Characterization and Prebiotic Effect of the Resistant Starch from Purple Sweet Potato
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Composition
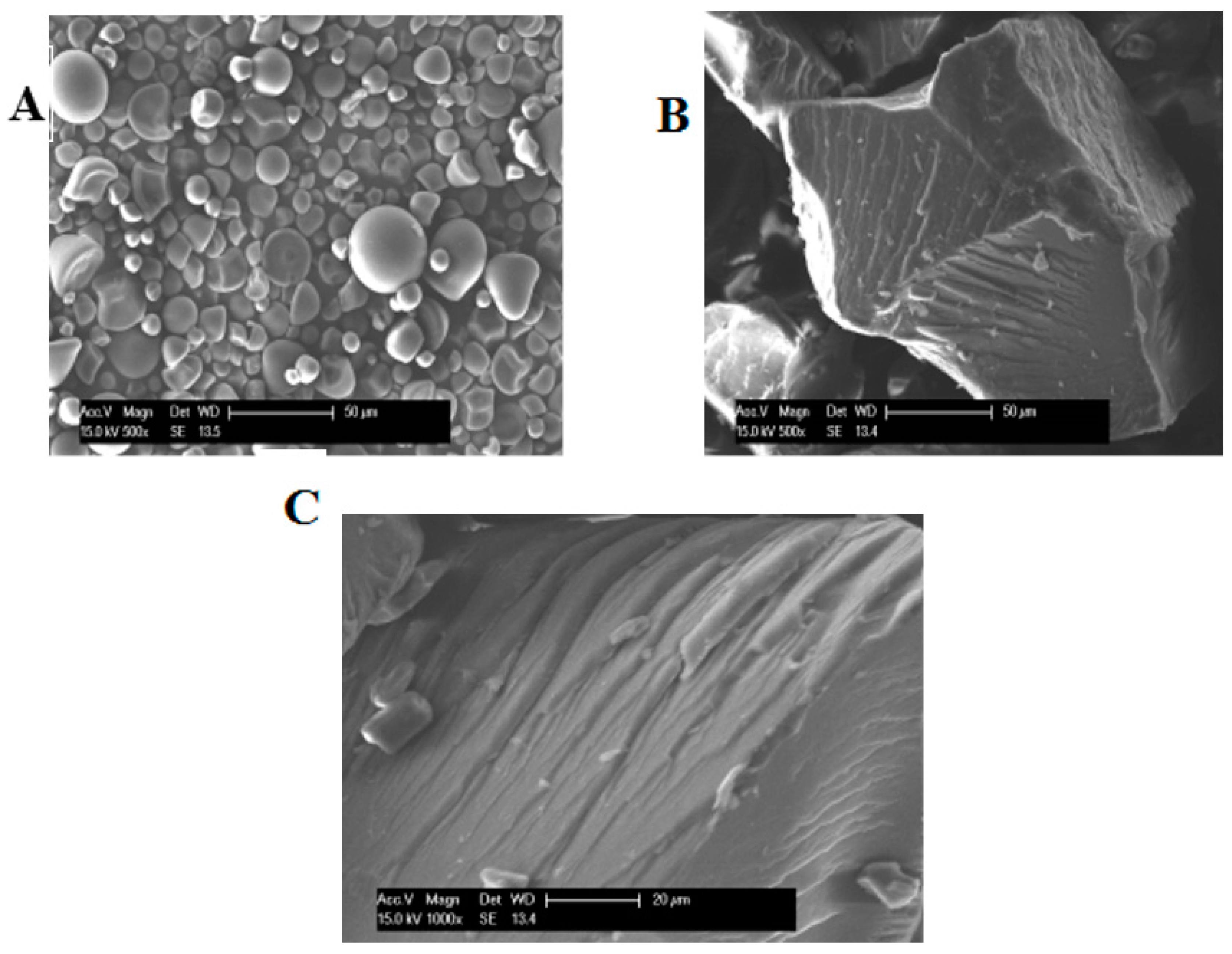
2.2. Morphological Characteristics
2.3. Swelling Power and Solubility
2.4. Thermal Properties
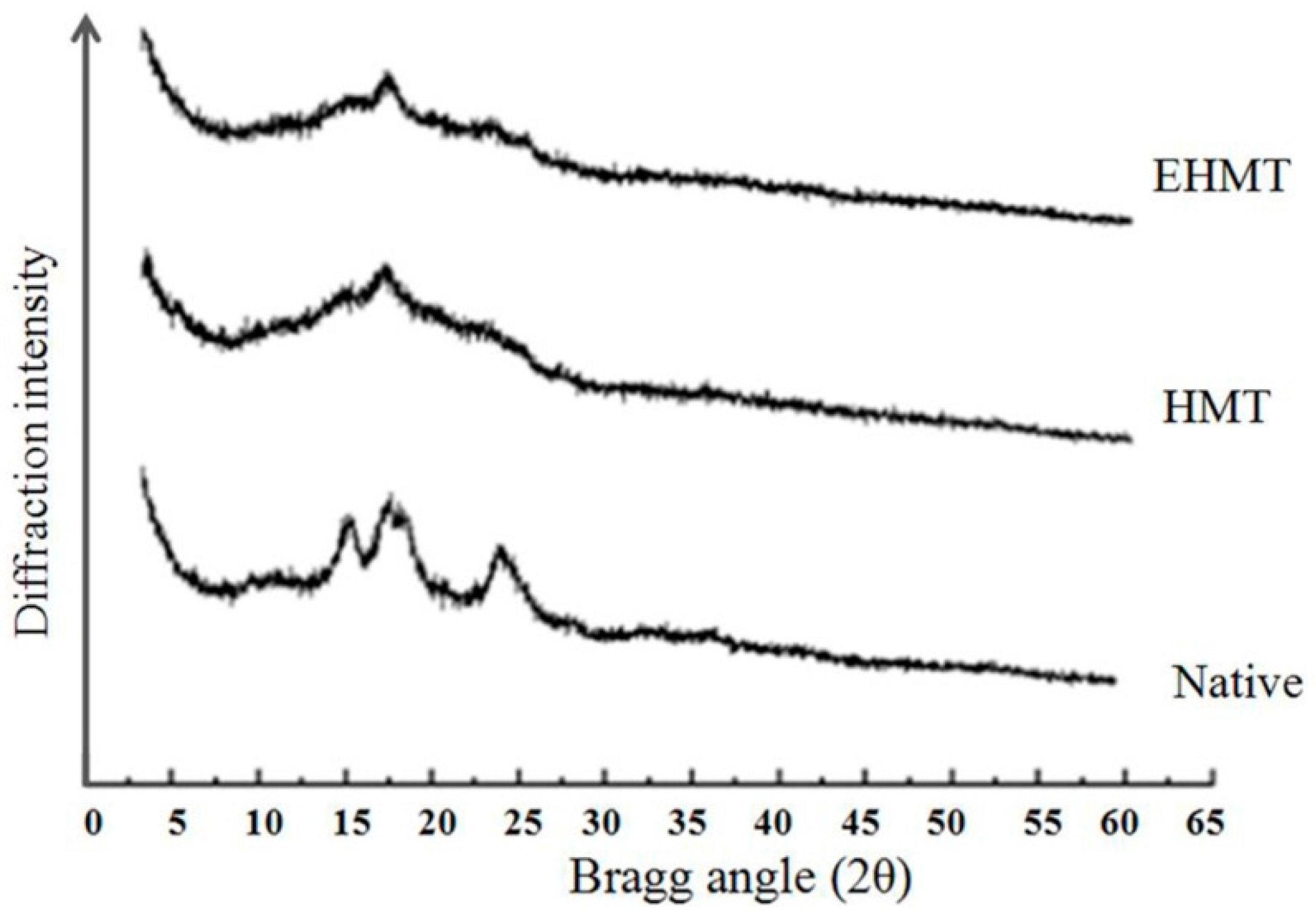
2.5. X-ray Diffraction and Relative Crystallinity
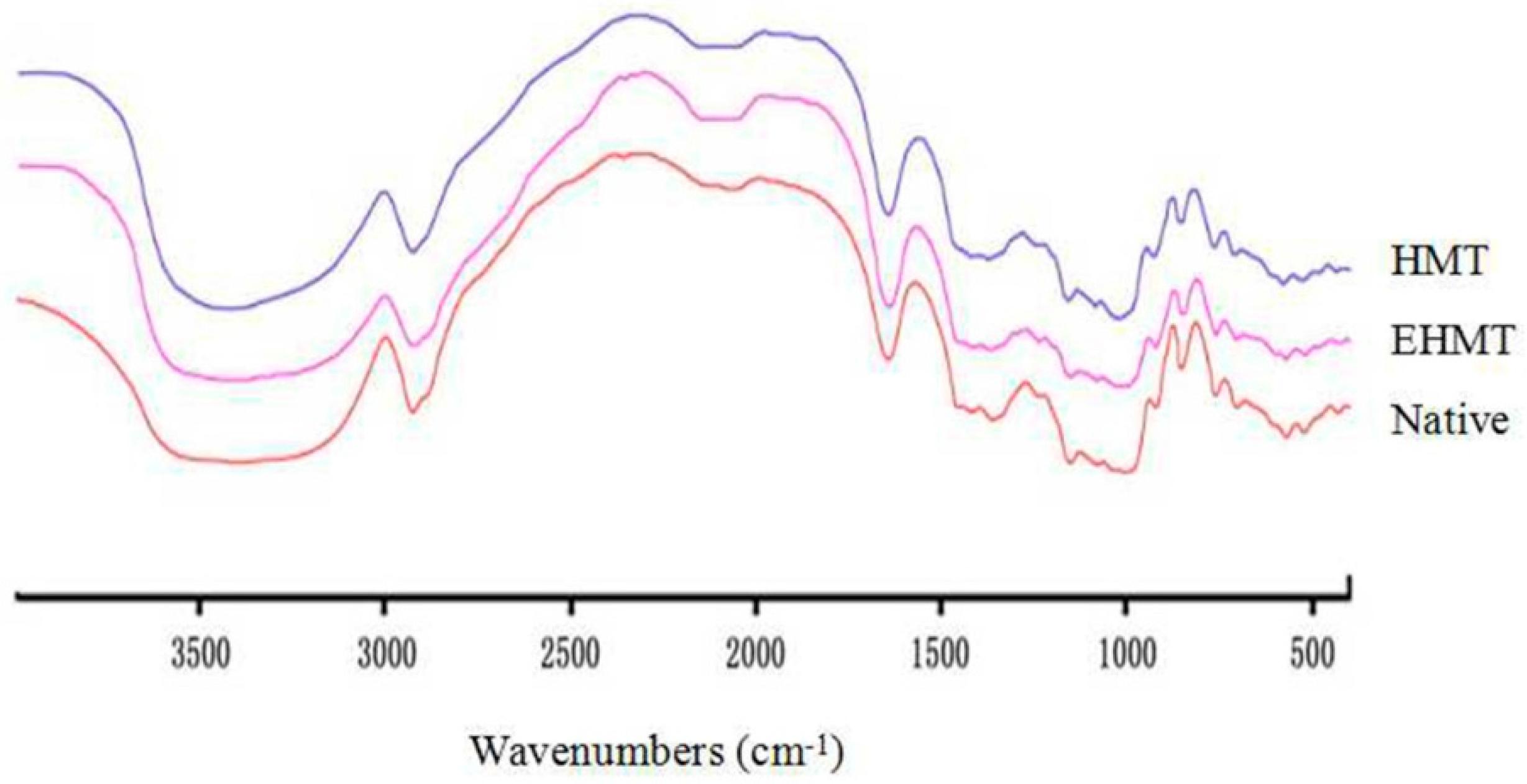
2.6. FT-IR Spectroscopy
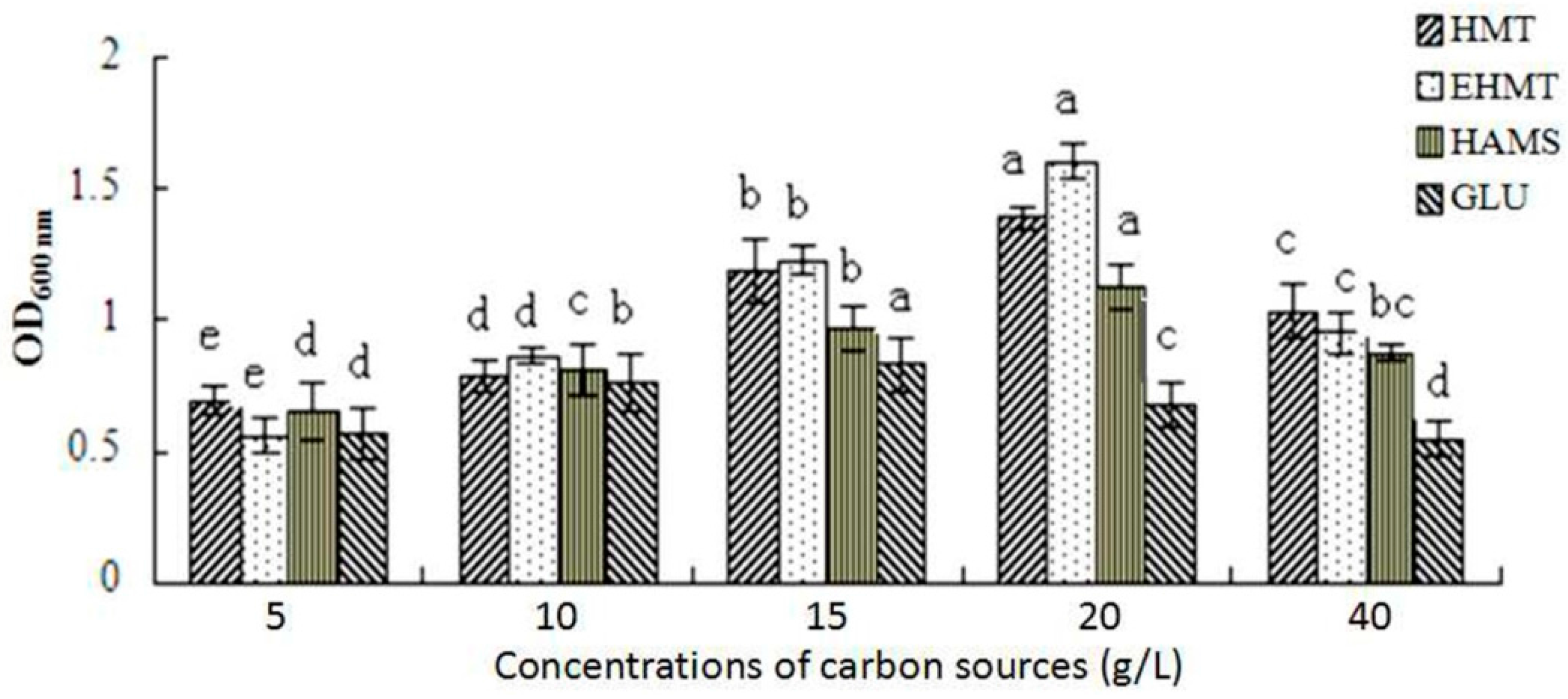
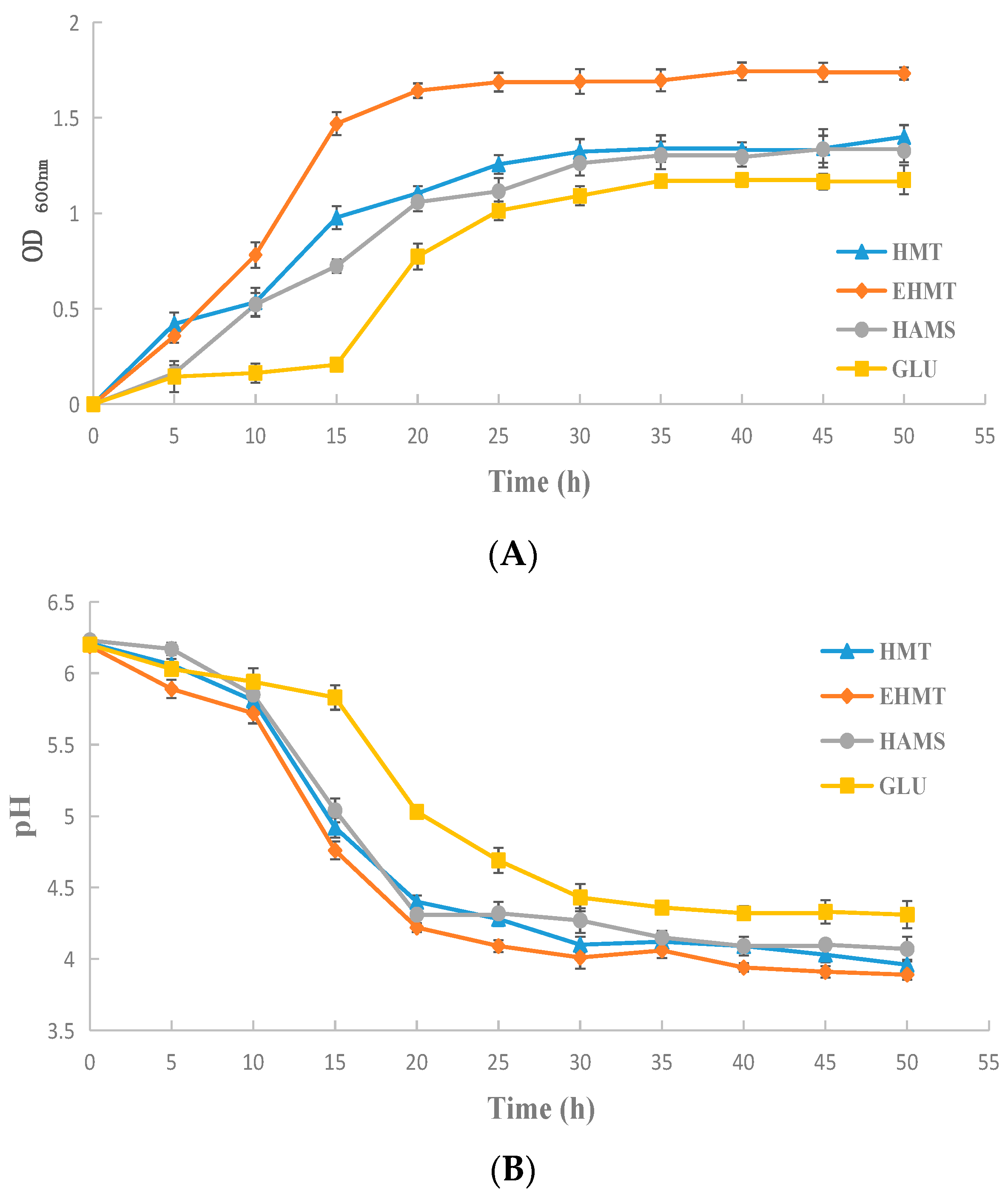
2.7. Probiotic Effects
3. Material and Methods
3.1. Preparation of Purple Sweet Potato Starch
3.2. Preparation of Purple Sweet Potato Resistant Starch
3.2.1. Preparation of Heat-Moisture-Treated (HMT) Starch
3.2.2. Preparation of Enzyme Debranching Combined Heat-Moisture-Treated Starch (EHMT)
3.3. Resistant Starch Determination
3.4. Scanning Electron Microscopy
3.5. Chemical Analysis
3.6. Water Solubility and Swelling Power
3.7. Thermal Analysis
3.8. X-ray Diffraction and Relative Crystallinity
3.9. Fourier Transform Infrared Spectroscopy (FT-IR)
3.10. Proliferation Rate of Bifidobacteria
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Truong, V.-D.; Deighton, N.; Thompson, R.T.; McFeeters, R.F.; Dean, L.O.; Pecota, K.V.; Yencho, G.C. Characterization of Anthocyanins and Anthocyanidins in Purple-Fleshed Sweetpotatoes by HPLC-DAD/ESI-MS/MS. J. Agric. Food Chem. 2010, 58, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Kano, N.; Takayanagi, T.; Harada, K.; Makino, K.; Ishikawa, F. Antioxidative activity of anthocyanins from purple sweet potato, Ipomoera batatas cultivar Ayamurasaki. Biosci. Biotechnol. Biochem. 2005, 69, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.-T.; Zhou, D.-N.; Jin, Z.-Y.; Xu, X.-M.; Chen, H.-Q. Effect of repeated heat-moisture treatments on digestibility, physicochemical and structural properties of sweet potato starch. Food Hydrocoll. 2016, 54, 202–210. [Google Scholar] [CrossRef]
- Xia, H.; Li, Y.; Gao, Q. Preparation and properties of RS4 citrate sweet potato starch by heat-moisture treatment. Food Hydrocoll. 2016, 55, 172–178. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46 (Suppl. 2), S33–S50. [Google Scholar] [PubMed]
- Bodinham, C.L.; Smith, L.; Thomas, E.L.; Bell, J.D.; Swann, J.R.; Costabile, A.; Russell-Jones, D.; Umpleby, A.M.; Robertson, M.D. Efficacy of increased resistant starch consumption in human type 2 diabetes. Endocr. Connect. 2014, 3, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Nichenametla, S.N.; Weidauer, L.A.; Wey, H.E.; Beare, T.M.; Specker, B.L.; Dey, M. Resistant starch type 4-enriched diet lowered blood cholesterols and improved body composition in a double blind controlled cross-over intervention. Mol. Nutr. Food Res. 2014, 58, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Y.; Zheng, B.; Lu, X.; Zhuang, W. The in vitro effects of retrograded starch (resistant starch type 3) from lotus seed starch on the proliferation of Bifidobacterium adolescentis. Food Funct. 2013, 4, 1609–1616. [Google Scholar] [CrossRef] [PubMed]
- Gibson, G.R.; Probert, H.M.; van Loo, J.; Rastall, R.A.; Roberfroid, M.B. Dietary modulation of the human colonic microbiota: Updating the concept of prebiotics. Nutr. Res. Rev. 2004, 17, 259–275. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Dai, L.; Nan, C.; Xiong, L. Effect of heat moisture treatment on physicochemical and morphological properties of wheat starch and xylitol mixture. Food Chem. 2014, 143, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Morales-Medina, R.; del Mar Munio, M.; Guadix, E.M.; Guadix, A. Production of resistant starch by enzymatic debranching in legume flours. Carbohydr. Polym. 2014, 101, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.I.; Lee, C.J.; Kim, D.-I.; Lee, H.A.; Cheong, J.-J.; Chung, K.M.; Baik, M.-Y.; Park, C.S.; Kim, C.H.; Moon, T.W. Formation, characterization, and glucose response in mice to rice starch with low digestibility produced by citric acid treatment. J. Cereal Sci. 2007, 45, 24–33. [Google Scholar] [CrossRef]
- Aparicio-Saguilan, A.; Gutierrez-Meraz, F.; Garcia-Suarez, F.J.; Tovar, J.; Bello-Perez, L.A. Physicochemical and functional properties of cross-linked banana resistant starch. Effect of pressure cooking. Starch Starke 2008, 60, 286–291. [Google Scholar] [CrossRef]
- Guo, J.; Liu, L.; Lian, X.; Li, L.; Wu, H. The properties of different cultivars of Jinhai sweet potato starches in China. Int. J. Biol. Macromol. 2014, 67, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.J.; Rickard, J.E.; Blanshard, J.M.V. Physicochemical properties of sweet potato starch. J. Sci. Food Agric. 1991, 57, 459–491. [Google Scholar] [CrossRef]
- Abegunde, O.K.; Mu, T.-H.; Chen, J.-W.; Deng, F.-M. Physicochemical characterization of sweet potato starches popularly used in Chinese starch industry. Food Hydrocoll. 2013, 33, 169–177. [Google Scholar] [CrossRef]
- Reddy, C.K.; Suriya, M.; Haripriya, S. Physico-chemical and functional properties of resistant starch prepared from red kidney beans (Phaseolus vulgaris L.) starch by enzymatic method. Carbohydr. Polym. 2013, 95, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zeng, H.; Wang, Y.; Zeng, S.; Zheng, B. Structural characteristics and crystalline properties of lotus seed resistant starch and its prebiotic effects. Food Chem. 2014, 155, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Gerits, L.R.; Pareyt, B.; Delcour, J.A. Wheat starch swelling, gelatinization and pasting: Effects of enzymatic modification of wheat endogenous lipids. LWT Food Sci. Technol. 2015, 63, 361–366. [Google Scholar] [CrossRef]
- Hung, P.V.; Vien, N.L.; Lan Phi, N.T. Resistant starch improvement of rice starches under a combination of acid and heat-moisture treatments. Food Chem. 2016, 191, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Han, Z.; Wang, L.; Xiong, L. Physicochemical differences between sorghum starch and sorghum flour modified by heat-moisture treatment. Food Chem. 2014, 145, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Kaur, A.; Singh, N.; Ezekiel, R.; Guraya, H.S. Physicochemical, thermal and pasting properties of starches separated from different potato cultivars grown at different locations. Food Chem. 2007, 101, 643–651. [Google Scholar] [CrossRef]
- Gomes, A.M.M.; Silva, C.E.M.D.; Ricardo, N.M.P.S. Effects of annealing on the physicochemical properties of fermented cassava starch (Polvilho azedo). Carbohydr. Polym. 2005, 60, 1–6. [Google Scholar] [CrossRef]
- Vermeylen, R.; Goderis, B.; Delcour, J.A. An X-ray study of hydrothermally treated potato starch. Carbohydr. Polym. 2006, 64, 364–375. [Google Scholar] [CrossRef]
- Singh, N.; Singh, J.; Kaur, L.; Singh Sodhi, N.; Singh Gill, B. Morphological, thermal and rheological properties of starches from different botanical sources. Food Chem. 2003, 81, 219–231. [Google Scholar] [CrossRef]
- Cheetham, N.W.H.; Tao, L. Variation in crystalline type with amylose content in maize starch granules: An X-ray powder diffraction study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Zobel, H.F. Molecules to Granules: A Comprehensive Starch Review. Starch Starke 1988, 40, 44–50. [Google Scholar] [CrossRef]
- Huang, T.T.; Zhou, D.N.; Jin, Z.Y.; Xu, X.M.; Chen, H.Q. Effect of debranching and heat-moisture treatments on structural characteristics and digestibility of sweet potato starch. Food Chem. 2015, 187, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Zeng, S.; Wu, X.; Lin, S.; Zeng, H.; Lu, X.; Zhang, Y.; Zheng, B. Structural characteristics and physicochemical properties of lotus seed resistant starch prepared by different methods. Food Chem. 2015, 186, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Diop, C.I.; Li, H.L.; Xie, B.J.; Shi, J. Effects of acetic acid/acetic anhydride ratios on the properties of corn starch acetates. Food Chem. 2011, 126, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Flores-Morales, A.; Jiménez-Estrada, M.; Mora-Escobedo, R. Determination of the structural changes by FT-IR, Raman, and CP/MAS 13C NMR spectroscopy on retrograded starch of maize tortillas. Carbohydr. Polym. 2012, 87, 61–68. [Google Scholar] [CrossRef]
- Joshi, M.; Aldred, P.; McKnight, S.; Panozzo, J.F.; Kasapis, S.; Adhikari, R.; Adhikari, B. Physicochemical and functional characteristics of lentil starch. Carbohydr. Polym. 2013, 92, 1484–1496. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, F.; Liu, F.; Wang, Z.-W. Study on structural changes of microwave heat-moisture treated resistant Canna edulis Ker starch during digestion in vitro. Food Hydrocoll. 2010, 24, 27–34. [Google Scholar] [CrossRef]
- Wei, C.; Qin, F.; Zhou, W.; Xu, B.; Chen, C.; Chen, Y.; Wang, Y.; Gu, M.; Liu, Q. Comparison of the crystalline properties and structural changes of starches from high-amylose transgenic rice and its wild type during heating. Food Chem. 2011, 128, 645–652. [Google Scholar] [CrossRef]
- Van Soest, J.J.G.; Tournois, H.; de Wit, D.; Vliegenthart, J.F.G. Short-range structure in (partially) crystalline potato starch determined with attenuated total reflectance Fourier-transform IR spectroscopy. Carbohydr. Res. 1995, 279, 201–214. [Google Scholar] [CrossRef]
- Sevenou, O.; Hill, S.E.; Farhat, I.A.; Mitchell, J.R. Organisation of the external region of the starch granule as determined by infrared spectroscopy. Int. J. Biol. Macromol. 2002, 31, 79–85. [Google Scholar] [CrossRef]
- Bird, A.R.; Vuaran, M.; Crittenden, R.; Hayakawa, T.; Playne, M.J.; Brown, I.L.; Topping, D.L. Comparative effects of a high-amylose starch and a fructooligosaccharide on fecal bifidobacteria numbers and short-chain fatty acids in pigs fed Bifidobacterium animalis. Dig. Dis. Sci. 2009, 54, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Jiang, H.; Jiang, B.; Cui, S.W.; Jin, Z.; Zhang, T. Structure and functional properties of starches from Chinese ginkgo (Ginkgo biloba L.) nuts. Food Res. Int. 2012, 49, 303–310. [Google Scholar] [CrossRef]
- Wang, L.; Xie, B.; Shi, J.; Xue, S.; Deng, Q.; Wei, Y.; Tian, B. Physicochemical properties and structure of starches from Chinese rice cultivars. Food Hydrocoll. 2010, 24, 208–216. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
| Samples | Moisture (%) | Protein (%) | Lipid (%) | Amylose (%) | RS (%) |
|---|---|---|---|---|---|
| Native | 5.03 ± 0.19 a | 0.41 ± 0.01 a | 0.11 ± 0.01 a | 29.91 ± 0.14 a | 5.02 ± 0.21 a |
| HMT | 4.92 ± 0.08 a | 0.38 ± 0.04 a | 0.17 ± 0.01 b | 32.83 ± 0.13 b | 14.23 ± 0.55 b |
| EHMT | 4.67 ± 0.07 b | 1.67 ± 0.07 b | 0.19 ± 0.02 c | 36.62 ± 0.25 c | 17.16 ± 0.63 c |
| Samples | SP (g/g) | S (%) | To (°C) | Tp (°C) | Tc (°C) | Tc–To (°C) | ΔH (J/g) |
|---|---|---|---|---|---|---|---|
| Native | 26.21 | 9.46 | 67.2 | 80.6 | 87.8 | 20.6 | 21.26 |
| HMT | 20.72 | 8.74 | 106.7 | 115.2 | 124.1 | 17.4 | 25.53 |
| EHMT | 17.21 | 7.41 | 135.9 | 143.3 | 152.8 | 16.9 | 26.78 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Wang, Q.; Li, B.; Lin, L.; Tundis, R.; Loizzo, M.R.; Zheng, B.; Xiao, J. Characterization and Prebiotic Effect of the Resistant Starch from Purple Sweet Potato. Molecules 2016, 21, 932. https://doi.org/10.3390/molecules21070932
Zheng Y, Wang Q, Li B, Lin L, Tundis R, Loizzo MR, Zheng B, Xiao J. Characterization and Prebiotic Effect of the Resistant Starch from Purple Sweet Potato. Molecules. 2016; 21(7):932. https://doi.org/10.3390/molecules21070932
Chicago/Turabian StyleZheng, Yafeng, Qi Wang, Baoyu Li, Liangmei Lin, Rosa Tundis, Monica R. Loizzo, Baodong Zheng, and Jianbo Xiao. 2016. "Characterization and Prebiotic Effect of the Resistant Starch from Purple Sweet Potato" Molecules 21, no. 7: 932. https://doi.org/10.3390/molecules21070932
APA StyleZheng, Y., Wang, Q., Li, B., Lin, L., Tundis, R., Loizzo, M. R., Zheng, B., & Xiao, J. (2016). Characterization and Prebiotic Effect of the Resistant Starch from Purple Sweet Potato. Molecules, 21(7), 932. https://doi.org/10.3390/molecules21070932












