New Isoxazolidine-Conjugates of Quinazolinones—Synthesis, Antiviral and Cytostatic Activity
Abstract
:1. Introduction
2. Results and Discussion
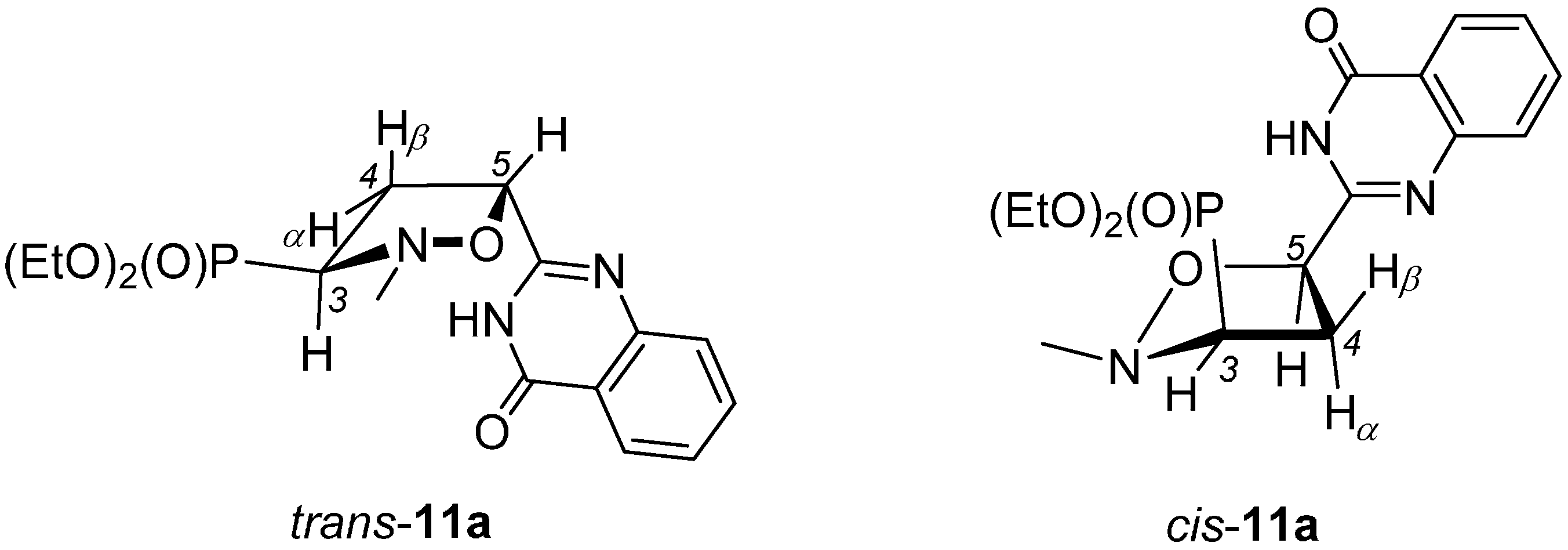
2.1. Chemistry
2.2. Antiviral and Cytostatic Evaluation
2.2.1. Antiviral Activity
2.2.2. Cytostatic Activity
3. Experimental Section
3.1. General
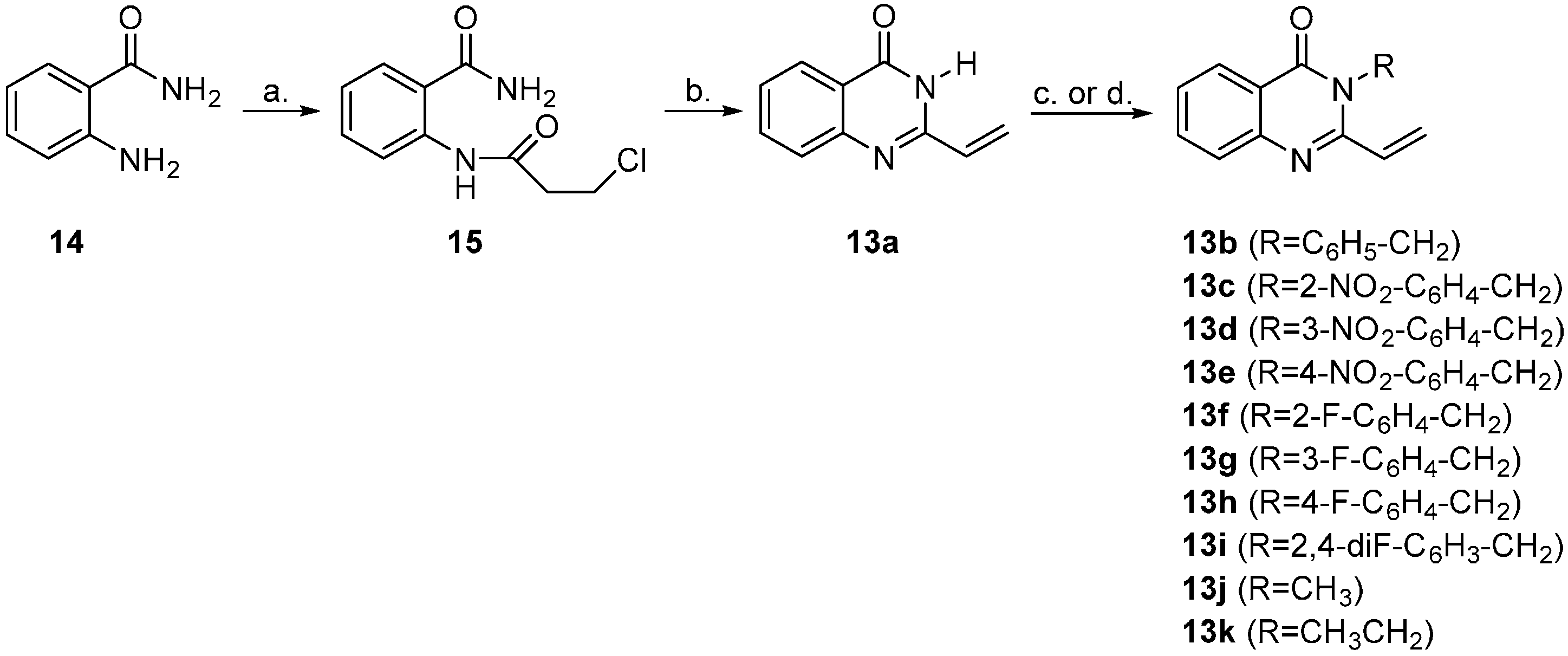
3.2. General Procedure for the Synthesis of N3-Benzylated 2-Vinyl-3H-quinazolin-4-ones 13b–i
3.3. General Procedure for the Synthesis of N3-Alkylated 2-Vinyl-3H-quinazolin-4-ones 13j and 13k
3.4. General Procedure for the Synthesis of Isoxazolidines Trans-11 and Cis-11
3.5. Antiviral Activity Assays
3.6. Cytostatic Activity against Immortalized Cell Lines
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hammer, H.; Bader, B.M.; Ehnert, C.; Bundgaard, C.; Bunch, L.; Hoestgaard-Jensen, K.; Schroeder, O.H.-U.; Bastlund, J.F.; Gramowski-Voss, A.; Jensen, A.A. A multifaceted GABAA receptor modulator: Functional properties and mechanism of action of the sedative-hypnotic and recreational drug methaqualone (quaalude). Mol. Pharmacol. 2015, 88, 401–420. [Google Scholar] [CrossRef] [PubMed]
- Tyurenkov, I.N.; Ozerov, A.A.; Solodunova, E.A.; Archakova, Y.V.; Shmatova, E.N.; Sampieva, K.T. Synthesis and Anxiosedative and Antidepressant Properties of α-(4-Oxoquinazolin-3(4H)-yl)carboxylic Acid Anilides. Pharm. Chem. J. 2013, 47, 239–242. [Google Scholar] [CrossRef]
- Kashaw, S.K.; Gupta, V.; Kashaw, V.; Mishra, P.; Stables, J.P.; Jain, N.K. Anticonvulsant and sedative-hypnotic activity of some novel 3-(5-(4-substituted) phenyl-1,3,4-oxadiazole-2yl)-2-styrylquinazoline-4(3H)-ones. Med. Chem. Res. 2010, 19, 250–261. [Google Scholar] [CrossRef]
- Chandregowda, V.; Kus, A.K.; Chandrasekara Reddy, G. Synthesis and in vitro antitumor activities of novel 4-anilinoquinazoline derivatives. Eur. J. Med. Chem. 2009, 44, 3046–3055. [Google Scholar] [CrossRef] [PubMed]
- Abbas, S.E.; Abdel Gawad, N.M.; Georgey, H.H.; Abdullah, J.H. New Quinazolinone Derivatives: Synthesis, Anti-inflammatory and Antitumor Activities. Int. J. Chemtech Res. 2010, 2, 1560–1578. [Google Scholar]
- Noolvi, M.N.; Patel, H.M.; Bhardwaj, V.; Chauhan, A. Synthesis and in vitro antitumor activity of substituted quinazoline and quinoxaline derivatives: Search for anticancer agent. Eur. J. Med. Chem. 2011, 46, 2327–2346. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.F.; Youns, M. Synthesis and Biological Evaluation of a Novel Series of 6,8-Dibromo-4(3H)quinazolinone Derivatives as Anticancer Agents. Arch. Pharm. Chem. Life Sci. 2013, 346, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.D.; Singh, J.; Kinger, M.; Arora, A.K.; Jaswal, V.S. Synthesis and antiviral activities of some 2,3-disubstituted quinazoline derivatives. Asian J. Chem. 2015, 27, 4379–4382. [Google Scholar] [CrossRef]
- Chen, M.; Li, P.; Hu, D.; Zeng, S.; Li, T.; Jin, L.; Xue, W.; Song, B. Synthesis, antiviral activity, 3D-QSAR, and interaction mechanisms study of novel malonate derivatives containing quinazolin-4(3H)-one moiety. Bioorg. Med. Chem. Lett. 2016, 26, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Li, P.; Chen, M.; Dong, L.; Hu, D.; Song, B. Synthesis, anti-tobacco mosaic virus and cucumber mosaic virus activity, and 3D-QSAR study of novel 1,4-pentadien-3-one derivatives containing 4-thioquinazoline moiety. Eur. J. Med. Chem. 2015, 102, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Swastika, G.; Ravichandran, V.; Pandy, V. Synthesis, antiviral and antimicrobial activities of quinazoline urea analogues. Int. J. Drug Des. Discov. 2013, 4, 1215–1230. [Google Scholar]
- Schroeder, C.E.; Yao, T.; Sotsky, J.; Smith, R.A.; Roy, S.; Chu, Y.-K.; Guo, H.; Tower, N.A.; Noah, T.J.W.; McKellip, S.; et al. Development of (E)-2-((1,4-Dimethylpiperazin-2-ylidene)amino)-5-nitro-N-phenylbenzamide, ML336: Novel 2-Amidinophenylbenzamides as Potent Inhibitors of Venezuelan Equine Encephalitis Virus. J. Med. Chem. 2014, 57, 8608–8621. [Google Scholar] [CrossRef] [PubMed]
- Rohini, R.; Reddy, P.M.; Shanker, K.; Hu, A.; Ravinder, V. Antimicrobial study of newly synthesized 6-substituted indolo(1,2-c)quinazolines. Eur. J. Med. Chem. 2010, 45, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Raju, G.N.; Sai, K.B.; Resshma, V.; Sudarshini, N.; Sowmya, P.L.; Nalini, Y.; Nadendla, R.R. Potential antimicrobial activities of quinazolinone derivatives. J. Chem. Pharm. Res. 2015, 7, 1279–1287. [Google Scholar]
- Rakesh, K.P.; Ramesh, S.; Kumar, H.M.M.; Chandan, S.; Gowda, D.C. Quinazolinones linked amino acids derivatives as a new class of promising antimicrobial, antioxidant and anti-inflammatory agents. Eur. J. Chem. 2015, 6, 254–260. [Google Scholar] [CrossRef]
- Kumar, R.; Shukla, R.K.; Shukla, A.; Tyagi, N. Synthetic and Pharmacological Evaluation of Some 3-(4-{((Substitutedphenyl)methylene)amino}phenyl)-6-bromo-2-methylquinazolin-4-one derivatives. World J. Pharm. Res. 2015, 4, 1984–1991. [Google Scholar]
- Serya, R.A.T.; Abbas, A.H.; Ismail, N.S.M.; Esmat, A.; Abou El Ella, D.A. Design, synthesis and biological evaluation of novel quinazoline-based anti-inflammatory agents acting as PDE4B inhibitors. Chem. Pharm. Bull. 2015, 63, 102–116. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, K.P.; Manukumar, H.M.; Gowda, D.C. Schiff's bases of quinazolinone derivatives: Synthesis and SAR studies of a novel series of potential anti-inflammatory and antioxidants. Bioorg. Med. Chem. Lett. 2015, 25, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Lal, R.; Rani, S. Synthesis of some new substituted azetidinonyl and thiazolidinonyl quinazolin-4(3H)-ones as potential non-steroidal anti-inflammatory and analgesic agents. IJIAS 2014, 8, 1798–1813. [Google Scholar]
- Pines, M.; Spector, I. Halofuginone—The Multifaceted Molecule. Moecules 2015, 20, 573–594. [Google Scholar] [CrossRef] [PubMed]
- Marzaro, G.; Castagliuolo, I.; Schirato, G.; Palu, G.; Dalla Via, M.; Chilin, A.; Brun, P. Substituted quinazolinones as kinase inhibitors endowed with anti-fibroic properties. Eur. J. Med. Chem. 2016, 115, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Nagarajan, R. Deep eutectic solvent mediated synthesis of quinazolinones and dihydroquinazolinones: Synthesis of natural products and drugs. RSC Adv. 2016, 6, 27378–27387. [Google Scholar] [CrossRef]
- Asif, M. Chemical Characteristics, Synthetic Methods, and Biological Potential of Quinazoline and Quinazolinone Derivatives. Int. J. Med. Chem. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Chawla, A.; Batra, C. Recent advances of quinazolinone derivatives as marker for various biological activities. Int. Res. J. Pharm. 2013, 4, 49–58. [Google Scholar] [CrossRef]
- Rajput, R.; Mishra, A.P. A Review on Biological Activity of Quinazolinones. Int. J. Pharm. Sci. 2012, 4, 66–70. [Google Scholar]
- Rajput, R.; Mishra, A.P. Quinazolinones as antimicrobial agents: A review. Int. J. Res. Pharm. Biomed. Sci. 2012, 3, 82–89. [Google Scholar]
- Arora, R.; Kappor, A.; Gill, N.S.; Rana, A.C. Quinazolinone: An overview. Int. Res. J. Pharm. 2011, 2, 22–28. [Google Scholar]
- Selvam, T.; Kumar, P.V. Quinazoline marketed drugs—A Review. Res. Pharm. 2011, 1, 1–21. [Google Scholar]
- Vogtle, M.M.; Marzinzik, A.L. Synthetic Approaches towards Quinazolines, Quinazolinones and Quinazolinediones on Solid Phase. QSAR Comb. Sci. 2004, 23, 440–459. [Google Scholar] [CrossRef]
- Shen, S.; Li, W.; Wang, J. A novel and other bioactive secondary metabolites from a marine fungus Penicillium oxalicum 0312F1. Nat. Prod. Res. 2013, 27, 2286–2291. [Google Scholar] [CrossRef] [PubMed]
- An, C.-Y.; Li, X.-M.; Li, C.-S.; Gao, S.-S.; Shang, Z.; Wang, B.-G. Triazoles and Other N-Containing Metabolites from the Marine-Derived Endophytic Fungus Pencillinum chrysogenum EN-118. Helv. Chim. Acta 2013, 96, 682–687. [Google Scholar] [CrossRef]
- Li, C.-S.; An, C.-Y.; Li, X.-M.; Gao, S.-S.; Cui, C.-M.; Sun, H.-F.; Wang, B.-G. Triazole and Dihydroimidazole Alkaloids from the Marine Sediment-Derived Fungus Penicillium paneum SD-44. J. Nat. Prod. 2011, 74, 1331–1334. [Google Scholar] [CrossRef] [PubMed]
- Kornsakulkarn, J.; Saepua, S.; Srijomthong, K.; Rachtawee, P.; Thongpanchang, C. Quinazolinone alkaloids from actinomycete Streptomyces sp. BCC 21795. Phytochem. Lett. 2015, 12, 6–8. [Google Scholar] [CrossRef]
- Liu, S.; Wang, W.; Jiang, L.; Wan, S.; Zhang, L.; Yu, R.; Jiang, T. 2-Pyridinyl-4(3H)-Quinazolinone: A Scaffold for Anti-influenza A Virus Compounds. Chem. Biol. Drug Des. 2015, 86, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.F.; Belal, A. Design, Synthesis, and Molecular Docking Studies of 2-(Furan-2-yl)-quinazolin-4-one Derivatives as Potential Antiproliferative Agents. Arch. Pharm. Chem. Life Sci. 2015, 348, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, D.G. N-Substituted C-diethoxyphosphorylated nitrones as useful synthons for the synthesis of α-aminophosphonates. Tetrahedron Lett. 2006, 47, 5363–5366. [Google Scholar] [CrossRef]
- Witt, A.; Bergman, J. Synthesis and reaction of some 2-vinyl-3H-quinazolin-4-ones. Tetrahedron 2000, 56, 7245–7253. [Google Scholar] [CrossRef]
- Makhloufi, A.; Wahl, M.; Frank, W.; Ganter, C. A new Mixed Amino-Amido N-Heterocyclic Carbene Based on Antranilin Acid. Organomettalics 2013, 32, 854–861. [Google Scholar] [CrossRef]
- Piotrowska, D.G.; Cieslak, M.; Krolewska, K.; Wróblewski, A.E. Design, synthesis and cytotoxicity of a new series of isoxazolidine based nucleoside analogues. Arch. Pharm. Chem. Life Sci. 2011, 11, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Grabkowska-Drużyc, M.; Balzarini, J.; Piotrowska, D.G. Design, synthesis, antiviral and cytostatic evaluation of novel isoxazolidine analogues of C-nucleotides. Nucleosides Nucleotides Nucleic Acids 2013, 32, 682–699. [Google Scholar] [CrossRef] [PubMed]
- Karplus, M. Vicinal Proton Coupling in Nuclear Magnetic Resonance. J. Am. Chem. Soc. 1963, 85, 2870–2871. [Google Scholar] [CrossRef]
- Benezra, C. NMR of phosphonates. VI. Variation of vicinal phosphorus-31-carbon-carbon-proton couplings with dihedral angle in phosphonates. J. Am. Chem. Soc. 1973, 95, 6890–6894. [Google Scholar] [CrossRef]
- Neeser, J.-R.; Tronchet, J.M.J.; Charollais, E.J. Structural analysis of 3-C-phosphonates, -phosphinates, and -phosphine oxides of branched-chain sugars. Can. J. Chem. 1983, 61, 2112–2120. [Google Scholar] [CrossRef]
- Adiwidjaja, G.; Meyer, B.; Thiem, J. Darstellung und Kristallstruktur von endo-2-Dimethylphosphono-exo-2-hydroxy-(–)-camphan zur Bestimmung von 3J(CCCP)-Vicinalkopplungen. Z. Naturforsch. 1979, 34, 1547–1551. [Google Scholar]
- Buchanan, G.W.; Bourque, K.; Seeley, A. 13C- and 31P-NMR spectra of 1-diethylphosphono-1-hydroxycycloalkanes. Magn. Res. Chem. 1986, 24, 360–367. [Google Scholar] [CrossRef]
- Hayato, N.; Noriki, K.; Takao, S. Functionalized Carbodiimide Mediated Synthesis of 2,3-Disubstituted Quinazolin-4(3H)-ones via the Tandem Strategy of C-Nucleophilic Addition and Intramolecular NH-Substitution Cyclization. Synthesis 2012, 44, 3179–3184. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
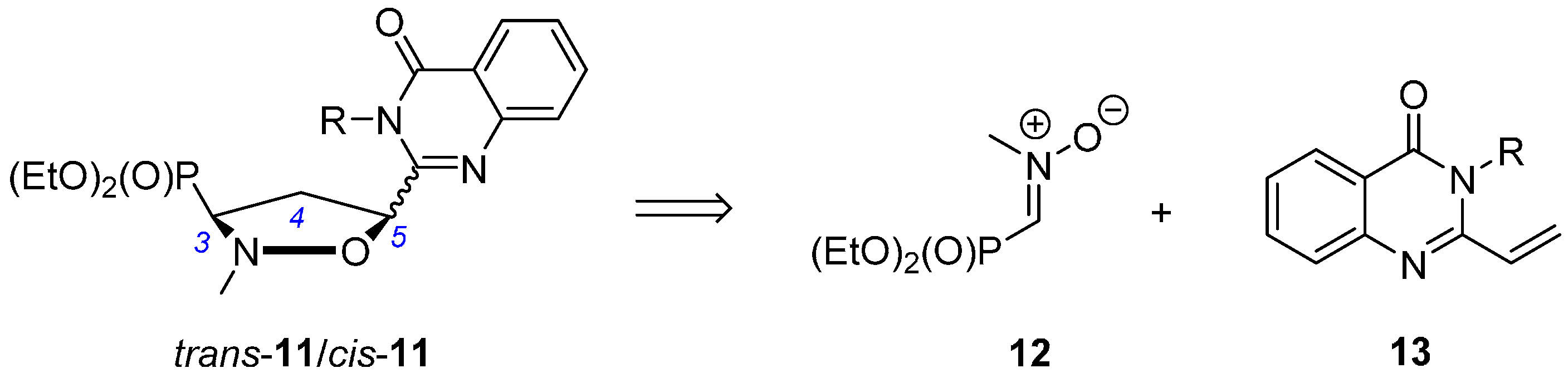
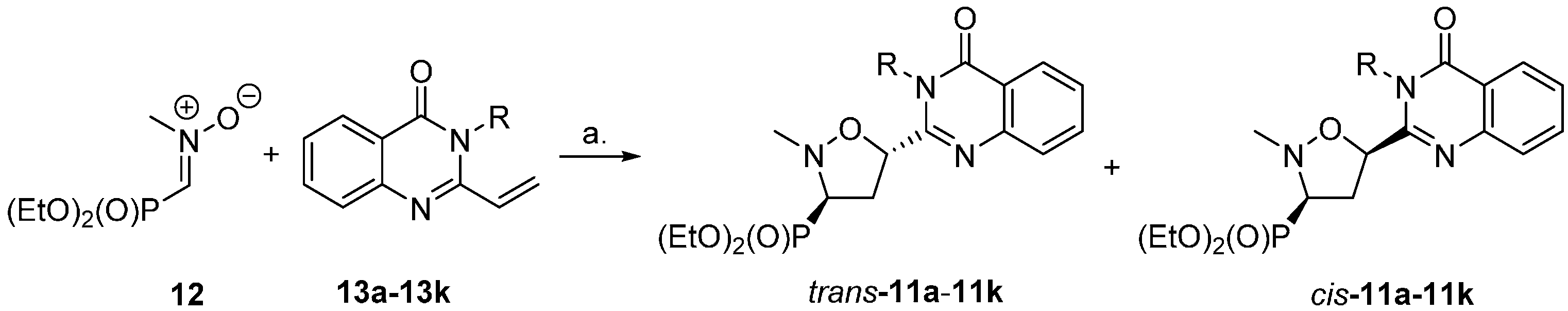
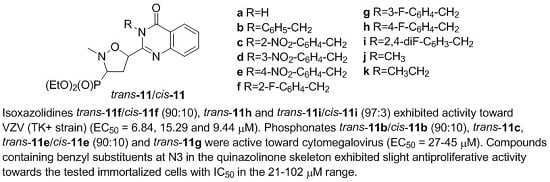
| Entry | Quinazolinone 13 | Ratio of Trans-11:Cis-11 | Yield (%) |
|---|---|---|---|
| R | |||
| a | H | 92:8 | trans-11a (23) a + trans-11a and cis-11a (70) b |
| b | C6H5-CH2 | 90:10 | trans-11b and cis-11b (84) b |
| c | 2-NO2-C6H4-CH2 | 90:10 | trans-11c (20) a + trans-11c and cis-11c (73) b |
| d | 3-NO2-C6H4-CH2 | 92:8 | trans-11d and cis-11d (98) b |
| e | 4-NO2-C6H4-CH2 | 90:10 | trans-11e and cis-11e (91) b |
| f | 2-F-C6H4-CH2 | 90:10 | trans-11f and cis-11f (94) b |
| g | 3-F-C6H4-CH2 | 90:10 | trans-11g (7) a + trans-11g and cis-11g (89) b |
| h | 4-F-C6H4-CH2 | 90:10 | trans-11h (22) a + trans-11h and cis-11h (70) b |
| i | 2,4-diF-C6H3-CH2 | 92:8 | trans-11i and cis-11i (94) b |
| j | Me | 94:6 | trans-11j (19) a + trans-11j and cis-11j (78) b |
| k | Et | 92:8 | trans-11k and cis-11k (86) b |
| Compound | R | Antiviral Activity EC50 (μM) a | Cytotoxicity (μM) | |
|---|---|---|---|---|
| TK+ VZV Strain | TK− VZV Strain | Cell Morphology MCC b | ||
| trans-11c | 2-NO2-C6H4-CH2 | 46.47 | 100 | >100 |
| trans-11e/cis-11e (90:10) | 4-NO2-C6H4-CH2 | 34.20 | 42.87 | 100 |
| trans-11f/cis-11f (90:10) | 2-F-C6H4-CH2 | 6.84 | >20 | 100 |
| trans-11h | 4-F-C6H4-CH2 | 15.29 | >20 | 100 |
| trans-11i/cis-11i (97:3) | 2,4-diF-C6H3-CH2 | 9.44 | >20 | 100 |
| trans-11k | CH3CH2 | 38.80 | 41.57 | >100 |
| Acyclovir | 0.71 | 39.69 | >100 | |
| Brivudin | 0.019 | 25.59 | >100 | |
| Compound | R | Antiviral Activity EC50 (μM) a | Cytotoxicity (μM) | |
|---|---|---|---|---|
| AD-169 Strain | Davis Strain | Cell Morphology MCC b | ||
| trans-11b/cis-11b (90:10) | C6H5-CH2 | 44.72 | >20 | ≥100 |
| trans-11c | 2-NO2-C6H4-CH2 | >100 | 44.72 | ≥20 |
| trans-11e/cis-11e (90:10) | 4-NO2-C6H4-CH2 | 44.72 | 20 | >100 |
| trans-11g | 3-F-C6H4-CH2 | >100 | 27.59 | 100 |
| Ganciclovir | 10.52 | 0.63 | >350 | |
| Cidofovir | 1.49 | 0.23 | >300 | |
| Compound | R | IC50 a (μM) | |||
|---|---|---|---|---|---|
| L1210 | CEM | HeLa | HMEC-1 | ||
| trans-11a | H | >250 | >250 | >250 | >250 |
| trans-11b/cis-11b (90:10) | C6H5-CH2 | 49 ± 23 | 28 ± 4 | 82 ± 5 | 83 ± 16 |
| trans-11c | 2-NO2-C6H4-CH2 | 87 ± 22 | 76 ± 9 | 97 ± 0 | 92 ± 1 |
| trans-11d/cis-11d (90:10) | 3-NO2-C6H4-CH2 | 28 ± 11 | 21 ± 4 | 50 ± 4 | 58 ± 0 |
| trans-11e/cis-11e (90:10) | 4-NO2-C6H4-CH2 | 59 ± 32 | 34 ± 12 | 76 ± 4 | 102 ± 3 |
| trans-11f/cis-11f (90:10) | 2-F-C6H4-CH2 | 33 ± 7 | 29 ± 13 | 76 ± 11 | 77 ± 0 |
| trans-11g | 3-F-C6H4-CH2 | 35 ± 12 | 26 ± 6 | 62 ± 9 | 74 ± 3 |
| trans-11h | 4-F-C6H4-CH2 | 26 ± 1 | 30 ± 12 | 58 ± 5 | 78 ± 1 |
| trans-11i/cis-11i (97:3) | 2,4-diF-C6H3-CH2 | 26 ± 2 | 24 ± 8 | 55 ± 10 | 67 ± 4 |
| trans-11j | Me | >250 | >250 | >250 | >250 |
| trans-11k/cis-11k (97:3) | Et | 101 ± 17 | 85 ± 13 | 97 ± 10 | 86 ± 11 |
| 5-Fluorouracil | 0.33 ± 0.17 | 18 ± 5 | 0.54 ± 0.12 | n.d. | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piotrowska, D.G.; Andrei, G.; Schols, D.; Snoeck, R.; Grabkowska-Drużyc, M. New Isoxazolidine-Conjugates of Quinazolinones—Synthesis, Antiviral and Cytostatic Activity. Molecules 2016, 21, 959. https://doi.org/10.3390/molecules21070959
Piotrowska DG, Andrei G, Schols D, Snoeck R, Grabkowska-Drużyc M. New Isoxazolidine-Conjugates of Quinazolinones—Synthesis, Antiviral and Cytostatic Activity. Molecules. 2016; 21(7):959. https://doi.org/10.3390/molecules21070959
Chicago/Turabian StylePiotrowska, Dorota G., Graciela Andrei, Dominique Schols, Robert Snoeck, and Magdalena Grabkowska-Drużyc. 2016. "New Isoxazolidine-Conjugates of Quinazolinones—Synthesis, Antiviral and Cytostatic Activity" Molecules 21, no. 7: 959. https://doi.org/10.3390/molecules21070959
APA StylePiotrowska, D. G., Andrei, G., Schols, D., Snoeck, R., & Grabkowska-Drużyc, M. (2016). New Isoxazolidine-Conjugates of Quinazolinones—Synthesis, Antiviral and Cytostatic Activity. Molecules, 21(7), 959. https://doi.org/10.3390/molecules21070959