Abstract
Several new pyrazole, pyridine, [1,2,4]triazolo[1,5-α]pyrimidine, benzimidazo[1,2-a]pyrimidine and 1,2,4-triazolo[3,4-c][1,2,4]triazine derivatives incorporating a thiophene moiety were synthesized from (E)-ethyl 5-(3-(dimethylamino)acryloyl)-4-phenyl-2-(phenylamino)thiophene-3-carboxylate (1). The structures of the newly synthesized compounds were confirmed by IR, 1H-, 13C-NMR, mass spectral data and elemental analysis. The antibacterial and antifungal activities of all the synthesized compounds were evaluated. The results indicated that compounds 9, 12, and 19 were found to be more potent than the standard drug Amphotericin B against Aspergillus fumigates. Additionally, compound 12 exhibited higher activity than the standard drug Amphotericin B against Syncephalastrum racemosum.
1. Introduction
The synthesis and study of thiophene derivatives have been of interest due to their facile synthesis and broad spectrum of biological and pharmacological activities. A literature survey indicated that heterocyclic compounds containing thiophene ring were found to exhibit wide range of biological activities, such as antioxidant [1], anti HIV [2], anesthetic [3], bactericidal [4], antifungal [5], anti-inflammatory [1,6], antitubercular [7], and antitumor [8,9,10] activities. Additionally, there is a thiophene ring embedded in a large number of biologically important compounds, such as vitamin H, anthelmintic Banminth [11], and insecticidal Xanthopappin A [12]. Moreover, many thiophene derivatives are documented as chromophoric components for photonic polymers [13], such as dyes [14] and as air stable semiconductors [15]. Enaminones are versatile starting materials for the synthesis of fused ring systems. These synthons were reacted with several nucleophilic and electrophilic reagents to construct new heterocyclic systems [16,17]. The aforementioned facts stimulated our interest for the synthesis of novel heterocyclic compounds containing thiophene ring utilizing enaminone 1 [18] as a key precursor to fulfill our objectives. We anticipated that the synthesized compounds would possess diverse pharmacological activities.
2. Results and Discussion
2.1. Chemistry
In continuation of our interest in the chemistry of thiophene compounds [19,20,21,22] to develop novel library of pharmaceutical targets, we started from the enaminone 1 as a precursor for the synthesis of novel heterocycles incorporating a thiophene core. The enaminone 1 was synthesized according to the procedure reported previously by our group [18].
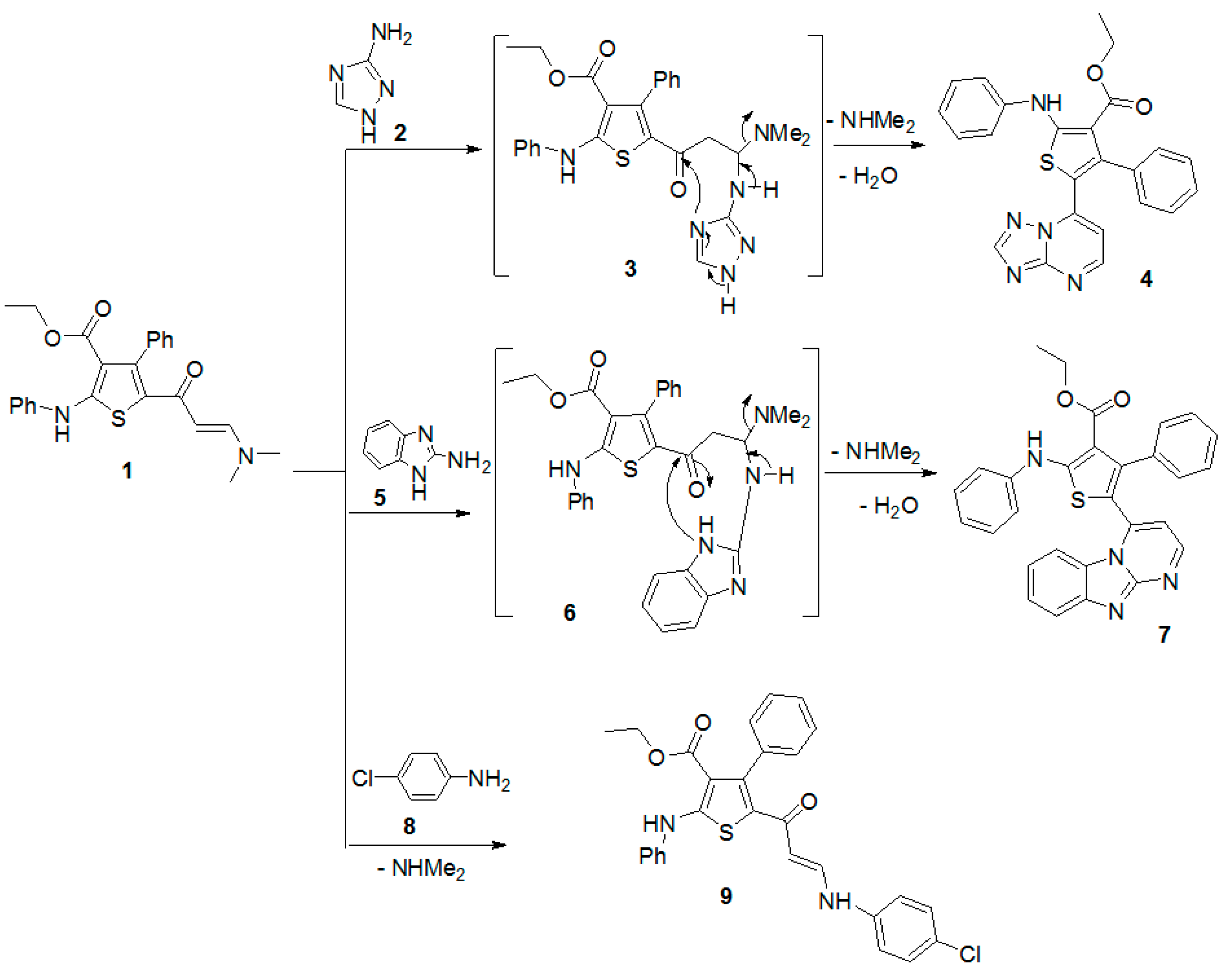
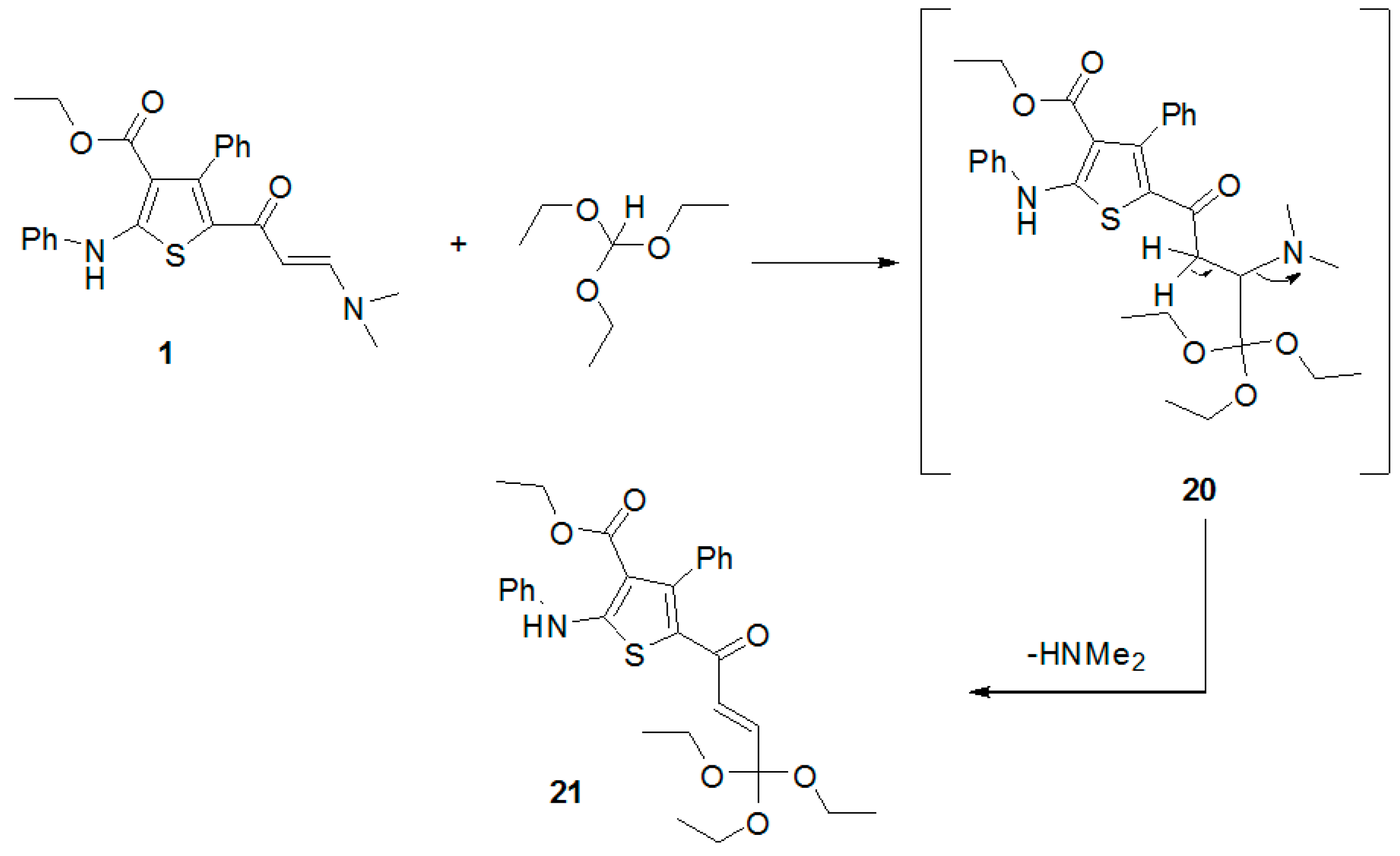
Firstly, the reactivity of this enaminone with some nitrogen nucleophiles was explored. Thus, condensation of enaminone 1 with 3-amino-1H-[1,2,4]triazole (2) afforded ethyl 5-([1,2,4]triazolo[1,5-a]pyrimidin-7-yl)-4-phenyl-2-(phenylamino)thiophene-3-carboxylate (4) (Scheme 1). The structure of the latter compound was established by its spectral data and elemental analysis. For example, its IR spectrum revealed the absence of the ketonic carbonyl group present in the spectrum of the enaminone 1. Additionally, its 1H-NMR spectrum revealed the disappearance of the signals corresponding to the two methyl protons of the starting enaminone 1. In addition, its mass spectrum showed a peak at m/z 441 due to its molecular ion. It was suggested that this reaction is started by nucleophilic attack of the exocyclic amino group of the triazole to the activated double bond of the enaminone 1 to afford the Michael-type product 3, which underwent intramolecular cyclization and the elimination of a dimethylamine and water molecules to afford the final product 4, as outlined below in Scheme 1.
Scheme 1.
The reaction of enaminone 1 with some nitrogen nucleophiles.
In a similar manner, the reaction of the enaminone 1 with 2-aminobenzimidazole (5) under the same experimental conditions gave only one isolable product, as examined by TLC. The structure of the obtained products were established as ethyl 5-(benzimidazo[1,2-a]pyrimidin-4-yl)-4-phenyl-2-(phenylamino)thiophene-3-carboxylate (7) (Scheme 1). The suggested chemical structure is in agreement with the experimental spectral data. Furthermore, the reaction of enaminone 1 with the 4-chloroaniline (8) gave the corresponding thiophene derivative 9 (Scheme 1). The structure of compound 9 was confirmed by its spectral and elemental data (see Experimental section). For example, its proton NMR spectrum revealed the absence of the protons of the dimethylamino group present in the spectrum of the enaminone 1.
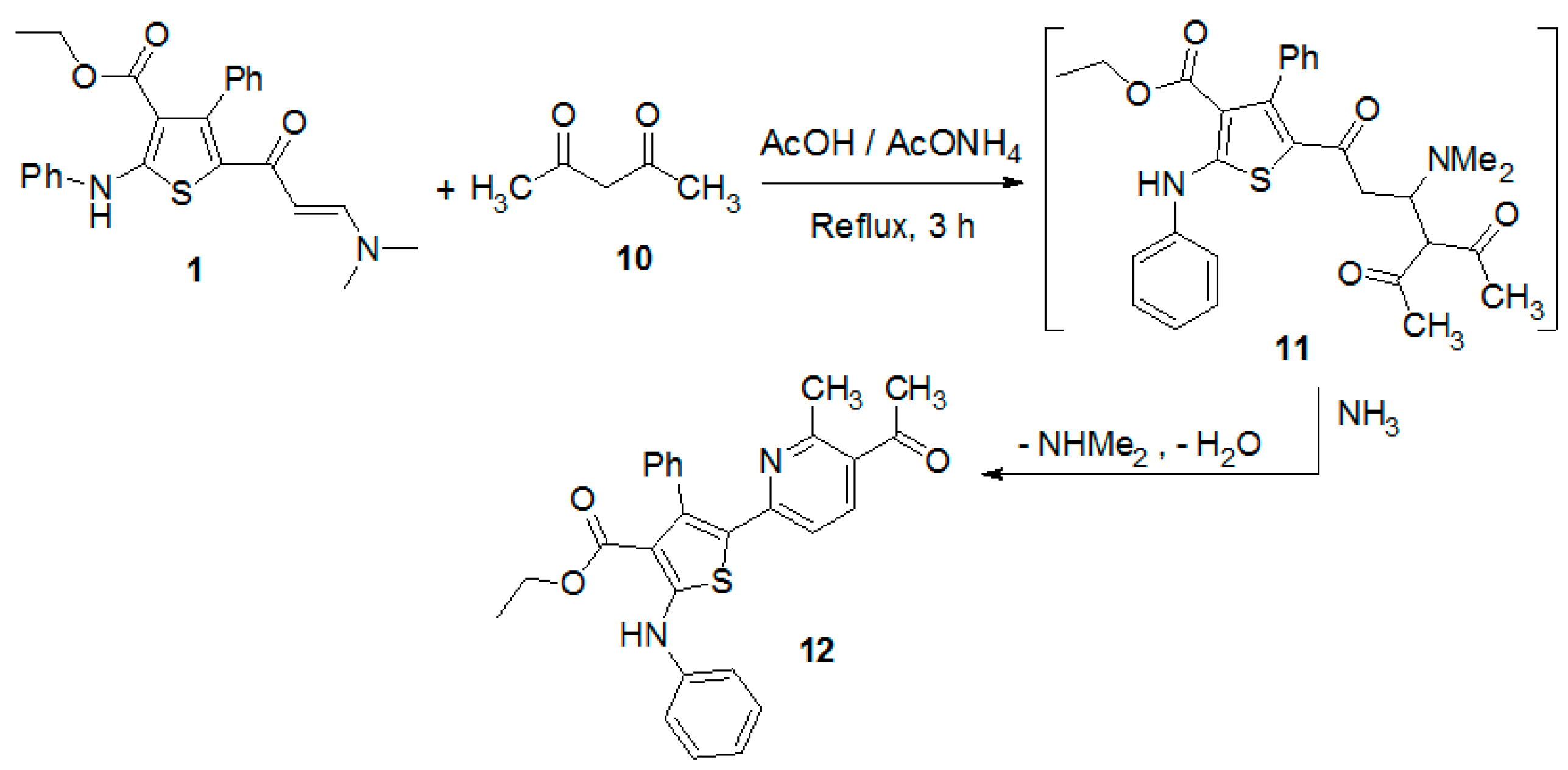
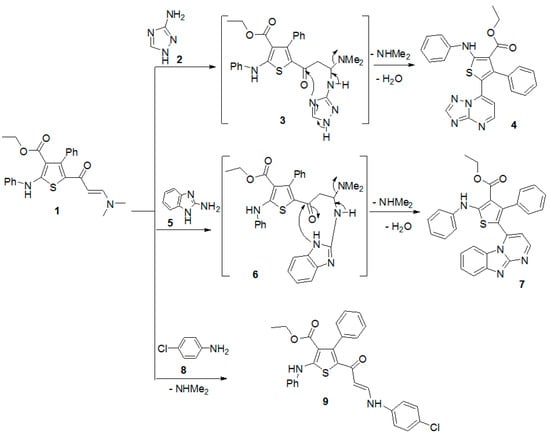
Next, The reaction of the enaminone 1 with 2,4-pentanedione in glacial acetic acid in the presence of ammonium acetate gave the pyridine derivative 12 (Scheme 2). The structure of the product was verified by spectral data and elemental analyses (see Experimental Section).
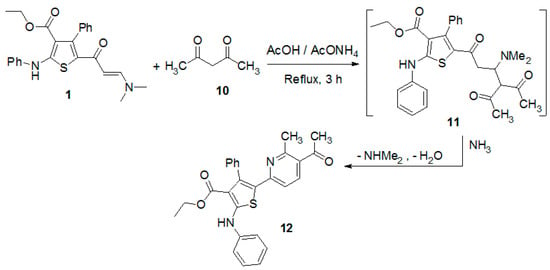
Scheme 2.
Reaction of enaminone 1 with 2,4-pentanedione.
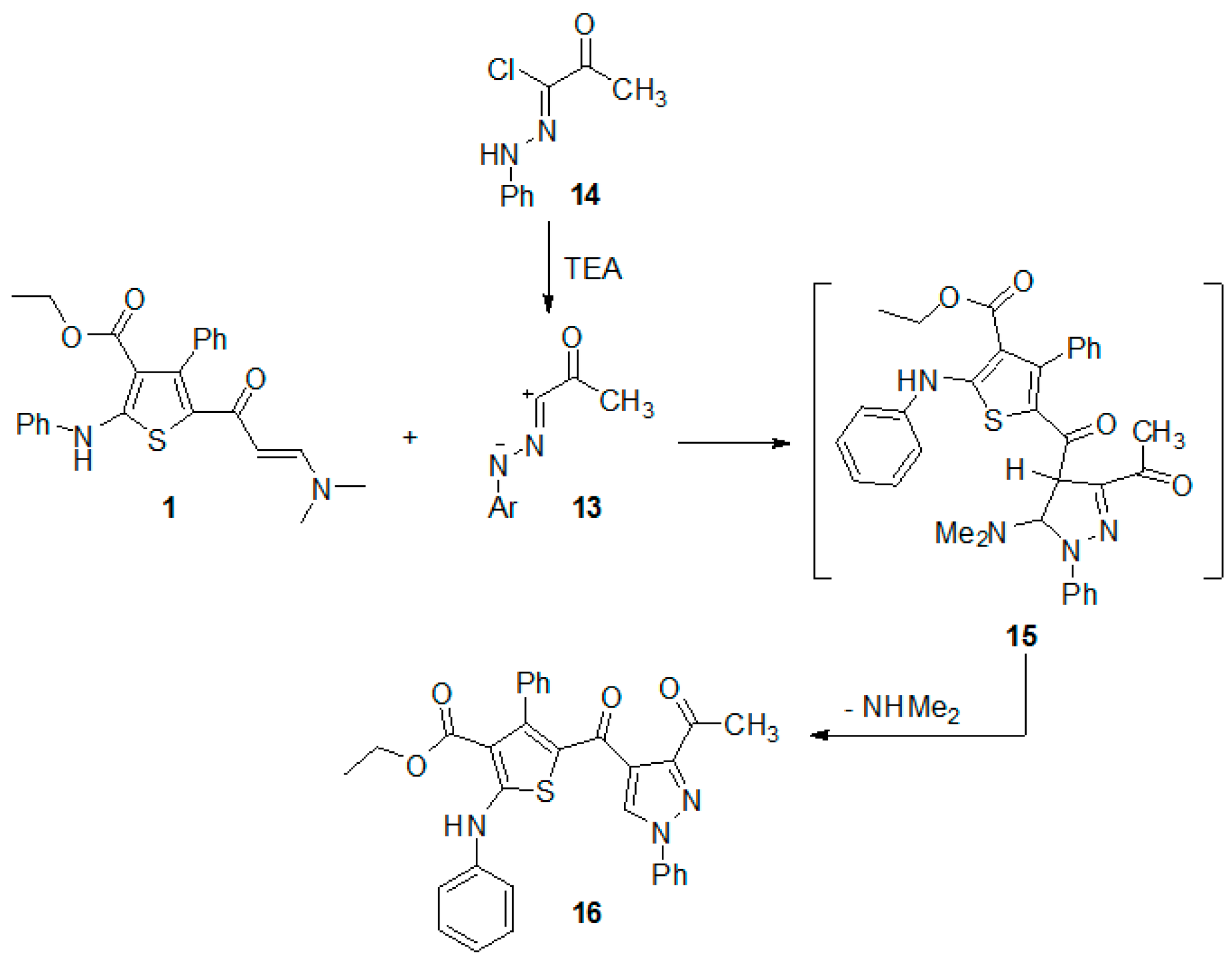
The regioselective synthesis of new pyrazole attached to the thiophene ring system via 1,3-dipolar cycloaddition reaction of imine 13 with the enaminone 1 was also studied.
Treatment of the enaminone 1 with the imine 13 (generated in situ from 2-oxo-N′-phenylpropanehydrazonoyl chloride (14) [23,24] by the action of triethylamine in benzene) afforded a single product that was identified, on the basis of its elemental analysis and spectral (IR, 1H-NMR and MS) data. The structure of the isolated cycloadduct was assigned as ethyl 5-(3-acetyl-1-phenyl-1H-pyrazole-4-carbonyl)-4-phenyl-2-(phenylamino)thiophene-3-carboxylate (16) based on its elemental analyses and spectroscopic data. The 1H-NMR spectrum of the formed cycloadduct showed a singlet signal at δ 7.46, which corresponds to H-5 of the pyrazole ring in the product. This assignment is based on the fact that in the pyrazole ring, H-4, is more shielded than H-5. Thus, the signal of H-5 usually appears at δ 8.66–8.69 [25], whereas that of H-4 appears at δ 5.81–5.89 [26,27]. The above-mentioned information indicated that the reaction of enaminone 1 with hydrazonoyl chloride 14 is regiospecific.
To account for the formation of pyrazole 16, we suggested that the reaction proceed via 1,3-dipolarcycloaddition of the imine 13, derived from hydrazonoyl chloride 14, to the activated double bond in enaminone 1 to give the respective nonisolable cycloadduct 15, which underwent aromatization via elimination of the dimethylamine molecule to give the final pyrazole derivative 16 (Scheme 3).
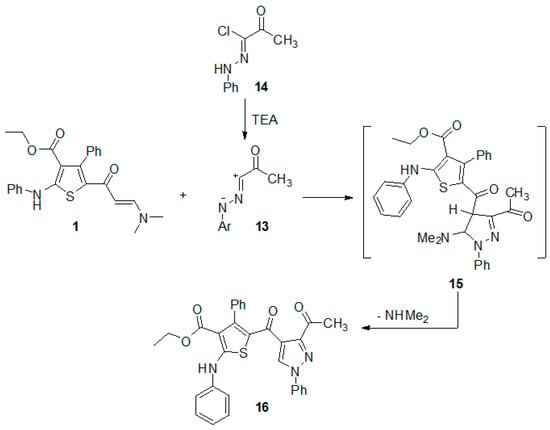
Scheme 3.
Regioselective synthesis of pyrazole derivative 16.
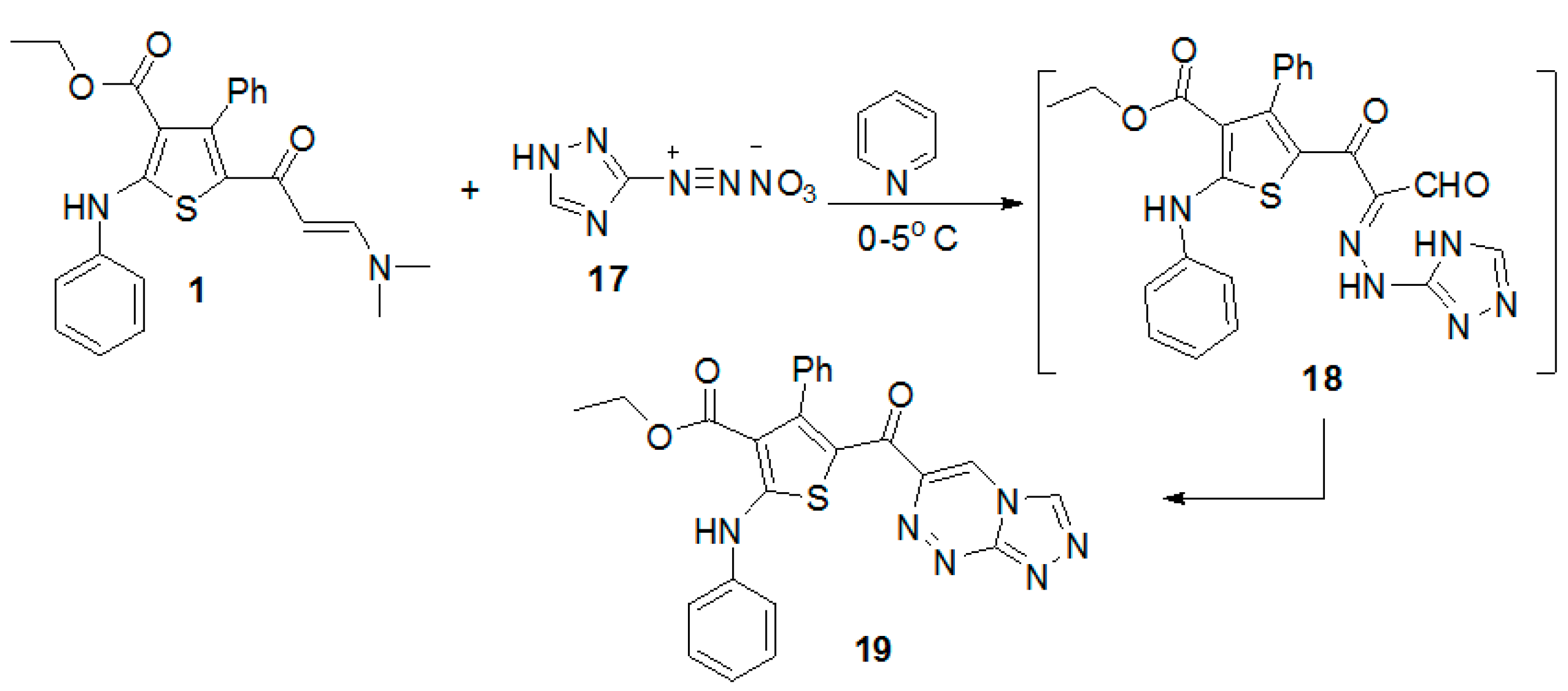
Finally, the coupling of enaminone 1 with the diazonium salt of 3-amino-1H-[1,2,4]triazole in pyridine at low temperature gave ethyl 5-([1,2,4]triazolo[3,4-c][1,2,4]triazine-6-carbonyl)-4-phenyl-2-(phenylamino)thiophene-3-carboxylate (19) (Scheme 4). The reaction proceeded by an initial formation of nonisolable intermediate 18 [28,29,30], followed by elimination of water molecule to give the final product 19.
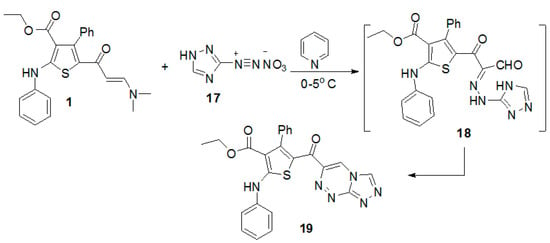
Scheme 4.
Synthesis of ethyl 5-([1,2,4]triazolo[3,4-c][1,2,4]triazine-6-carbonyl)-4-phenyl-2-(phenylamino)thiophene-3-carboxylate (19).
Refluxing enaminone 1 with triethylorthoformate gave the triethoxymethyl derivative 21 (Scheme 5). Its 1H-NMR spectrum showed the disappearance of the signals due to dimethylamino protons and presence of signals due to the ethoxy protons. In addition, its 13C-NMR spectrum revealed presence of ethoxy carbons. Moreover, its mass spectrum showed a peak at m/z 523 due to its molecular ion.
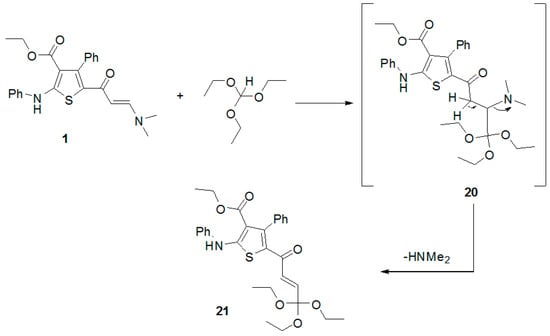
Scheme 5.
Synthesis of thiophene derivative 21.
2.2. Antimicrobial Evaluation
The newly synthesized compounds were evaluated in vitro for their antibacterial activity against Streptococcus pneumonia and Bacillis subtilis as examples of Gram-positive bacteria, and Pseudomonas aeruginosa and Escherichia coli as examples of Gram-negative bacteria. Additionally, they were evaluated in vitro for their antifungal activity against Aspergillus fumigates, Syncephalastrum racemosum, Geotricum candidum, and Candida albicans. Ampicillin and Gentamicin were used as reference drugs for antibacterial activity, and Amphotericin B was used as reference drugs for antifungal activity [31]. These assays were conducted at the Regional Center for Mycology and Biotechnology of Al-Azhar University, Cairo, Egypt. The antimicrobial results are expressed as the mean zone of inhibition in mm ± standard deviation beyond well diameter (6 mm) produced on the microorganisms, shown in Table 1 and Table 2.

Table 1.
Antibacterial activity of the synthesized compounds.

Table 2.
Antifungal activity of the synthesized compounds.
The results in Table 1 revealed that compound 16 with a pyrazole moiety revealed a high degree of antibacterial activity towards Pseudomonas aeruginosa and inhibition effects against Escherichia coli.
Data from the antifungal evaluation in Table 2 shows that compounds 9, 12, and 19 were found to be more potent than the standard drug, Amphotericin B, against Aspergillus fumigates. Additionally, compound 12 was more potent than Amphotericin B against Syncephalastrum racemosum. All the compounds revealed moderate activity towards Candida albicans.
2.3. Structure Activity Relationship (SAR)
By inspection of the experimental results of the antimicrobial activity of the synthesized compounds, the following structural activity relationship assumptions are suggested.
- The pyrazole moiety in compound 16 is necessary to have higher antibacterial activities towards Pseudomonas aeruginosa, and Escherichia coli.
- It is interesting to point out that 4-chlorophenylaminoacryloylmoiety in 9, pyridine moiety in 12, and triazolo[3,4-c]triazine moiety in 19 are necessary to have higher antifungal activity against Aspergillus fumigates.
In general, the above-mentioned results suggest that the new thiophene derivatives 9, 12 and 19 may provide valuable leads for the synthesis and development of novel antifungal agents.
3. Experimental Section
3.1. Chemistry
3.1.1. General
All the chemicals were purchased from various suppliers, including Sigma-Aldrich (St. Louis, MO, USA), and were used without further purification, unless otherwise stated. Melting points were measured on a Gallenkamp melting point apparatus (Thermo Fisher Scientific, Paisley, UK) in open glass capillaries and are uncorrected. Infrared spectra (IR) were recorded using the KBr disc technique using a Perkin Elmer FT-IR spectrophotometer 1000 (PerkinElmer, Waltham, MA, USA). 1H- and 13C-NMR spectra were recorded on either a JEOL ECP 400 NMR spectrometer (Tokyo, Japan) operating at 400 MHz or on a JEOL ECP 300 NMR spectrometer operating at 300 MHz in deuterated chloroform (CDCl3) or dimethyl sulphoxide (DMSO-d6). Chemical shifts are referred to in terms of ppm and J-coupling constants are given in Hz. Mass spectra were recorded on a Shimadzu GCMS-QP 1000 EX mass spectrometer (Tokyo, Japan) at 70 eV. Elemental analysis was carried out on a Perkin Elmer 2400 Elemental Analyzer; CHN mode. The biological evaluations of the products were carried out in the Medical Mycology Laboratory of the Regional Center for Mycology and Biotechnology of Al-Azhar University, Cairo, Egypt.
3.1.2. Synthetic Procedures
Reaction of Enaminone 1 with Heterocyclic and Aromatic Amines
General procedure: A mixture of enaminone 1 (0.42 g, 1 mmol) and the appropriate amine derivatives 2 or 5 or 8 (1 mmol each), in absolute ethanol (20 mL) were heated under reflux for 6 h and then left to cool. The precipitated solid product was filtered off, dried and recrystallized from EtOH to afford the corresponding thiophene derivatives 4, 7 and 9, respectively.
Ethyl 5-([1,2,4]triazolo[1,5-a]pyrimidin-7-yl)-4-phenyl-2-(phenylamino)thiophene-3-carboxylate (4). Yield (90%), yellow powder, m.p. 230–232 °C; IR (KBr) v 3422 (NH), 1619 (C=O) cm−1; 1H-NMR (CDCl3, 300 MHz) δ 0.71 (t, 3H, CH3, J = 7.0 Hz), 3.90 (q, 2H, CH2, J = 7.0 Hz), 6.5 (d, 1H, CH, J = 12 Hz), 7.02–8.01 (m, 12H, ArH), 10.62 (s, 1H, NH); MS m/z (%): 442 (2.7%), 441 (M+, 8.5%), 440 (5.5%), 194 (99.9%). Anal Calcd. for C24H19N5O2S (441.50): C, 65.29; H, 4.34; N, 15.86. Found: C, 65.18; H, 4.22; N, 15.92%.
Ethyl 5-(benzimidazo[1,2-a]pyrimidin-4-yl)-4-phenyl-2-(phenylamino)thiophene-3-carboxylate (7). Yield (85%), yellow powder, m.p. 214–216 °C; IR (KBr) v 3428 (NH), 1632 (C=O) cm−1; 1H-NMR (CDCl3, 300 MHz) δ 0.71 (t, 3H, CH3, J = 7.0 Hz), 3.89 (q, 2H, CH2, J = 7.0 Hz), 7.19–7.35 (m, 14H, ArH), 7.74 (d, 1H, J = 12 Hz), 8.12 (d, 1H, J = 12 Hz), 10.62 (s, 1H, NH); MS m/z (%): 490 (M+, 4.1%), 368 (99.9%), 255 (19.9%), 105 (35.9%). Anal Calcd. for C29H22N4O2S (490.58): C, 71.00; H, 4.52; N, 11.42. Found: C, 71.12; H, 4.48; N, 11.35%.
Ethyl 5-(3-(4-chlorophenylamino)acryloyl)-4-phenyl-2-(phenylamino)thiophene-3-carboxylate (9). Yield (92%), yellow powder, m.p. 222–225 °C; IR (KBr) v 3426 (NH), 1652, 1622 (2 C=O) cm−1; 1H-NMR (CDCl3, 300 MHz) δ 0.73 (t, 3H, CH3, J = 7.0 Hz), 3.90 (q, 2H, CH2, J = 7.0 Hz), 4.63 (d, 1H, CH, J = 8 Hz), 6.82–7.44 (m, 15H, ArH), 10.68 (s, 1H, NH), 11.70 (s, 1H, NH); MS m/z (%) 502 (M+, 2.5%), 460 (46.5%), 353 (99.9%), 309 (15.5%). Anal Calcd. for C28H23ClN2O3S (503.01): C, 66.86; H, 4.61; N, 5.57. Found: C, 66.75; H, 4.48; N, 5.65%.
Ethyl 5-(5-acetyl-6-methylpyridin-2-yl)-4-phenyl-2-(phenylamino)thiophene-3-carboxylate (12). Ammonium acetate (0.156 g, 2 mmol) was added to a solution of enaminone 1 (0.42 g, 1 mmol) and 2,4-pentanedione (0.1 g, 0.1 mL, 1 mmol) in glacial acetic acid (10 mL). The reaction mixture was heated under reflux for 6 h. After cooling, the reaction mixture was poured onto ice water mixture then the formed solid product was filtered and recrystallized from ethanol to afford compound 12 as deep yellow needles, in yield (97%), m.p. 197–200 °C; IR (KBr) v 3424 (NH), 1708, 1675 (2C=O) cm−1; 1H-NMR (CDCl3, 300 MHz) δ 0.74 (t, 3H, CH3, J = 7.0 Hz), 2.44 (s, 3H, CH3), 2.72 (s, 3H, CH3), 3.92 (q, 2H, CH2, J = 7.0 Hz), 6.24 (d, 1H, J = 8.4 Hz), 7.11–7.46 (m, 11H, ArH), 10.54 (s, 1H, NH); MS m/z (%) 456 (M+, 55.1%), 259 (99.9%), 238 (27.9%), 109 (55.1%), 55 (66.2%). Anal Calcd. for C27H24N2O3S (456.56): C, 71.03; H, 5.30; N, 6.14. Found: C, 71.15; H, 5.21; N, 6.22%.
Ethyl 5-(3-acetyl-1-phenyl-1H-pyrazole-4-carbonyl)-4-phenyl-2-(phenylamino)thiophene-3-carboxylate (16). Triethylamine (2 mmol) was added to a mixture of enaminone 1 (0.42 g, 1 mmol) and the hydrazonoyl chloride 14 in dry benzene (20 mL). The resulting reaction mixture was heated under reflux for 8 h then left to cool. The solvent was distilled under reduced pressure. The residue was triturated with methanol and the solid product was collected by filtration, washed with methanol and recrystallized from ethanol to afford the pyrazole derivative 16 as pale brown powder, in yield (89%), m.p. 205–206 °C; IR(KBr) v 3445 (NH), 1684, 1653, 1619 (3C=O) cm−1; 1H-NMR (CDCl3, 300 MHz) δ 0.6 (t, 3H, CH3, J = 7.3 Hz), 2.64 (s, 3H, CH3), 3.8 (q, 2H, CH2, J = 7.3 Hz), 6.89–7.43 (m, 15H, ArH), 7.46 (s, 1H, CH), 10.78 (s, 1H, NH); MS m/z (%) 535 (M+, 85.2%), 456 (99.9%), 413 (68.4%), 217 (51.1%). Anal Calcd. for C31H25N3O4S (535.61): C, 69.52; H, 4.70; N, 7.85. Found: C, 69.38; H, 4.56; N, 7.86%.
Ethyl 5-([1,2,4]triazolo[3,4-c][1,2,4]triazine-6-carbonyl)-4-phenyl-2-(phenyl amino)thiophene-3-carboxylate (19). To a cold solution of the enaminone 1 (0.42 g, 1mmol) in pyridine (10 mL) was added portion wise the diazonium salt of aminotriazole 17 (prepared by diazotizing 3-amino-1H-[1,2,4]triazole (0.084 g, 1 mmol) in concentrated HNO3 (2 mL) with a solution of sodium nitrite (0.07 g, 1 mmol) in H2O (2 mL). After complete addition of the diazonium salt the reaction mixture was stirred in an ice bath for 30 min then filtered off, dried, and recrystallized from EtOH to afford the title compound as deep yellow powder (92% yield), m.p. >300 °C; IR (KBr) v 3418 (NH), 1657, 1622 (2C=O) cm−1; 1H-NMR (CDCl3, 300 MHz) δ 0.71 (t, 3H, CH3, J = 7.0 Hz), 3.88 (q, 2H, CH2, J = 7.0 Hz), 7.50–7.25 (m, 10H, ArH), 8.45 (s, 1H, CH), 9.00 (s, 1H, CH), 10.61 (s, 1H, NH); MS m/z (%) 470 (M+, 1.8%) for C24H18N6O3S, 455 (75.3%), 227 (35.4%), 155 (99.9%), 91 (91.1%), 69 (35.2%). Anal Calcd. for C24H18N6O3S (470.50): C, 61.27; H, 3.86; N, 17.86. Found: C, 61.38; H, 3.77; N, 17.93%.
(E)-Ethyl 4-phenyl-2-(phenylamino)-5-(4,4,4-triethoxybut-2-enoyl)thiophene-3-carboxylate (21). A mixture of enaminone derivative 1 (0.42 g, 1 mmol) and triethylorthoformate (2 mL) was heated under reflux for 2 h. After cooling, ethanol was added and the formed solid product was filtered off and recrystallized from ethanol to afford pale red needles (86% yield), m.p. 204–206 °C; IR (KBr) v 3462 (NH), 1658, 1619 (2C=O), 1554 (C=C) cm−1; (DMSO-d6, 400 MHz) δ 0.66 (t, 3H, CH3, J = 7.3 Hz), 1.24 (t, 9H, 3CH3, J = 7.3 Hz), 3.3 (q, 6H, 3CH2, J = 7.3 Hz), 3.85 (q, 2H, CH2, J = 7.3 Hz), 4.31 (d, 1H, CH, J = 12.4 Hz, CHα=CHβ), 5.11 (d, 1H, CH, J = 12.4 Hz, CHα=CHβ), 7.2–7.4 (m, 10H, ArH), 10.28 (s, 1H, NH); 13C-NMR (DMSO-d6, 100 MHz;) 179.9, 162.4, 157.8, 149.7, 147.3, 140.4, 135.9, 133.0, 128.4, 127.9, 127.8, 126.5, 126.1, 120.8, 118.4, 117.8, 92.6, 58.9, 53.4, 22.7, 12.2; MS m/z (%) 523 (M+, 1.3%), 511 (16%), 270 (40%), 180 (29.8%), 91 (99.9%). Anal Calcd. for C29H33NO6S (523.64): C, 66.52; H, 6.35; N, 2.67. Found: C, 66.48; H, 6.42; N, 2.55%.
3.2. Agar Diffusion Well Method to Determine the Antimicrobial Activity
The microorganism inoculums were uniformly spread using sterile cotton swabs on a sterile Petri dish malt extract agar (for fungi) and nutrient agar (for bacteria). One hundred microliters of each sample were added to each well (10 mm diameter holes cut in the agar gel, 20 mm apart from one another). Then, the systems were incubated for 24–48 h at 37 °C (for bacteria) and at 28 °C (for fungi). After incubation, the microorganism’s growth was observed. Inhibition of the bacterial and fungal growth were measured in mm. Tests were performed in triplicate [31].
4. Conclusions
In conclusion various thiophene derivatives were synthesized in efficient and easy protocol. Antimicrobial studies revealed that compounds 9, 12, and 19 showed a higher activity towards Aspergillus fumigates than the standard drug Amphotericin B.
Acknowledgments
The authors extend their sincere appreciation to the Deanship of Scientific Research at the King Saud University for its funding of this Prolific Research Group (PRG-1437-29).
Author Contributions
Yahia Nasser Mabkhot conceived and designed the experiments; Fatima Alatibi performed the experiments; Nahed Nasser E. El-Sayed, Nabila Abdelshafy Kheder and Salim S. Al-Showiman analyzed the data and helped in results and discussion.
Conflicts of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- Abdel-Wahab, B.F.; Abdel-Gawad, H.; Ghada, E.A.A.; Badria, F.A. Synthesis, antimicrobial, antioxidant, antiinflammatory and analgesic activities of some new 3-(2′-thienyl)pyrazolebased heterocycles. Med. Chem. Res. 2012, 21, 1418–1426. [Google Scholar] [CrossRef]
- Bonini, C.; Chiummiento, L.; Bonis, M.D.; Funicello, M.; Lupattelli, P.; Suanno, G.; Berti, F.; Campaner, P. Synthesis, biological activity and modelling studies of two novel anti HIV PR inhibitors with a thiophene containing hydroxyethylamino core. Tetrahedron 2005, 61, 6580–6589. [Google Scholar] [CrossRef]
- Geronikaki, A.; Theophilidis, G. Synthesis of 2-(aminoacetylamino)thiazole derivatives and comparison of their local anaesthetic activity by the method of action potential. Eur. J. Med. Chem. 1992, 27, 709–716. [Google Scholar] [CrossRef]
- Rejon, G.J.M.; Castell, R.O.M. Synthesis and primary evaluation of the bactericide and fungicide activity of some 5-halofuranic and 5-halo-4-phenylthiazolic derivatives. Afinidad 1993, 50, 319–322. [Google Scholar]
- Ahmad, V.U.; Alam, N. New antifungal bithienylacetylenes from Blumea obliqua. J. Nat. Prod. 1995, 58, 1426–1429. [Google Scholar] [CrossRef] [PubMed]
- Giridhar, T.; Reddy, R.B.; Prasanna, B.; Chandra Mouli, G.V.P. Aminothiazoles: Part 1 Synthesis and pharmacological evaluation of 4-[isobutylphenyl]-2-substituted aminothiazoles. Indian J. Chem. 2001, 40, 1279–1281. [Google Scholar]
- Lu, X.; Wan, B.; Franzblau, S.G.; You, Q. Design, synthesis and anti-tubercular evaluation of new 2-acylated and 2-alkylated amino-5-(4-(benzyloxy)phenyl)thiophene-3-carboxylic acid derivatives. Eur. J. Med. Chem. 2011, 46, 3551–3563. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, N.K.; Kim, H.S.; Chae, Y.J.; Lee, Y.N.; Kwon, G.; Choi, E.H.; Kim, I.T. Synthesis and Anticancer Activity of Di(3-thienyl)methanol and Di(3-thienyl)methane. Molecules 2012, 17, 11456–11468. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.D.; Johnson, S.; Powell, D.; McGinnis, J.P.; Miranda, M.; Rabindran, S.K. Inhibition of tumor cell proliferation by thieno[2,3-d]pyrimidin-4(1H)-one-based analogs. Bioorg. Med. Chem. Lett. 2005, 15, 3763–3766. [Google Scholar] [CrossRef] [PubMed]
- Jennings, L.D.; Kincaid, S.L.; Wang, Y.D.; Krishnamurthy, G.; Beyer, C.F.; McGinnis, J.P.; Miranda, M.; Discafani, C.M.; Rabindran, S.K. Parallel synthesis and biological evaluation of 5,6,7,8-tetrahydrobenzothieno[2,3-d]pyrimidin-4(3H)-one cytotoxic agents selective for p21-deficient cells. Bioorg. Med. Chem. Lett. 2005, 15, 4731–4735. [Google Scholar] [CrossRef] [PubMed]
- Aubry, M.L.; Cowell, P.; Davey, M.J.; Shevde, S. Aspects of the pharmacology of a new anthelmintic: Pyrantel. Br. J. Pharmacol. 1970, 38, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wei, X.; Xu, H.J. Photoactivated Insecticidal Thiophene Derivatives from Xanthopappus subacaulis. Nat. Prod. 2006, 69, 1241–1244. [Google Scholar]
- Shen, Y.; Zhao, Y.; Li, Z.; Wang, J.; Qiu, L.; Liu, S.; Zhai, J.; Zhou, J. The synthesis of highly active thiophene ring-containing chromophore components for photonic polymers based on a newly designed route. J. Chem. Soc. Perkin Trans. 1 1999, 3691–3695. [Google Scholar] [CrossRef]
- Zhiryakov, V.G.; Abramenko, P.I. Polymethylene dyes derived from heterocyclic bases containing condensed thiophene rings IV. Derivatives of thienopyridines-5 and-6. Chem. Heterocycl. Compd. 1967, 3, 501–504. [Google Scholar] [CrossRef]
- Heeney, M.; Bailey, C.; Genevicius, K.; Shkunov, M.; Sparrowe, D.; Tierney, S.; McCulloch, I. Stable Polythiophene Semiconductors Incorporating Thieno[2,3-b]thiophene. J. Am. Chem. Soc. 2005, 127, 1078–1079. [Google Scholar] [CrossRef] [PubMed]
- Kheder, N.A.; Mabkhot, Y.N.; Farag, A.M. Facile and Convenient Synthesis of Pyrazole, Pyridine, Pyridazine, Pyrazolo[3,4-b]pyridine, and Pyrazolo[5,1-c][1,2,4]triazine Derivatives. Synth. Commun. 2008, 38, 3170–3182. [Google Scholar] [CrossRef]
- Mabkhot, Y.N.; Kheder, N.A.; Al-Majid, A.M. Facile and convenient synthesis of new thieno[2,3-b]-thiophene derivatives. Molecules 2010, 15, 9418–9426. [Google Scholar] [CrossRef] [PubMed]
- Mabkhot, Y.N.; Brakat, A.; Alatibi, F.A.; Choudharyc, M.I.; Yousuf, S. (E)-Ethyl 2-anilino-5-[3-(dimethylamino)-acryloyl]-4-phenylthiophene-3-carboxylate. Acta Crystallogr. Sect. E 2013, 69. [Google Scholar] [CrossRef] [PubMed]
- Mabkhoot, Y.N. Synthesis and analysis of some bis-heterocyclic compounds containing sulphur. Molecules 2009, 14, 1904–1914. [Google Scholar] [CrossRef] [PubMed]
- Kheder, N.A.; Mabkhot, Y.N. Synthesis and Antimicrobial Studies of Some Novel bis-[1,3,4]thiadiazole and bis-thiazole Pendant to Thieno[2,3-b]thiophene moiety. Int. J. Mol. Sci. 2012, 13, 3661–3670. [Google Scholar] [CrossRef] [PubMed]
- Mabkhot, Y.N.; Kheder, N.A.; Farag, A.M. Synthesis and Antimicrobial Activity of Some New Thieno[2,3-b]thiophene Derivatives. Molecules 2013, 18, 4669–4678. [Google Scholar] [CrossRef] [PubMed]
- Mabkhot, Y.N.; Alatibi, F.; El-Sayed, N.N.; Al-Showiman, S.; Kheder, N.A.; Wadood, A.; Rauf, A.; Bawazeer, S.; Hadda, T. Antimicrobial Activity of Some Novel Armed Thiophene Derivatives and Petra/Osiris/Molinspiration (POM) Analyses. Molecules 2016, 21, 222. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, W.; Platz, L. Ueber eine neue Bildungsweise von Osotetrazonen. Ber. Dtsch. Chem. Ges. 1905, 38, 2986–2990. [Google Scholar] [CrossRef]
- Abushamleh, A.S.; Al-Aqarbeh, M.M.; Day, V. Transition Metal Complexes of Derivatized Chiral Dihydro-1,2,4-triazin-6-ones. Template Synthesis of Nickel(II) Tetraaza-(4N-M) Complexes Incorporating the Triazinone Moiety. Am. J. Appl. Sci. 2008, 5, 750–754. [Google Scholar] [CrossRef]
- Komarova, E.S.; Makarov, V.A.; Alekseeva, G.V.; Granik, V.G. Synthesis of derivatives of a new heterocyclic system pyrazolo[3,4-b]pyrido[1′,2′:1,2]imidazo[4,5-d]pyridine. Russ. Chem. Bull. 2006, 55, 735–740. [Google Scholar] [CrossRef]
- He, F.Q.; Liu, X.H.; Wang, B.L.; Li, Z.M. Synthesis and biological activities of novel bis-heterocyclic pyrrodiazole derivatives. Heteroat. Chem. 2008, 19, 21–27. [Google Scholar] [CrossRef]
- Amer, F.A.; Hammouda, M.; El-Ahl, A.S.; Abdelwahab, B.F. Synthesis of Important New Pyrrolo[3,4-c]pyrazoles and Pyrazolyl-Pyrrolines from Heterocyclic β-Ketonitriles. J. Chin. Chem. Soc. 2007, 54, 1543–1552. [Google Scholar] [CrossRef]
- Salaheldin, A.M.; Al-Sheikh, M.A. β-Enamino Esters in Heterocyclic Synthesis: Synthesis of Pyrazolone and Pyridinone Derivatives. Molecules 2010, 15, 4359–4368. [Google Scholar] [CrossRef] [PubMed]
- Al-Shiekh, M.A.; Salah El-Din, A.M.; Hafez, E.A.; Elnagdi, M.H. α-Enones in heterocyclic synthesis, Part I. Classical synthetic and environmentally friendly synthetic approaches to alkyl and acyl azoles and azines. J. Chem. Res. 2004, 174–179. [Google Scholar] [CrossRef]
- Riyadh, S.M. Enaminones as Building Blocks for the Synthesis of Substituted Pyrazoles with Antitumor and Antimicrobial Activities. Molecules 2011, 16, 1834–1853. [Google Scholar] [CrossRef] [PubMed]
- Smânia, A., Jr.; Monache, F.D.; Smânia, E.F.A.; Cuneo, R.S. Antibacterial activity of steroidal compounds isolated from Ganoderma applanatum (Pers.) Pat. (Aphyllophoromycetideae) fruit body. Int. J. Med. Mushrooms 1999, 1, 325–330. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).