Simultaneous Determination of Eight Alkaloids in Rat Plasma by UHPLC-MS/MS after Oral Administration of Coptis deltoidea C. Y. Cheng et Hsiao and Coptis chinensis Franch
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Development
2.1.1. UHPLC-MS/MS Optimization
2.1.2. Selection of the Internal Standard and the Extraction Method
2.2. Method Validation
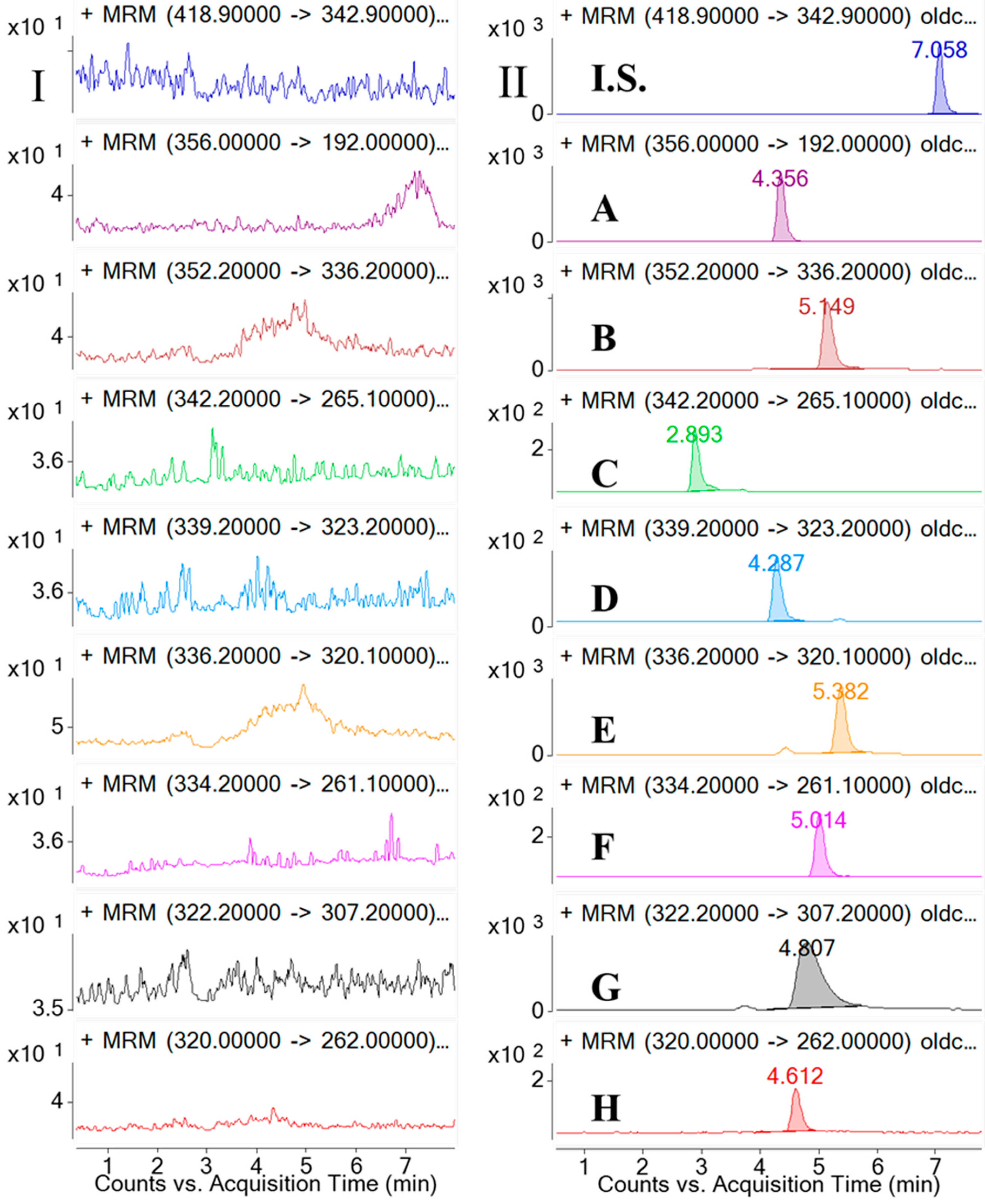
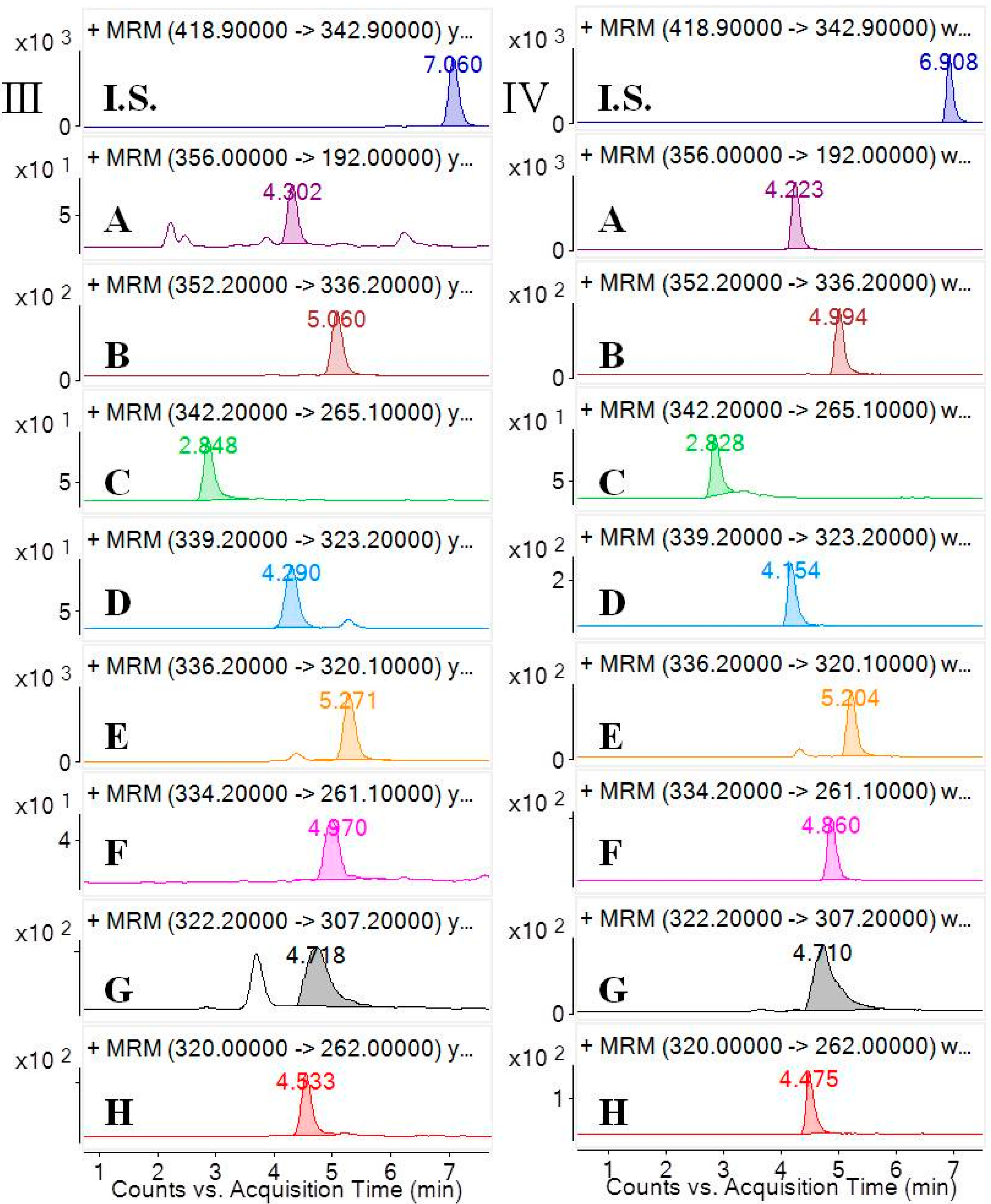
2.2.1. Selectivity and Specificity
2.2.2. Linearity and Lower Limit of Quantification
2.2.3. Precision and Accuracy
2.2.4. Extraction Recovery and Matrix Effects
2.2.5. Stability
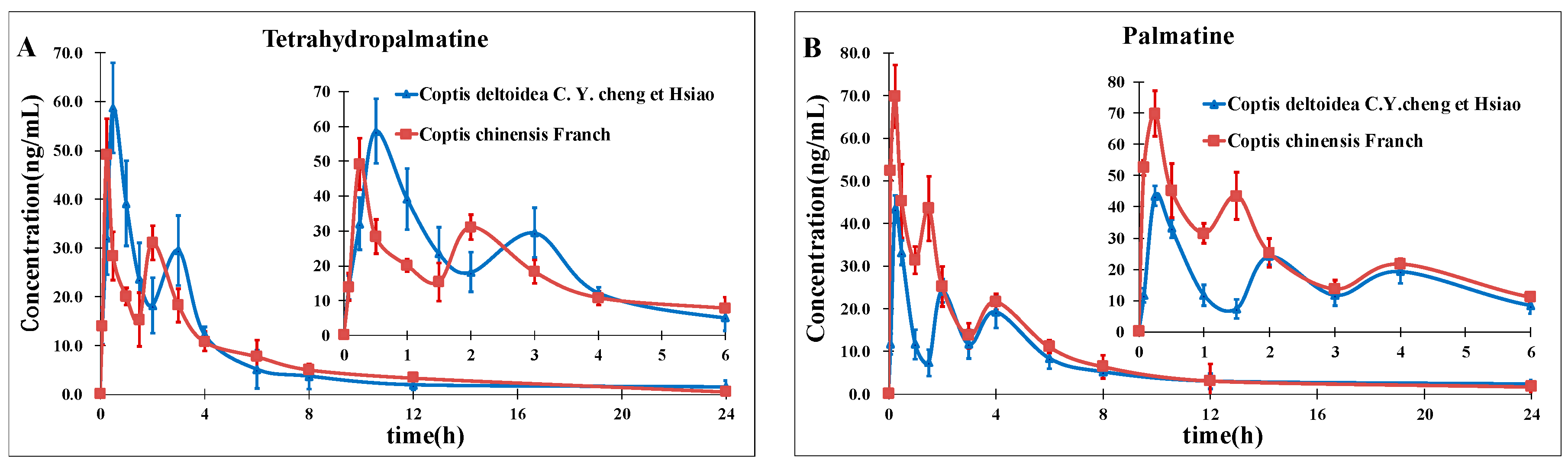
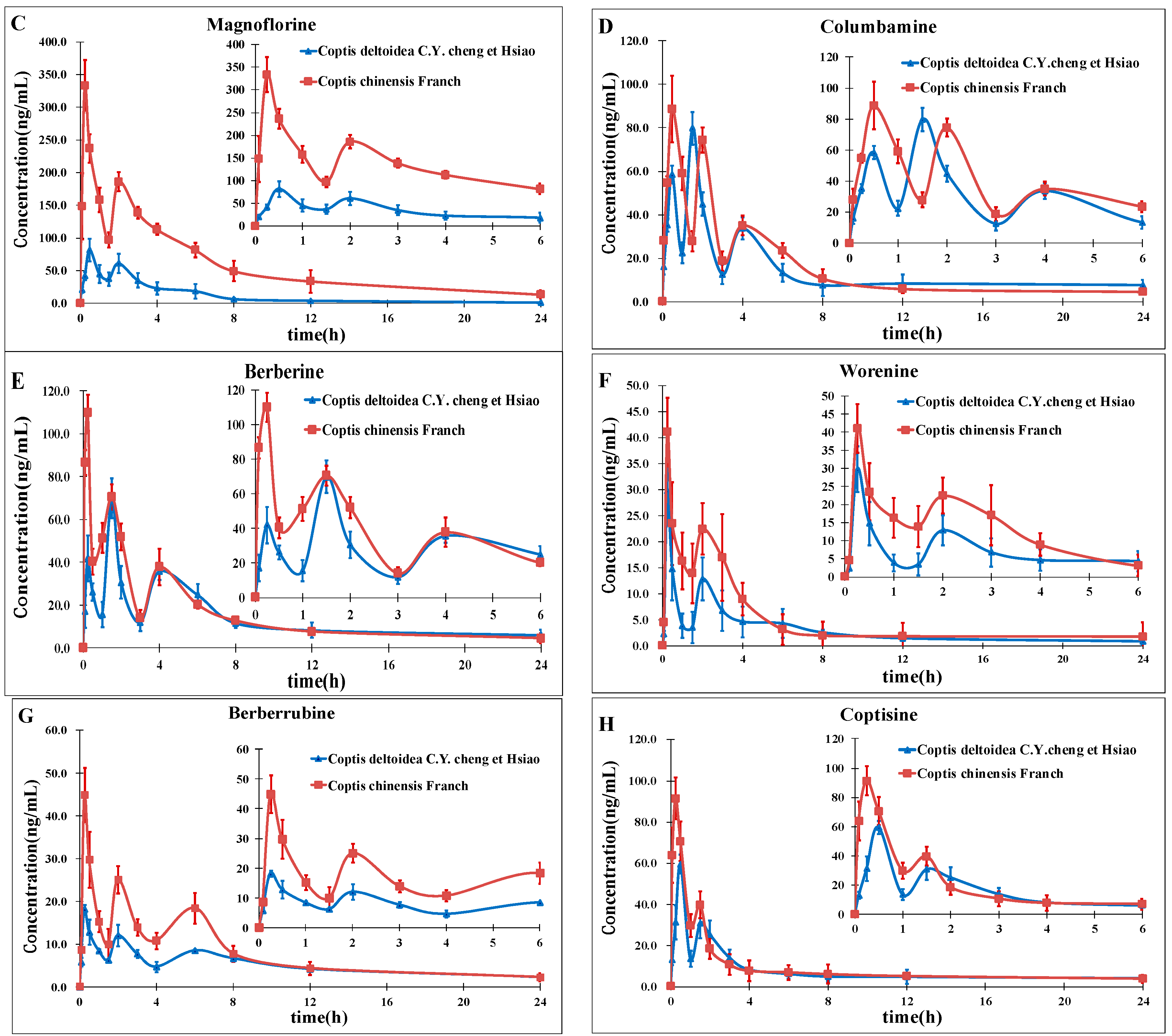
2.3. Method Comparison with Existing Reports
3. Experimental Section
3.1. Material and Regents
3.2. Preparation of CCY Extract and CF Extract
3.3. Instrumental and Chromatographic Conditions
3.4. Preparation of Calibration Standards and Quality Control Samples
3.5. Animals and Sample Preparation
3.6. Method Validation
3.6.1. Selectivity and Specificity
3.6.2. Linearity and Lower Limit of Quantification
3.6.3. Precision and Accuracy
3.6.4. Recovery and Matrix Effect
3.6.5. Stability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Jiang, X.; Huang, L.F.; Wu, L.B.; Wang, Z.H.; Chen, S.L. UPLC-QTOF/MS Analysis of alkaloids in traditional processed Coptis chinensis Franch. Evid. Based Complement. Altern. Med. 2012, 2012. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Hu, Y.R.; Zou, Z.Y.; Feng, M.; Ye, X.L.; Li, X.G. Antihyperglycemia and antihyperlipidemia effect of protoberberine alkaloids from Rhizoma coptidis in HepG2 cell and diabetic KK-Ay mice. Drug Dev. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.E.; Goh, Y.L.; Zhang, C. From prejudice to evidence: The case of rhizoma coptidis in Singapore. Evid. Based Complement. Altern. Med. 2014. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.B.; Lee, Y.J.; Park, S.K.; Kim, H.C.; Bae, H.; Kim, H.M.; Ko, S.G.; Choi, H.Y.; Oh, M.S.; Park, W. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7macrophages. J. Ethnopharmacol. 2009, 125, 286–290. [Google Scholar] [CrossRef] [PubMed]
- Linn, Y.C.; Lu, J.; Lim, L.C.; Sun, H.; Sun, J.; Zhou, Y.; Ng, H.S. Berberine-induced haemolysis revisited: Safety of Rhizoma coptidis and Cortex phellodendri in chronic haematological diseases. Phytother. Res. 2012, 26, 682–686. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Cheng, R.; Xiong, F.Y.; Chen, X.; Ma, Y.T.; Yan, Z.Y. Embryological studies of Coptis deltoidea C.Y. Cheng et Hsiao. Pharm. Clin. Chin. Mater. Med. 2011, 2, 7–10. [Google Scholar]
- Yuan, L.; Tu, D.; Ye, X.; Wu, J. Hypoglycemic and hypocholesterolemic effects of Coptis chinensis Franch inflorescence. Plant Foods Hum. Nutr. 2006, 61, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Qian, X.; Li, J.S.; Cui, X.B.; Chen, L.H.; Cai, B.C.; Tan, S.Z. Simultaneous determination of 11 alkaloids in crude and wine-processed Rhizoma coptidis by HPLC-PAD. J. Chromatogr. Sci. 2015, 53, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wang, J.; Xiao, X.; Chen, S.; Yang, M. Evaluation of antibacterial effect and mode of Coptidis rhizoma by microcalorimetry coupled with chemometric techniques. Analyst 2012, 137, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Li, X.K.; Motwani, M.; Tong, W.; Bornmann, W.; Schwartz, G.K. Huanglian, a Chinese herbal extract, inhibits cell growth by suppressing the expression of cyclin B1 and inhibiting CDC2 kinase activity in human cancer cells. Mol. Pharmacol. 2001, 58, 1287–1293. [Google Scholar]
- Lin, H.L.; Liu, T.Y.; Wu, C.W.; Chi, C.W. Berberine modulates expression of mdr1 gene product and the responses of digestive track cancer cells to Paclitaxel. Br. J. Cancer 1999, 81, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Chinami, M.; Emiko, S.S.; Minako, N.Y.; Megumi, U.; Ikuo, N.; Yuji, O.; Masato, F.; Yoshio, K.; Kenichiro, M. Analysis of the antioxidative function of the radioprotective Japanese traditional (Kampo) medicine, hangeshashinto, in an aqueous phase. J. Radiat. Res. 2015, 56, 669–677. [Google Scholar]
- Zeng, X.H.; Zeng, X.J.; Li, Y.Y. Efficacy and safety of berberine for congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am. J. Cardiol. 2003, 92, 173–176. [Google Scholar] [CrossRef]
- Zhao, G.L.; Yu, L.M.; Gao, W.L.; Duan, W.X.; Jiang, B.; Liu, X.D.; Zhang, B.; Liu, Z.H.; Zhai, M.E.; Jin, Z.X.; et al. Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacol. Sin. 2016, 37, 354–367. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Kou, S.; Zou, Z.; Hu, Y.; Feng, M.; Han, B.; Li, X.; Ye, X. Hypolipidemic effects of alkaloids from Rhizoma coptidis in diet-induced hyperlipidemic hamsters. Planta Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Jin, C.; Xiao, X.H.; Dong, X.P. Antimicrobial properties of berberines alkaloids in Coptis chinensis Franch by microcalorimetry. J. Biochem. Biophys. Methods 2008, 70, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Küpeli, E.; Kosar, M.; Yesilada, E.; Hüsnü, K.; Baser, C. Acomparative study on the anti-inflammatory, antinociceptive and antipyreticeffects of isoquinoline alkaloids from the roots of Turkish Berberis species. Life Sci. 2002, 72, 645–657. [Google Scholar] [CrossRef]
- Lam, P.; Kok, S.H.L.; Lee, K.K.H.; Lam, K.H.; Hau, D.K.P.; Wong, W.Y.; Bian, Z.X.; Gambari, R.; Chui, C.H. Sensitization of Candida albicans to terbinafine by berberine and berberrubine. Biomed. Rep. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Sharma, U.; Bala, M.; Kumar, N.; Singh, B.; Munshi, R.K.; Bhalerao, S. Immunomodulatory active compounds from Tinospora cordifolia. J. Ethnopharmacol. 2012, 141, 918–926. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.; Hassan, E.; Ibrahim, N. Cytotoxic and antiviral activities of aporphine alkaloids of Magnolia grandiflora L. Nat. Prod. Res. 2010, 24, 1395–1402. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Ma, Y.; Shi, R.; Wang, T. Simultaneous quantification of three alkaloids of Coptidis rhizoma in rat urine by high-performance liquid chromatography: Application to pharmacokinetic study. Biopharm. Drug Dispos. 2007, 28, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.W.; Katharina, W.; Tang, L.Y.; Kopp, B. Influence of vinegar and wine processing on the alkaloid content and composition of the Traditional Chinese Medicine Corydalis Rhizoma (Yanhusuo). Molecules 2014, 19, 11487–11504. [Google Scholar] [CrossRef] [PubMed]
- Kushida, H.; Matsumoto, T.; Igarashi, Y.; Nishimura, H.; Watanabe, J.; Maemura, K.; Kase, Y. Metabolic profiling of the Uncaria hook alkaloid geissoschizine methyl ether in rat and human liver microsomes using high-performance liquid chromatography with tandem mass spectrometry. Molecules 2015, 20, 2100–2114. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yu, Y.L.; Lu, S.S.; Liu, L.; Liu, X.D. Pharmacokinetics of berberine, palmatine, coptisine, epiberberine and jatrorrhizine from Coptidis rhizoma in diabetic rats. J. China Pharm. Univ. 2008, 39, 526–529. [Google Scholar]
- Ma, H.; Wang, Y.; Guo, T.; He, Z.; Chang, X.; Pu, X. Simultaneous determination of tetrahydropalmatine, protopine, and palmatine in rat plasma by LC-ESI-MS and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2009, 49, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Xu, W.; Tao, X.; Wei, H.; Cai, F.; Jiang, B.; Chen, W. Simultaneous determination of baicalin, baicalein, wogonin, berberine, palmatine and jatrorrhizine in rat plasma by liquid chromatography-tandem mass spectrometry and application in pharmacokinetic studies after oral administration of traditional Chinese medicinal preparations containing scutellaria—Coptis herb couple. J. Pharm. Biomed. Anal. 2010, 53, 591–598. [Google Scholar] [PubMed]
- Wu, H.; Liu, H.; Bai, J.; Lu, Y.; Du, S. Simultaneous determination of notoginsenoside R1, ginsenoside Rg1, ginsenoside Re and 20(S) protopanaxatriol in beagle dog plasma by ultra-high performance liquid mass spectrometry after oral administration of a Panax notoginseng saponin preparation. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2015, 974, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xian, Y.; Lai, Z.; Loo, S.; Chan, W.; Lin, Z.X. Anti-inflammatory and anti-allergic effects and underlying mechanisms of Huang-Lian-Jie-Du Extract: Implication for atopic dermatitis treatment. J. Ethnopharmacol. 2016, 16, 30132–30135. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Liu, J.X.; Zhang, Y.; Lin, C.R.; Duan, C.L. Pharmacokinetic studies of tetrahydropalmatine and dehydrocorydaline in rat after oral administration of Yanhusuo extraction by LC-MS/MS method. Acta Pharm. Sin. 2008, 43, 1123–1127. [Google Scholar]
- Xue, B.; Zhao, Y.; Miao, Q.; Miao, P.; Yang, X.; Sun, G.; Su, J.; Ye, J.; Wei, B.; Zhang, Y.; et al. In vitro and in vivo identification of metabolites of magnoflorine by LC LTQ-Orbitrap MS and its potential pharmacokinetic interaction in Coptidis rhizoma decoction in rat. Biomed. Chromatogr. BMC 2015, 29, 1235–1248. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.Y.; Jiang, H.Z.; Lin, L. Study on the Fingerprint of Coptis deltoidea C.Y. Cheng et Hsiao Produced from Emei. J. Anhui Agri. Sci. 2008, 36, 11806–11838. [Google Scholar]
- Maureen, B.; Philip, Y.; Maurice, Y.; Pauline, C. Determination of cytotoxicity of traditional Chinese medicine herbs, Rhizoma coptidis, Radix scutellariae, and Cortex phellodendri, by three methods. Contact Lens Ant. Eye J. Br. Contact Lens Assoc. 2016, 39, 128–132. [Google Scholar]
- Ma, L.Y.; Zhang, Y.B.; Zhou, Q.L.; Yang, Y.F.; Yang, X.W. Simultaneous determination of eight Ginsenosides in rat plasma by Liquid Chromatography-Electrospray Ionization Tandem Mass Spectrometry: Application to Their Pharmacokinetics. Molecules 2015, 20, 21597–21608. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds named tetrahydropalmatine, palmatine chloride, magnoflorine, columbamine, berberine hydrochloride, worenine, berberrubine, coptisine and bifendate are available from the authors.
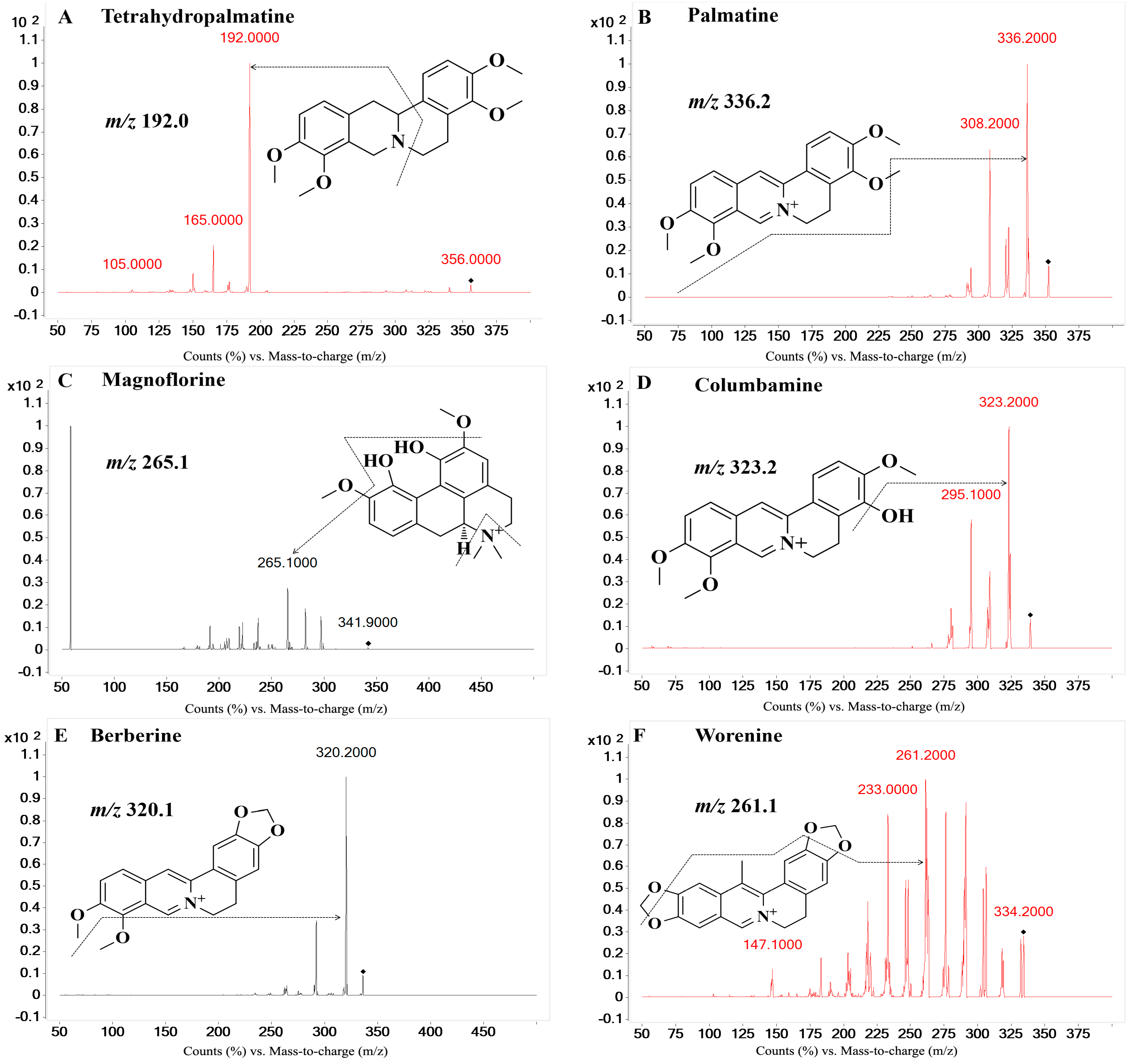
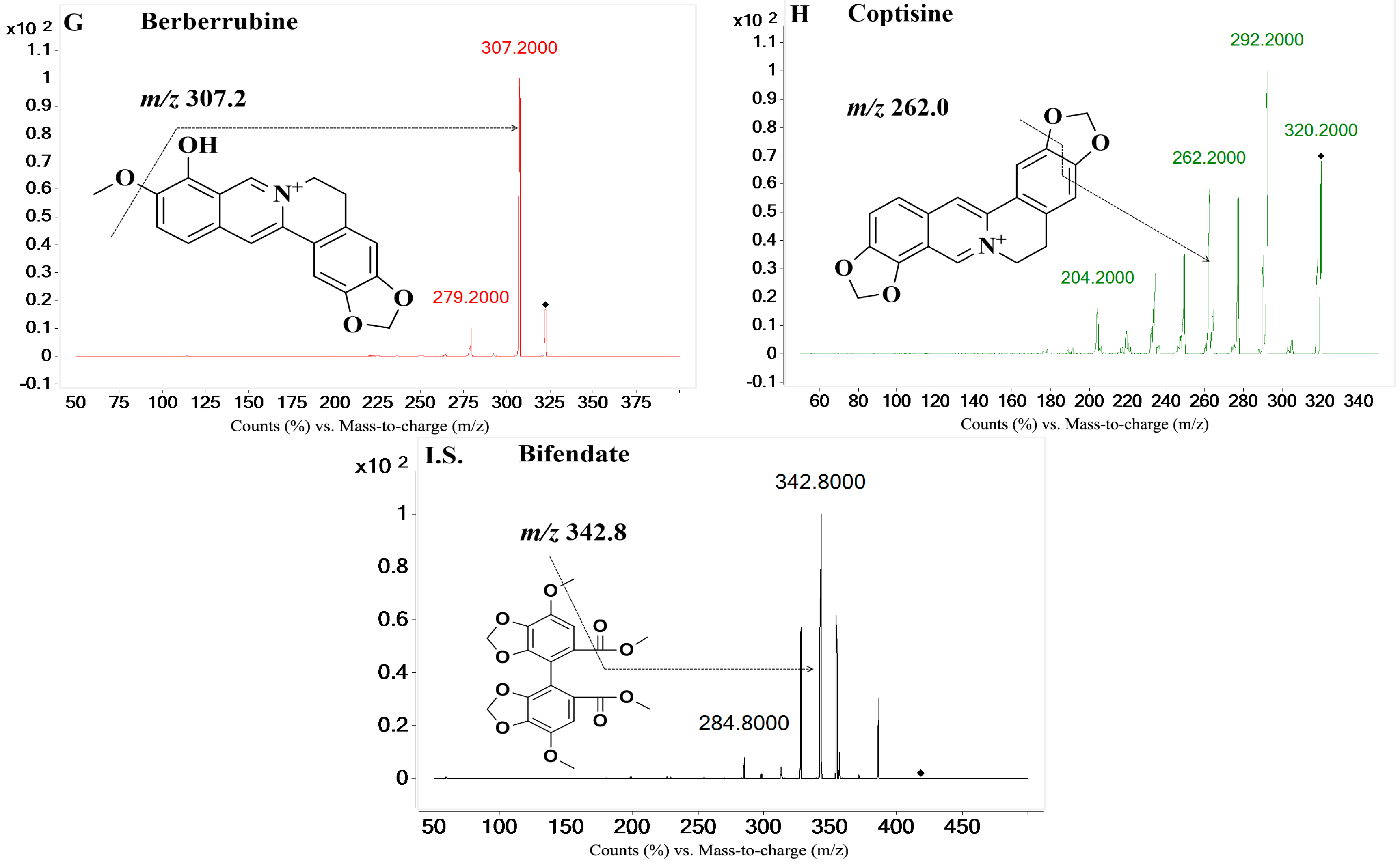
| Compounds | Ion Pair (m/z) | Qualifier Ion (m/z) | Fragmentor (V) | Collision Energy (V) | Polarity 1 |
|---|---|---|---|---|---|
| Bifendate (I.S.) | 418.9→342.8 | 284.8 | 78 | 18 | Positive |
| Tetrahydropalmatine | 356.0→192.0 | 165.0 | 159 | 27 | Positive |
| Palmatine | 352.2→336.2 | 308.2 | 158 | 30 | Positive |
| Magnoflorine | 342.2→265.1 | 58.2 | 134 | 22 | Positive |
| Columbamine | 339.2→323.2 | 295.1 | 160 | 29 | Positive |
| Berberine | 336.2→320.1 | 292.2 | 136 | 30 | Positive |
| Worenine | 334.2→261.1 | 233.0 | 181 | 49 | Positive |
| Berberrubine | 322.2→307.2 | 279.2 | 160 | 29 | Positive |
| Coptisine | 320.0→262.0 | 292.2 | 167 | 29 | Positive |
| Time (min) | A% | B% |
|---|---|---|
| 0 | 65 | 35 |
| 0–2 | 45 | 55 |
| 2–3 | 38 | 62 |
| 3–4.5 | 38 | 62 |
| 4.5–7.0 | 30 | 70 |
| 7.0–7.5 | 65 | 35 |
| Compounds | Regression Equation | R2 | Linear Range (ng/mL) | LLOQ (ng/mL) |
|---|---|---|---|---|
| Tetrahydropalmatine | Y = 15.358X + 1.720 × 10−2 | 0.9964 | 0.5–2028 | 0.5 |
| Palmatine | Y = 112.57X + 5.3976 × 10−2 | 0.9928 | 0.1–428 | 0.1 |
| Magnoflorine | Y = 0.3350X + 3.272 × 10−3 | 0.9906 | 1.1–4320 | 1.1 |
| Columbamine | Y = 2.3498X + 5.334 × 10−2 | 0.9956 | 0.6–2230 | 0.6 |
| Berberine | Y = 18.189X + 0.117 × 10−1 | 0.9985 | 0.1–422 | 0.1 |
| Worenine | Y = 2.4691X + 3.378 × 10−3 | 0.9907 | 0.6–2220 | 0.6 |
| Berberrubine | Y = 12.738X + 1.730 × 10−3 | 0.9945 | 1.1–4420 | 1.1 |
| Coptisine | Y = 2.5543X + 3.665 × 10−2 | 0.9905 | 0.2–800 | 0.2 |
| Compounds | Spiked Concentration (ng/mL) | Measured CONC (ng/mL) | Accuracy (%) | Intra-Day Precision (%) | Inter-Day Precision (%) |
|---|---|---|---|---|---|
| Tetrahydropalmatine | 0.5 | 0.5 ± 0.1 | −1.0 | 17.6 | 18.8 |
| 2.0 | 2.1 ± 0.3 | −3.4 | 13.6 | 3.7 | |
| 50.7 | 53.1 ± 6.9 | −3.0 | 12.9 | 12.9 | |
| 1622 | 1759 ± 172 | 8.4 | 9.1 | 13.8 | |
| Palmatine | 0.1 | 0.1 ± 0.02 | 8.5 | 18.5 | 15.6 |
| 0.4 | 0.5 ± 0.07 | 11.2 | 13.4 | 11.9 | |
| 10.7 | 11.1 ± 1.5 | 3.4 | 14.2 | 10.3 | |
| 342 | 368 ± 41.8 | 7.5 | 11.8 | 6.7 | |
| Magnoflorine | 1.1 | 1.3 ± 0.1 | 14.1 | 11.7 | 6.6 |
| 4.3 | 4.3 ± 1.1 | 7.1 | 14.3 | 4.7 | |
| 108 | 116 ± 13.8 | 7.5 | 12.0 | 10.3 | |
| 3456 | 3817 ± 485 | 10.4 | 12.5 | 14.4 | |
| Columbamine | 0.6 | 0.6 ± 0.04 | −14.4 | 14.9 | 14.2 |
| 2.2 | 2.5 ± 0.3 | 9.8 | 14.3 | 3.5 | |
| 55.8 | 53.9 ± 6.7 | −3.4 | 12.3 | 13.5 | |
| 1784 | 1713 ± 193 | −4.0 | 11.0 | 13.4 | |
| Berberine | 0.1 | 0.1 ± 0.01 | −2.9 | 10.0 | 17.8 |
| 0.4 | 0.5 ± 0.1 | 5.5 | 14.3 | 6.3 | |
| 11.1 | 10.7 ± 1.0 | −7.3 | 8.7 | 12.4 | |
| 354 | 317 ± 30.7 | −10.4 | 10.0 | 6.8 | |
| Worenine | 0.6 | 0.6 ± 0.1 | −0.1 | 16.8 | 13.9 |
| 2.2 | 2.2 ± 0.3 | −4.3 | 14.0 | 10.3 | |
| 55.5 | 51.3 ± 6.1 | −7.4 | 12.0 | 12.0 | |
| 1776 | 1715 ± 90.6 | −3.4 | 4.9 | 7.6 | |
| Berberrubine | 1.1 | 1.1 ± 1.1 | 0.8 | 14.3 | 9.2 |
| 4.4 | 5.3 ± 0.6 | 14.1 | 11.6 | 8.4 | |
| 111 | 112 ± 14.0 | 1.5 | 13.1 | 6.2 | |
| 3536 | 3949 ± 437 | 11.7 | 11.3 | 9.4 | |
| Coptisine | 0.2 | 0.2 ± 0.03 | 13.4 | 13.8 | 1.8 |
| 0.8 | 0.8 ± 0.1 | 2.1 | 12.7 | 9.9 | |
| 20.0 | 22.4 ± 2.6 | 11.8 | 11.9 | 9.0 | |
| 640 | 555 ± 70.3 | −13.3 | 13.1 | 8.4 |
| Compounds | Spiked Concentration (ng/mL) | Matrix Effect | Extraction Recovery | ||
|---|---|---|---|---|---|
| Mean (%) | RSD (%) | Mean (%) | RSD (%) | ||
| Tetrahydropalmatine | 2.0 | 105.1 | 8.4 | 94.8 | 14.2 |
| 50.7 | 100.5 | 8.2 | 95.9 | 7.8 | |
| 1622 | 104.4 | 7.2 | 94.7 | 4.2 | |
| Palmatine | 0.4 | 101.7 | 6.2 | 93.1 | 13.6 |
| 10.7 | 93.7 | 11.2 | 86.4 | 5.1 | |
| 342 | 101.8 | 12.5 | 95.6 | 3.0 | |
| Magnoflorine | 4.3 | 99.1 | 11.4 | 93.4 | 5.5 |
| 108 | 85.2 | 13.1 | 90.0 | 4.1 | |
| 3456 | 98.5 | 13.4 | 86.4 | 14.3 | |
| Columbamine | 2.2 | 100.0 | 13.5 | 100.0 | 6.2 |
| 55.8 | 98.8 | 5.9 | 90.8 | 8.9 | |
| 1784 | 101.2 | 9.6 | 98.4 | 12.0 | |
| Berberine | 0.4 | 101.8 | 12.3 | 92.3 | 8.2 |
| 11.1 | 100.5 | 9.3 | 93.2 | 4.0 | |
| 354 | 102.3 | 5.0 | 97.6 | 11.1 | |
| Worenine | 2.2 | 92.5 | 7.8 | 98.9 | 12.1 |
| 55.5 | 87.7 | 10.0 | 88.0 | 9.8 | |
| 1776 | 105.6 | 5.1 | 93.4 | 1.8 | |
| Berberrubine | 4.4 | 94.7 | 2.4 | 90.6 | 10.2 |
| 111 | 98.0 | 11.3 | 91.2 | 2.6 | |
| 3536 | 100.8 | 4.3 | 93.4 | 11.5 | |
| Coptisine | 0.8 | 106.8 | 3.9 | 92.3 | 7.4 |
| 20.0 | 99.5 | 5.7 | 92.2 | 4.2 | |
| 640 | 89.1 | 5.1 | 89.0 | 8.4 | |
| I.S. | 2000 | 98.2 | 11.1 | 88.7 | 13.7 |
| Compounds | Spiked Concentration (ng/mL) | Stability (% RE) | |||
|---|---|---|---|---|---|
| Freeze-Thaw | Short Term | Long Term | Post Preparative | ||
| Tetrahydropalmatine | 2.0 | −10.9 | 13.3 | 13.6 | 2.8 |
| 50.7 | −13.8 | 12.5 | 13.5 | −0.8 | |
| 1622 | 6.2 | 8.0 | 14.5 | −5.5 | |
| Palmatine | 0.4 | −0.2 | 14.9 | 5.9 | 8.5 |
| 10.7 | −2.3 | −9.6 | −11.2 | −12.4 | |
| 342 | −2.8 | −1.7 | 12.9 | −3.0 | |
| Magnoflorine | 4.3 | 12.2 | 10.6 | 9.4 | 10.7 |
| 108 | −3.4 | 14.1 | 13.6 | −1.0 | |
| 3456 | 13.3 | 7.4 | 13.2 | 6.7 | |
| Columbamine | 2.2 | 12.3 | 11.9 | 11.7 | 6.9 |
| 55.8 | −10.2 | −0.3 | −2.5 | −11.2 | |
| 1784 | 1.9 | −4.2 | −5.9 | −1.8 | |
| Berberine | 0.4 | 9.4 | 1.2 | 2.4 | 2.9 |
| 11.1 | −12.5 | −1.0 | −14.6 | 2.6 | |
| 354 | −12.5 | −4.1 | −11.0 | −4.2 | |
| Worenine | 2.2 | 5.6 | −12.9 | −7.8 | 12.3 |
| 55.5 | 4.7 | 8.9 | 9.9 | 5.6 | |
| 1776 | −5.4 | −9.8 | −4.3 | −6.6 | |
| Berberrubine | 4.4 | 8.1 | 8.2 | 8.0 | 13.9 |
| 111 | 11.3 | 0.01 | −2.5 | 4.4 | |
| 3536 | 6.9 | 8.6 | −0.6 | 11.2 | |
| Coptisine | 0.8 | 13.1 | −14.4 | 8.7 | 5.1 |
| 20.0 | 2.1 | −6.1 | −5.6 | 1.8 | |
| 640 | 8.4 | −4.8 | 0.6 | 12.2 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Wang, Z.-B.; Song, Y.; Yang, J.; Wu, L.-J.; Yang, B.-Y.; Wang, Q.-H.; Wang, L.-Q.; Wang, R.-X.; Yang, C.-J. Simultaneous Determination of Eight Alkaloids in Rat Plasma by UHPLC-MS/MS after Oral Administration of Coptis deltoidea C. Y. Cheng et Hsiao and Coptis chinensis Franch. Molecules 2016, 21, 913. https://doi.org/10.3390/molecules21070913
Liu L, Wang Z-B, Song Y, Yang J, Wu L-J, Yang B-Y, Wang Q-H, Wang L-Q, Wang R-X, Yang C-J. Simultaneous Determination of Eight Alkaloids in Rat Plasma by UHPLC-MS/MS after Oral Administration of Coptis deltoidea C. Y. Cheng et Hsiao and Coptis chinensis Franch. Molecules. 2016; 21(7):913. https://doi.org/10.3390/molecules21070913
Chicago/Turabian StyleLiu, Lu, Zhi-Bin Wang, Yang Song, Jing Yang, Li-Jun Wu, Bing-You Yang, Qiu-Hong Wang, Li-Qian Wang, Ru-Xuan Wang, and Chun-Juan Yang. 2016. "Simultaneous Determination of Eight Alkaloids in Rat Plasma by UHPLC-MS/MS after Oral Administration of Coptis deltoidea C. Y. Cheng et Hsiao and Coptis chinensis Franch" Molecules 21, no. 7: 913. https://doi.org/10.3390/molecules21070913
APA StyleLiu, L., Wang, Z.-B., Song, Y., Yang, J., Wu, L.-J., Yang, B.-Y., Wang, Q.-H., Wang, L.-Q., Wang, R.-X., & Yang, C.-J. (2016). Simultaneous Determination of Eight Alkaloids in Rat Plasma by UHPLC-MS/MS after Oral Administration of Coptis deltoidea C. Y. Cheng et Hsiao and Coptis chinensis Franch. Molecules, 21(7), 913. https://doi.org/10.3390/molecules21070913






