.B. Honokiol potentiates apoptosis, suppresses osteoclastogenesis, and inhibits invasion through modulation of nuclear factor-κB activation pathway. Mol. Cancer Res. 2006, 4, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Hibasami, H.; Achiwa, Y.; Katsuzaki, H.; Imai, K.; Yoshioka, K.; Nakanis, K.; Ishii, Y.; Hasegawa, M.; Komiya, T. Honokiol induces apoptosis in human lymphoid leukemia Molt 4B cells. Int. J. Mol. Med. 1998, 2, 671–673. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wang, T.; Wu, Y.F.; Zheng, S.; Hu, X. Honokiol, A potent chemotherapy candidate for human colorectal carcinoma. World J. Gastroenterol. 2004, 10, 3459–3463. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.E.; Hsieh, M.T.; Tsai, T.H.; Hsu, S.L. Effector mechanism of magnolol-induced apoptosis in human lung squamous carcinoma CH27 cells. Br. J. Pharmacol. 2003, 138, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Chen, F.; Chen, Z.; Wu, Y.F.; Xu, X.L.; Zheng, S.; Hu, X. Honokiol induces apoptosis through p53-independent pathway in human colorectal cell line RKO. World J. Gastroenterol. 2004, 1015, 2205–2208. [Google Scholar]
- Fong, W.F.; Tse, A.K.; Poon, K.H.; Wang, C. Magnolol and honokiol enhance HL-60 human leukemia cell differentiation induced by 1,25-dihydroxyvitamin D3 and retinoic acid. Int. J. Biochem. Cell Biol. 2005, 37, 427–441. [Google Scholar] [CrossRef] [PubMed]
- Wolf, I.; O'Kelly, J.; Wakimoto, N.; Nguyen, A.; Amblard, F.; Karlan, B.Y.; Arbiser, J.L.; Koeffler, H.P. Honokiol, a natural biphenyl, inhibits in vitro and in vivo growth of breast cancer through induction of apoptosis and cell cycle arrest. Int. J. Oncol. 2007, 30, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.Q.; Fan, L.Y.; Yang, G.L.; Guo, W.H.; Hou, W.L.; Chen, L.J.; Wei, Y.Q. Improved therapeutic effectiveness by combining liposomal honokiol with cisplatin in lung cancer model. BMC Cancer 2008, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Adachi, N.; So, S.; Iiizumi, S.; Nomura, Y.; Murai, K.; Yamakawa, C.; Miyagawa, K.; Koyama, H. The human pre-B cell line Nalm-6 is highly proficient in gene targeting by homologous recombination. DNA Cell Biol. 2006, 25, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.M.; Scemama, J.L.; Panayiotidis, M.I.; Georgakilas, A.G. Compromised repair of clustered DNA damage in the human acute lymphoblastic leukemia MSH2-deficient NALM-6 cells. Mutat. Res. 2009, 674, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Pervin, S.; Tran, L.; Urman, R.; Braga, M.; Parveen, M.; Li, S.A.; Chaudhuri, G.; Singh, R. Oxidative stress specifically downregulates survivin to promote breast tumour formation. Br. J. Cancer. 2013, 108, 848–858. [Google Scholar] [CrossRef] [PubMed]
- Candé, C.; Vahsen, N.; Garrido, C.; Kroemer, G. Apoptosis-inducing factor (AIF), caspase-independent after all. Cell Death Differ. 2004, 11, 591–595. [Google Scholar] [CrossRef] [PubMed]
- Buggins, A.G.; Pepper, C.J. The role of Bcl-2 family proteins in chronic lymphocytic leukaemia. Leuk. Res. 2010, 34, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Smolewski, P.; Bedner, E.; Du, L.; Wu, J.M.; Phelps, D.J.; Darzynkiewicz, Z. Detection of caspase activation by fluorochrome-labeled inhibitors: Multiparameter analysis by laser scanning cytometry. Cytometry 2001, 44, 73–82. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all the compounds investigated in this work are available from the authors.
| Honokiol Analogues (HAs) | HA 1 IC50 | HA 2 IC50 | HA 4 IC50 | HA 5 IC50 |
|---|---|---|---|---|
| CLL | 10 μM | 5 μM | 10 μM | 5 μM |
| Raji | 2.5 μM | 2.5 μM | 2.5 μM | 0.25 μM |
| Toledo | 0.5 μM | 0.5 μM | 2.5 μM | 0.5 μM |
| RPMI 8226 | 0.5 μM | 0.5 μM | 5 μM | 1 μM |
| Nalm-6 | NR | NR | NR | NR |
| A. Apoptosis-Regulating Protein Expression in Response to HA1. | ||||||||||
| Cells | HA1 | |||||||||
| Bax | Bak | Bcl-2 | Mcl-1 | c-IAP1 | c-IAP2 | XIAP | Smac-Diablo | Survivin | HTRA2/Omi | |
| CLL | NS | NS | 0.78 * | 0.51 ** | 0.59 ** | 0.47 ** | 0.27 *** | 0.34 *** | 1.68 * | NS |
| Raji | 2.61 *** | 2.12 ** | 0.39 *** | NS | NS | NS | NS | NS | 1.73 * | NS |
| Toledo | 2.93 *** | NS | 0.49 ** | NS | NS | NS | 0.68 * | NS | 2.06 ** | NS |
| RPMI 8226 | 1.62 * | 1.53 * | 0.71 * | NS | NS | NS | 0.75 * | NS | 2.77 *** | NS |
| Nalm-6 | NS | NS | 0.82 * | NS | NS | NS | NS | NS | NS | NS |
| B. Apoptosis-Regulating Protein Expression in Response to HA2. | ||||||||||
| Cells | HA2 | |||||||||
| Bax | Bak | Bcl-2 | Mcl-1 | c-IAP1 | c-IAP2 | XIAP | Smac-Diablo | Survivin | HTRA2/Omi | |
| CLL | NS | NS | 0.65 * | 0.47 ** | 0.59 ** | NS | 0.29 *** | 0.78 * | 1.75 * | NS |
| Raji | 2.33 *** | 1.98 ** | 0.44 * | NS | NS | NS | NS | NS | 1.67 * | 1.92 * |
| Toledo | 2.02 ** | NS | 0.35 *** | NS | NS | NS | 0.52 ** | NS | 2.81 ** | NS |
| RPMI 8226 | 1.50 * | NS | 0.71 * | NS | NS | NS | 0.73 * | NS | 1.64 * | NS |
| Nalm-6 | NS | NS | 0.78 * | NS | NS | NS | NS | NS | NS | NS |
| C. Apoptosis-Regulating Protein Expression in Response to HA4. | ||||||||||
| Cells | HA4 | |||||||||
| Bax | Bak | Bcl-2 | Mcl-1 | c-IAP1 | c-IAP2 | XIAP | Smac-Diablo | Survivin | HTRA2/Omi | |
| CLL | NS | NS | 0.72 * | 0.38 * | NS | NS | 0.29 *** | 0.66 * | 1.91 * | NS |
| Raji | 2.29 * | 1.94 * | 0.59 * | NS | NS | NS | NS | NS | NS | 1.87 * |
| Toledo | 1.87 * | NS | 0.42 * | NS | NS | NS | 0.79 * | NS | 2.85 *** | NS |
| RPMI 8226 | 1.76 * | NS | 0.68 * | NS | NS | NS | 0.73 * | NS | NS | NS |
| Nalm-6 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS |
| D. Apoptosis-Regulating Protein Expression in Response to HA5. | ||||||||||
| Cells | HA5 | |||||||||
| Bax | Bak | Bcl-2 | Mcl-1 | c-IAP1 | c-IAP2 | XIAP | Smac-Diablo | Survivin | HTRA2/Omi | |
| CLL | NS | NS | 0.55 ** | 0.65 * | 0.72 * | NS | 0.64 * | NS | 0.42 ** | NS |
| Raji | 1.99 ** | 2.12 ** | 0.75 * | NS | NS | NS | NS | NS | 0.68 * | NS |
| Toledo | 2.23 ** | NS | 0.53 ** | NS | NS | NS | 0.44 ** | NS | 0.65 * | NS |
| RPMI 8226 | 1.68 * | 1.80 * | 0.72 * | NS | NS | NS | 0.73 * | NS | 0.73 * | NS |
| Nalm-6 | NS | NS | NS | NS | NS | NS | 0.60 * | NS | NS | NS |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mędra, A.; Witkowska, M.; Majchrzak, A.; Cebula-Obrzut, B.; Bonner, M.Y.; Robak, T.; Arbiser, J.L.; Smolewski, P. Pro-Apoptotic Activity of New Honokiol/Triphenylmethane Analogues in B-Cell Lymphoid Malignancies. Molecules 2016, 21, 995. https://doi.org/10.3390/molecules21080995
Mędra A, Witkowska M, Majchrzak A, Cebula-Obrzut B, Bonner MY, Robak T, Arbiser JL, Smolewski P. Pro-Apoptotic Activity of New Honokiol/Triphenylmethane Analogues in B-Cell Lymphoid Malignancies. Molecules. 2016; 21(8):995. https://doi.org/10.3390/molecules21080995
Chicago/Turabian StyleMędra, Aleksandra, Magdalena Witkowska, Agata Majchrzak, Barbara Cebula-Obrzut, Michael Y. Bonner, Tadeusz Robak, Jack L. Arbiser, and Piotr Smolewski. 2016. "Pro-Apoptotic Activity of New Honokiol/Triphenylmethane Analogues in B-Cell Lymphoid Malignancies" Molecules 21, no. 8: 995. https://doi.org/10.3390/molecules21080995
APA StyleMędra, A., Witkowska, M., Majchrzak, A., Cebula-Obrzut, B., Bonner, M. Y., Robak, T., Arbiser, J. L., & Smolewski, P. (2016). Pro-Apoptotic Activity of New Honokiol/Triphenylmethane Analogues in B-Cell Lymphoid Malignancies. Molecules, 21(8), 995. https://doi.org/10.3390/molecules21080995







