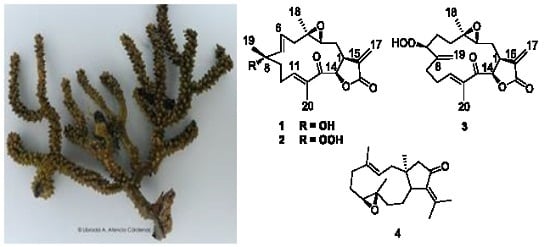
Uprolides N, O and P from the Panamanian Octocoral Eunicea succinea
Abstract
:1. Introduction
2. Results and Discussion
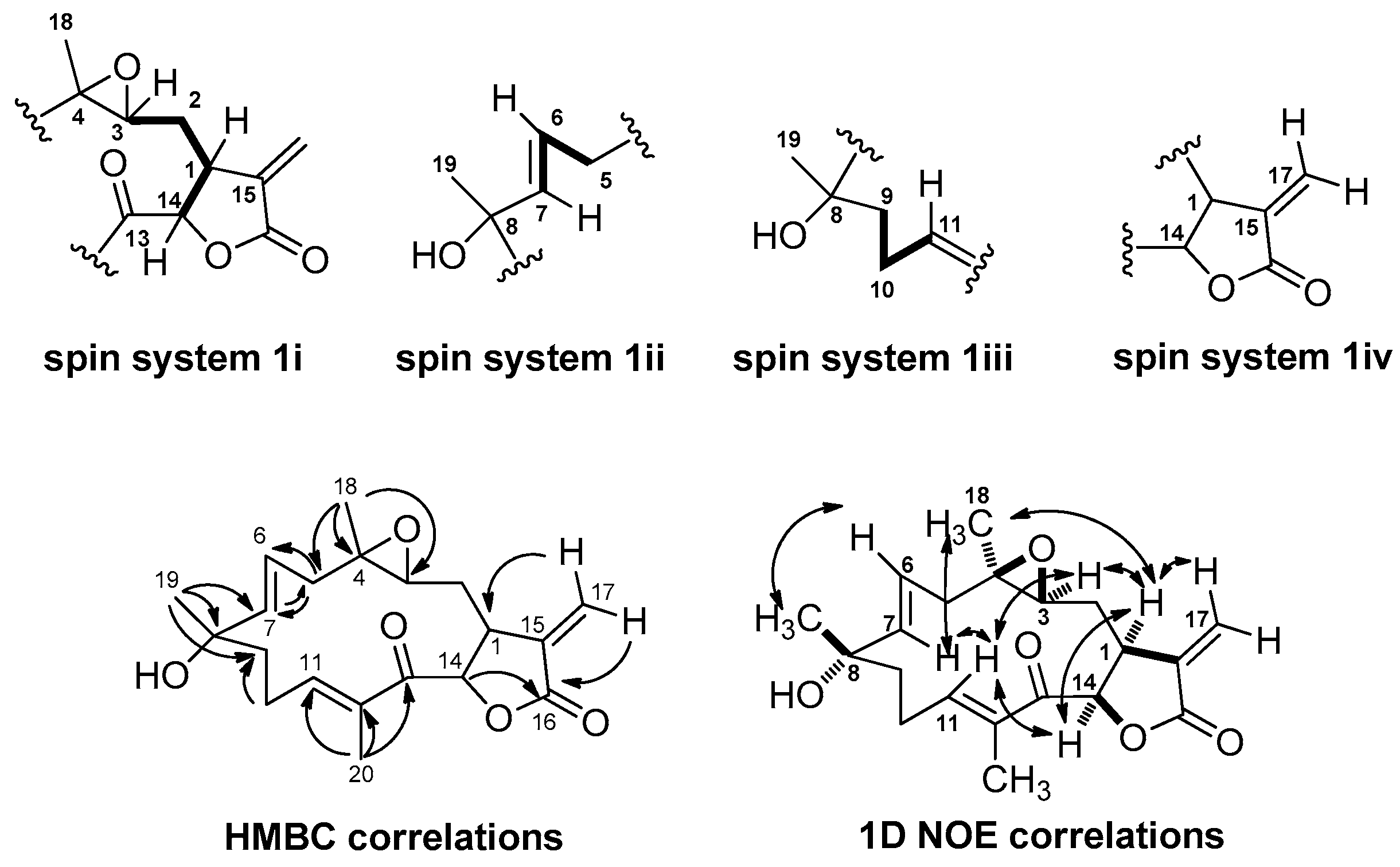
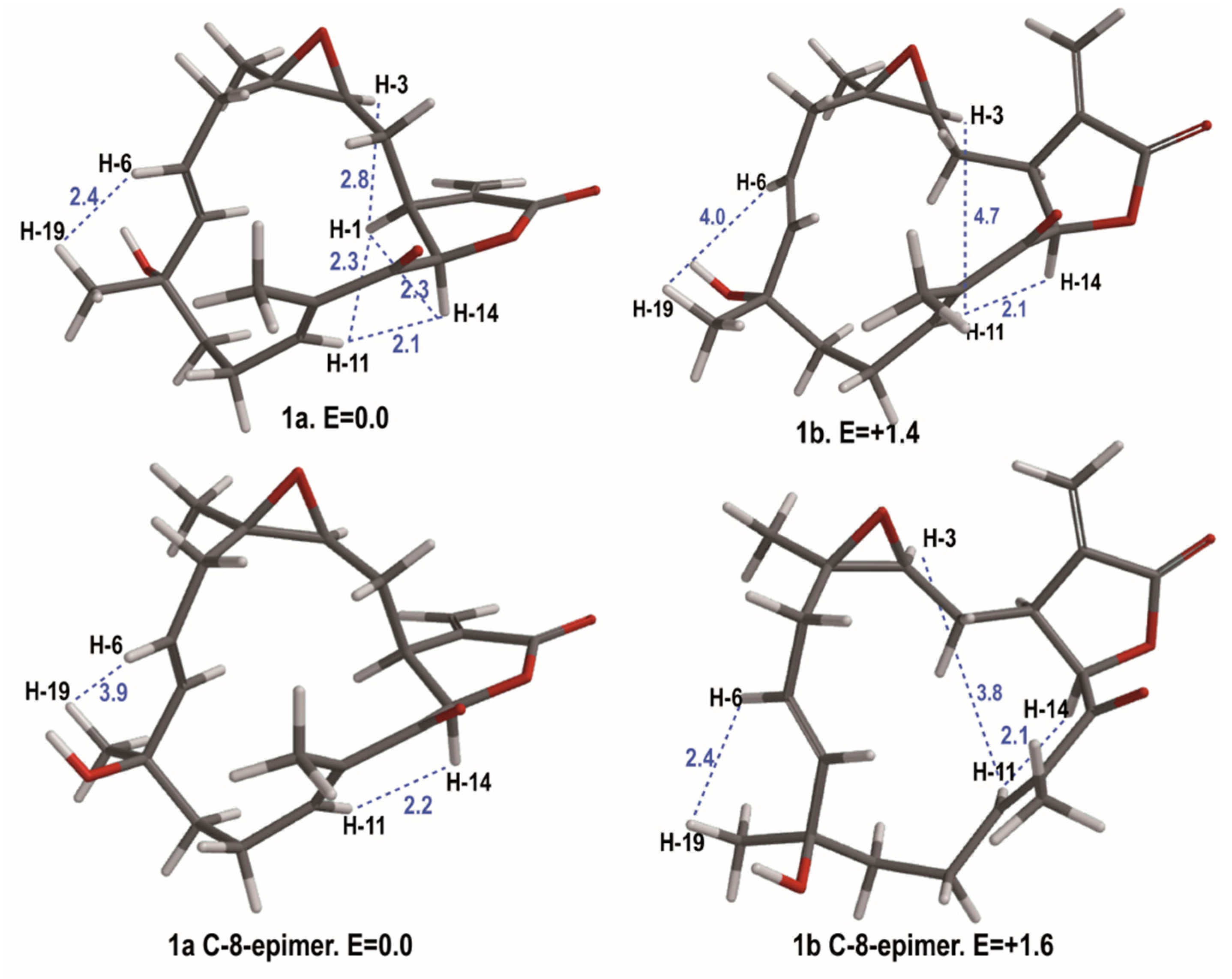
2.1. Isolation and Structural Elucidation
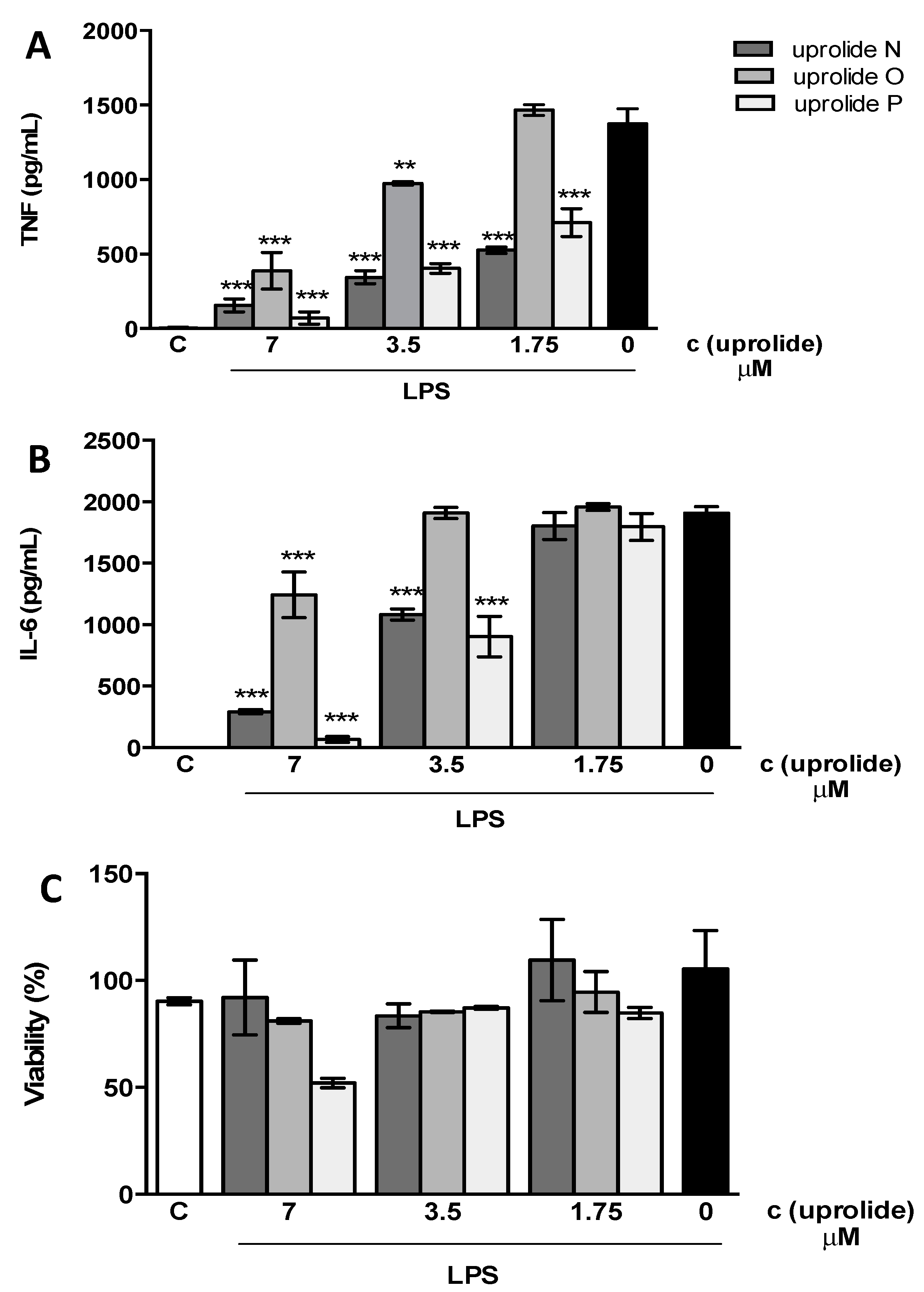
2.2. Anti-inflammatory Effect
3. Experimental Section
3.1. General Procedures
3.2. Animal Material
3.3. Extraction and Isolation
3.4. Compound Characterization
3.5. Molecular Modeling Studies
3.6. Cell Culture and Cytokines Determination
3.7. Cytotoxicity Assay
3.8. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Berrue, F.; Kerr, R.G. Diterpenes from gorgonian corals. Nat. Prod. Rep. 2009, 26, 681–710. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Keyzers, R.A.; Munro, M.H.G.; Prinsep, M.R. Marine Natural Products. Nat. Prod. Rep. 2016, 33, 382–431. [Google Scholar] [CrossRef] [PubMed]
- Sammarco, P.W.; Coll, J.C. Chemical adaptations in the Octocorallia: Evolutionary considerations. Mar. Ecol. Prog. Ser. 1992, 88, 93–104. [Google Scholar] [CrossRef]
- Weinheimer, A.; Middlebrook, R.E.; Bledsoe, J.O.; Marsico, W.E.; Karns, T.K.B. Eunicin, an oxa-bridged cembranolide of marine origin. Chem. Comm. 1968, 384–385. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Soto, J.J.; Piña, I.C. Uprolides D-G, 2. A rare family of 4,7-oxa-bridged cembranolides from the Caribbean gorgonian Eunicea mammosa. J. Nat. Prod. 1995, 58, 1209–1216. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, A.D.; Piña, I.C.; Soto, J.J.; Rojas, D.R.; Barnes, C.L. Isolation and structure of the uprolides. I. Thirteen new cytotoxic cembranolides from the Caribbean gorgonian Eunicea mammosa. Can. J. Chem. 1995, 73, 643–654. [Google Scholar] [CrossRef]
- Rodríguez, A.D.; Acosta, A.L. New Cembranolides from the Gorgonian Eunicea succinea. J. Nat. Prod. 1998, 61, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y-P.; Rodríguez, A.D.; Barnes, C.L.; Sánchez, J.A.; Raptis, R.G.; Baran, P. New Terpenoid Constituents from Eunicea pinta. J. Nat. Prod. 2002, 65, 1232–1241. [Google Scholar]
- Rodríguez, A.D.; Dhasmana, H. Further Bioactive Cembranolide Diterpenes from the Gorgonian Eunicea succinea. J. Nat. Prod. 1993, 56, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Rodríguez, A.D.; Baran, P.; Raptis, R.G.; Sánchez, J.A.; Ortega-Barría, E.; González, J. Antiplasmodial cembradiene diterpenoids from a Southwestern Caribbean gorgonian octocoral of the genus Eunicea. Tetrahedron 2004, 60, 11813–11819. [Google Scholar] [CrossRef]
- Chang, C.Y.; Ciereszko, L.S.; Hossain, M.B.; van Der Helm, D. Structure of Peunicin. Acta Cryst. 1980, B36, 731–733. [Google Scholar] [CrossRef]
- Kolossváry, I.; Guida, W.C. Low Mode Search. An Efficient, Automated Computational Method for Conformational Analysis: Application to Cyclic and Acyclic Alkanes and Cyclic Peptides. J. Am. Chem. Soc. 1996, 118, 5011–5019. [Google Scholar] [CrossRef]
- Mohamadi, F.; Richards, N.G.J.; Guida, W.C.; Liskamp, R.; Lipton, M.; Caufield, C.; Chang, G.; Hendrickson, T.; Still, W.C. MacroModel-An Integrated Software System for Modeling Organic and Bioorganic Molecules Using Molecular Mechanics. J. Comput. Chem. 1990, 11, 440–467. [Google Scholar] [CrossRef]
- Kawai, T.; Akira, S. The role of pattern-recognition receptors in innate immunity: Update on Toll-like receptors. Nat. Immunol. 2010, 11, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Mariottini, G.L.; Pane, L. Cytotoxic and Cytolytic Cnidarian Venoms. A Review on Health Implications and Possible Therapeutic applications. Toxins 2014, 6, 108–151. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.-Y.; Lu, Y.; Chen, B.-W.; Huang, C-Y.; Su, J-H.; Wen, Z-H.; Dai, C.-F.; Kuo, Y.-H.; Sheu, J.-H. Sarcocrassocolides M-O, Bioactive Cembranoids from the Dongsha Atoll Soft Coral Sarcophyton crassocaule. Mar. Drugs 2012, 10, 617–626. [Google Scholar] [CrossRef] [PubMed]
- Look, S.A.; Fenical, W. New Bicyclic Diterpenoids from the Caribbean Gorgonian Octocoral Eunicea calyculata. J. Org. Chem. 1982, 47, 4129–4134. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–3 are available from the authors.
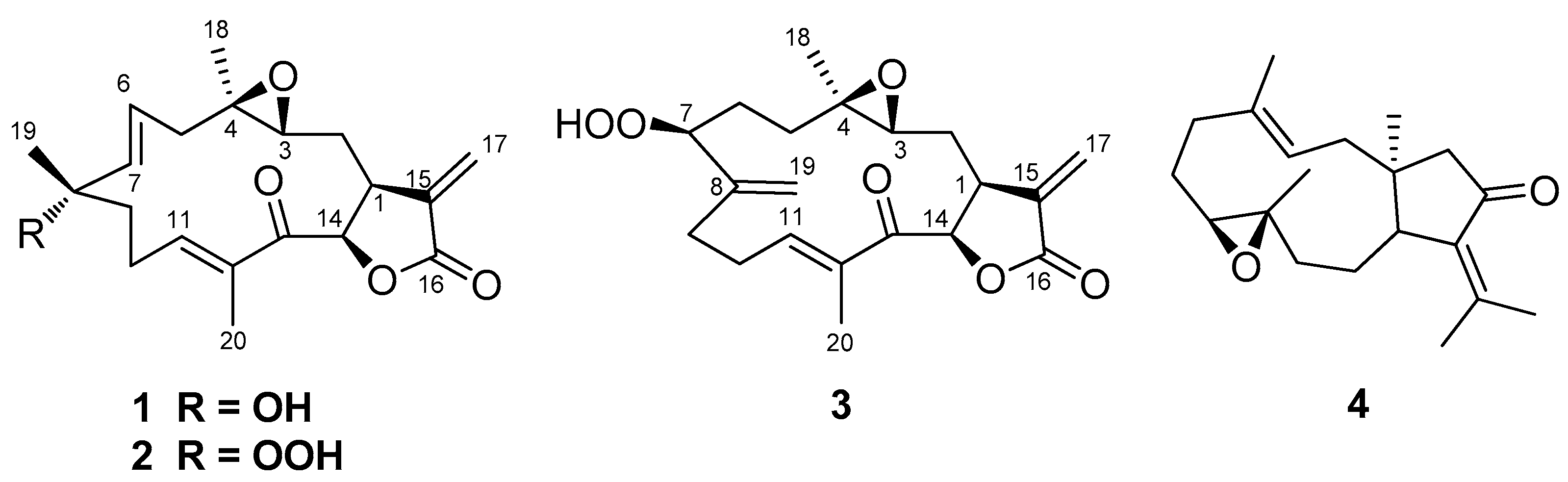
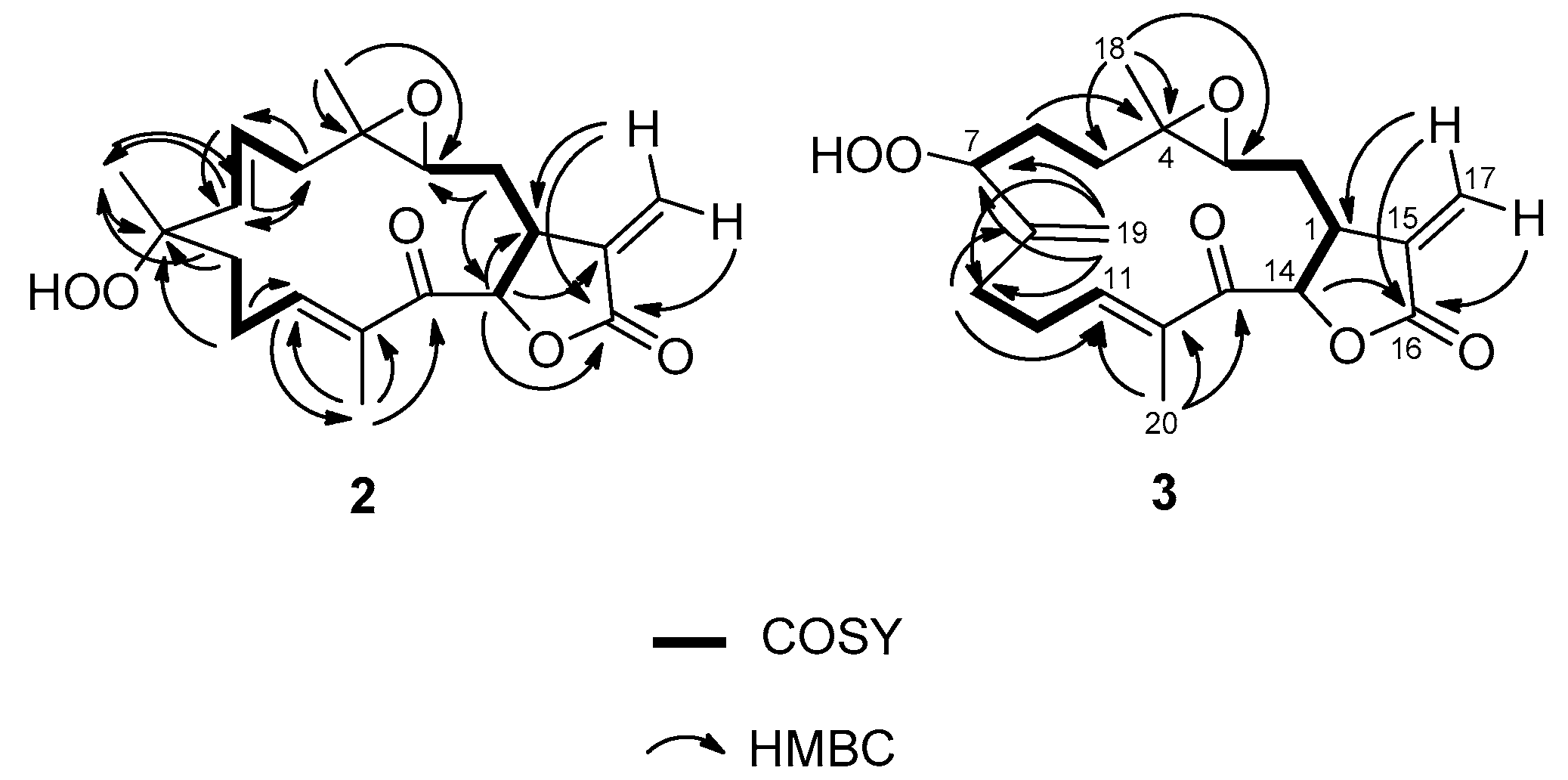
| Position | Uprolide N (1) | Uprolide O (2) | Uprolide P (3) | |||
|---|---|---|---|---|---|---|
| δC | δH (J in Hz) | δC | δH (J in Hz) | δC | δH (J in Hz) | |
| 1 | 41.7, CH | 3.17, m | 41.6, CH | 3.17, m | 40.8, CH | 3.26, m |
| 2 | 27.7,CH2 | 1.74, m | 27.6, CH2 | 1.74, m | 29.1, CH2 | 1.95, m 1.69, m |
| 3 | 56.3, CH | 2.65, dd (4.0, 9.9) | 56.2, CH | 2.72, m | 56.6, CH | 2.47, dd (4.7, 9.5) |
| 4 | 60.9, C | 60.8, C | 59.2, C | |||
| 5a 5b | 39.2, CH2 | 2.58, m 2.43, m | 39.1, CH2 | 2.51, m 2.65, m | 31.9, CH2 | 1.24, m |
| 6 | 124.2, CH | 5.34, ddd (4.7, 9.8, 15.3) | 128.3, CH | 5.45, ddd (4.3, 10.2, 16.5) | 30.8, CH2 | 1.84, m 1.61, m |
| 7 | 140.7, CH | 5.65, d (15.3) | 136.5, CH | 5.63, d (16.5) | 87.3, CH | 4.28, br t (6.9) |
| 8 | 73.1, C | 84.4, C | 145.7, C | |||
| 9 | 41.6, CH2 | 1.97, m | 36.9, CH2 | 2.01, m | 30.2, CH2 | 2.40, m 2.34, m |
| 10 | 25.3, CH2 | 2.40, m | 24.8, CH2 | 2.45, m | 28.3, CH2 | 2.72, m 2.51, m |
| 11 | 147.8, CH | 6.81, br t (6.6) | 147.2, CH | 6.76, br t (5.9) | 145.4, CH | 6.91 br dd (4.8, 9.1) |
| 12 | 137.2, C | 137.5, C | 138.0, C | |||
| 13 | 195.2, C | 195.2, C | 195.5, C | |||
| 14 | 72.5, CH | 5.80, br d (7.3) | 72.9, CH | 5.80, d (8.0) | 73.3, CH | 5.82, d (8.0) |
| 15 | 136.1, C | 136.1, C | 136.8, C | |||
| 16 | 169.4, C | 169.4, C | 169.3, C | |||
| 17a 17b | 120.0, CH2 | 6.28, d (3.3) 5.44, d (3.3) | 120.0, CH2 | 6.28, d (3.3) 5.44, d (3.3) | 120.8, CH2 | 6.28, d (2.2) 5.47, d (2.2) |
| 18 | 18.2, CH3 | 1.37, s | 18.2, CH3 | 1.36, s | 18.0, CH3 | 1.32, s |
| 19a 19b | 25.9, CH3 | 1.34, s | 19.5, CH3 | 1.35, s | 113.7, CH2 | 5.25, s 5.15, s |
| 20 | 11.4, CH3 | 1.83, s | 11.3, CH3 | 1.82, s | 11.2, CH3 | 1.86, s |
| Mediator | IC50 ± S.D. (μM) | |||
|---|---|---|---|---|
| Uprolide N (1) | Uprolide O (2) | Uprolide P (3) | Dolabellane (4) | |
| TNF | 1.39 ± 0.48 | 2.73 ± 0.03 | 2.27 ±0.91 | n.a. |
| IL-6 | 3.26 ± 0.32 | 4.22 ± 1.52 | 2.60 ±1.47 | n.a. |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Mendoza, D.; González, Y.; Gómez-Reyes, J.F.; Guzmán, H.M.; López-Perez, J.L.; Gerwick, W.H.; Fernandez, P.L.; Gutiérrez, M. Uprolides N, O and P from the Panamanian Octocoral Eunicea succinea. Molecules 2016, 21, 819. https://doi.org/10.3390/molecules21060819
Torres-Mendoza D, González Y, Gómez-Reyes JF, Guzmán HM, López-Perez JL, Gerwick WH, Fernandez PL, Gutiérrez M. Uprolides N, O and P from the Panamanian Octocoral Eunicea succinea. Molecules. 2016; 21(6):819. https://doi.org/10.3390/molecules21060819
Chicago/Turabian StyleTorres-Mendoza, Daniel, Yisett González, José Félix Gómez-Reyes, Héctor M. Guzmán, José Luis López-Perez, William H. Gerwick, Patricia L. Fernandez, and Marcelino Gutiérrez. 2016. "Uprolides N, O and P from the Panamanian Octocoral Eunicea succinea" Molecules 21, no. 6: 819. https://doi.org/10.3390/molecules21060819
APA StyleTorres-Mendoza, D., González, Y., Gómez-Reyes, J. F., Guzmán, H. M., López-Perez, J. L., Gerwick, W. H., Fernandez, P. L., & Gutiérrez, M. (2016). Uprolides N, O and P from the Panamanian Octocoral Eunicea succinea. Molecules, 21(6), 819. https://doi.org/10.3390/molecules21060819