Phenolic Profile and Antioxidant Potential of Leaves from Selected Cotoneaster Medik. Species
Abstract
:1. Introduction
2. Results and Discussion
2.1. Qualitative UHPLC-PDA-ESI-QTOF-MS Profiling of Cotoneaster Leaf Phenolics
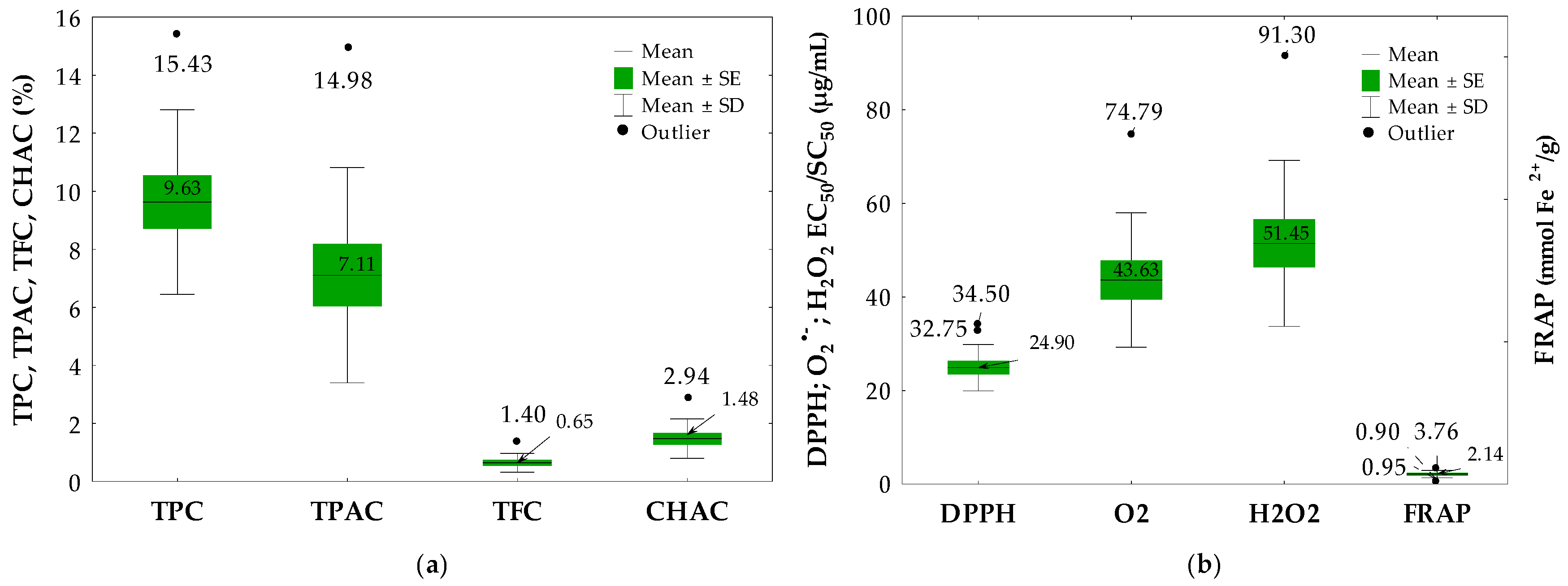
2.2. Quantitative Determination of Phenolics, Proanthocyanidins, Flavonoids and Chlorogenic Acid Isomers in the Cotoneaster Leaf Extracts
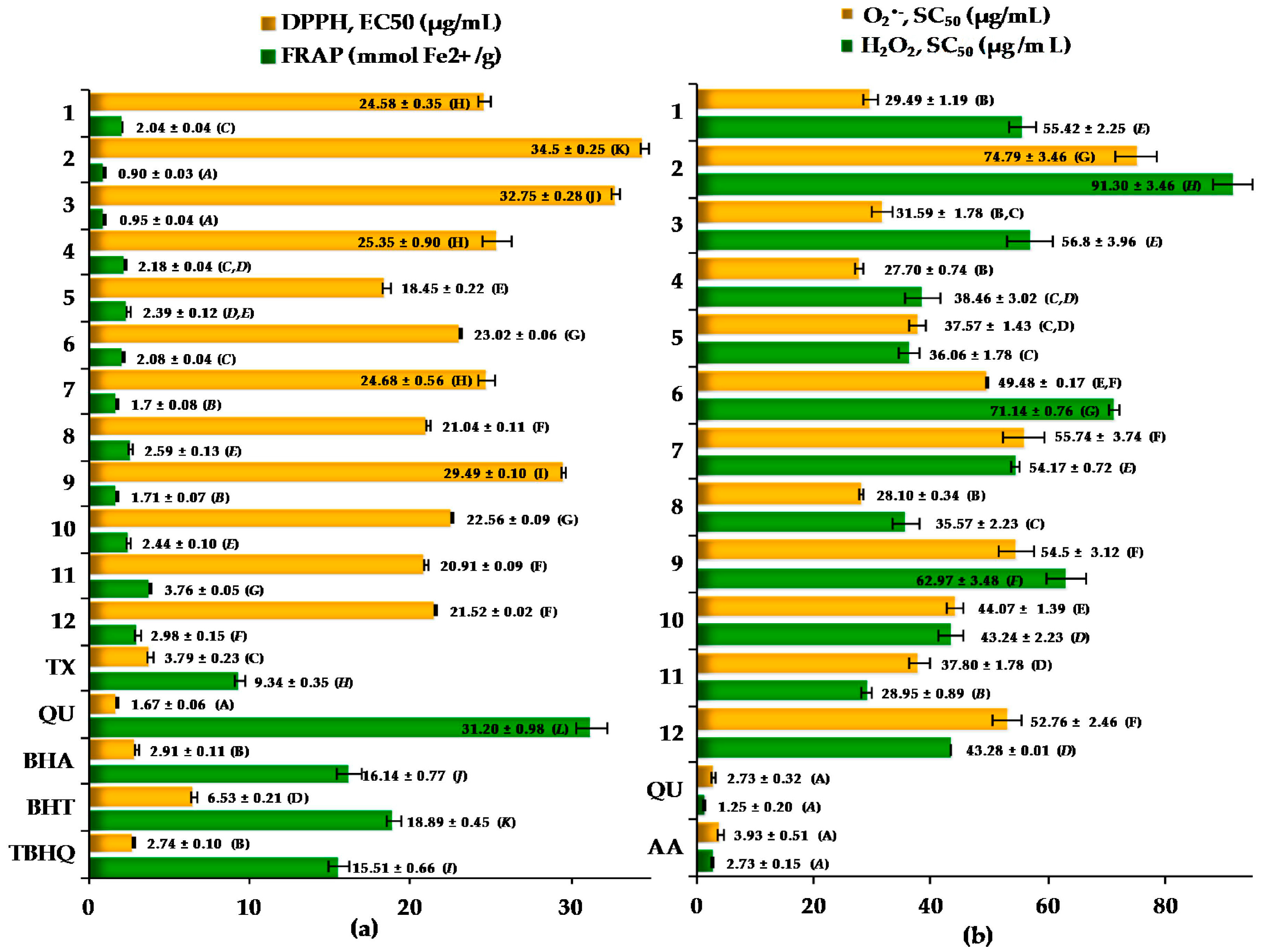
2.3. Antioxidant Activity of the Cotoneaster Leaf Extracts
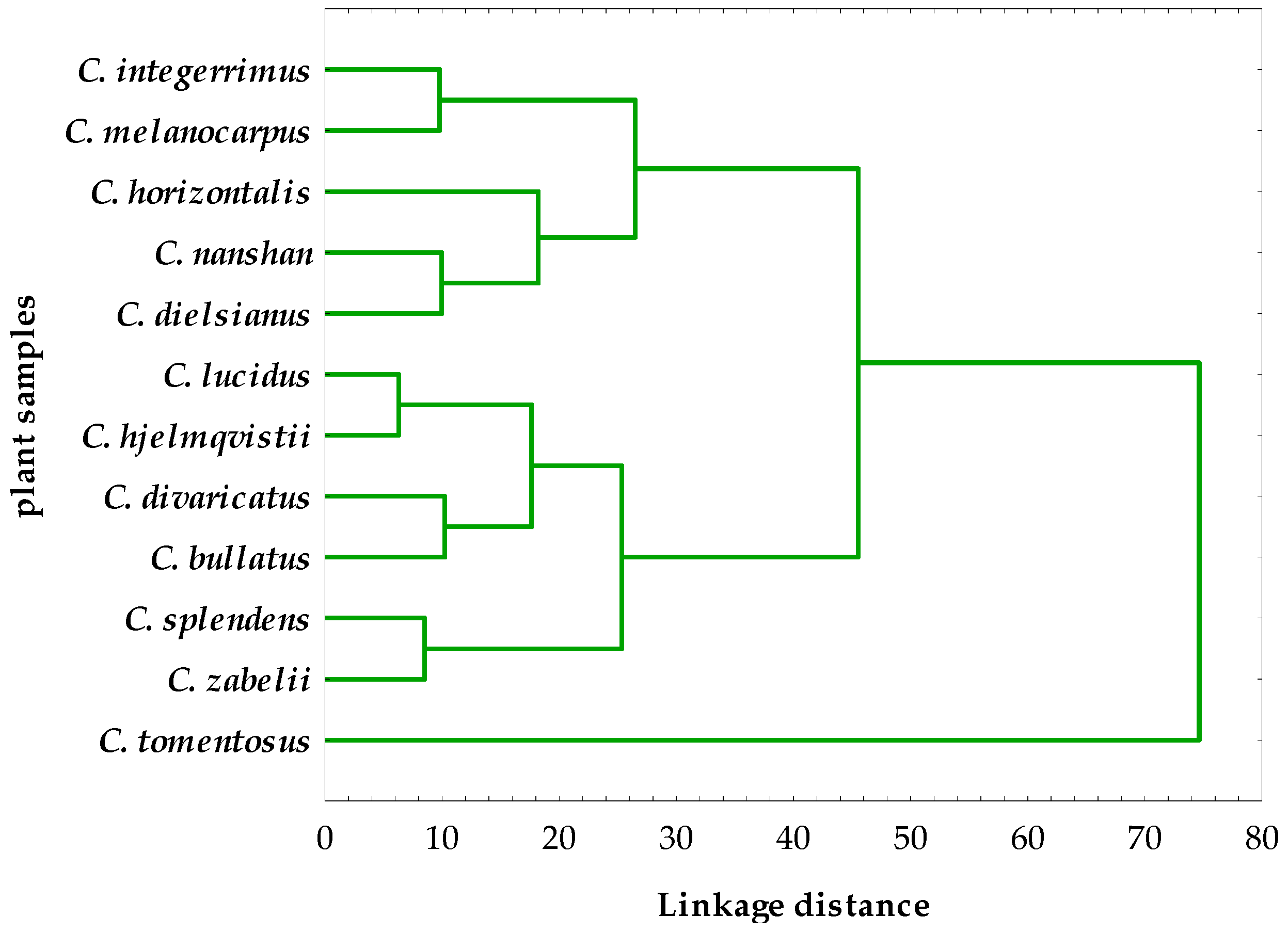
2.4. Hierarchical Cluster Analysis of the Phytochemical and Activity Data
3. Materials and Methods
3.1. Plant Material
3.2. General
3.3. Extraction and Hydrolysis Procedures
3.4. Phytochemical Profiling
3.4.1. UHPLC-PDA-ESI-QTOF-MS and HPLA-PDA Analyses
3.4.2. Determination of Total Flavonoid Content (TFC)
3.4.3. Determination of Chlorogenic Acid Isomers (CHAC)
3.4.4. Determination of Total Phenolic Content (TPC)
3.4.5. Determination of Total Proanthocyanidin Content (TPAC)
3.5. Antioxidant Activity Testing
3.5.1. DPPH Free Radical Scavenging Assay
3.5.2. Ferric Reducing Antioxidant Power (FRAP) Assay
3.5.3. Superoxide Anion Radical (O2•−) Scavenging Assay
3.5.4. Hydrogen Peroxide (H2O2) Scavenging Assay
3.6. Statistical and Data Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aseervatham, G.S.B.; Sivasudha, T.; Jeyadevi, R.; Ananth, D.A. Environmental factors and unhealthy lifestyle influence oxidative stress in humans—An overview. Environ. Sci. Pollut. Res. 2013, 20, 4356–4369. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Li, A.N.; Li, S.; Li, H.B.; Xu, D.P.; Xu, X.R.; Chen, P. Total phenolic contents and antioxidant capacities of 51 edible and wild flowers. J. Funct. Food 2014, 6, 319–330. [Google Scholar] [CrossRef]
- Fu, L.; Xu, B.-T.; Xu, X.-R.; Gan, R.-Y.; Zhang, Y.; Xia, E.-Q.; Li, H.-B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011, 129, 345–350. [Google Scholar] [CrossRef]
- Edwards, J.E.; Brown, P.N.; Talent, N.; Dickinson, T.A.; Shipley, P.R. A review of the chemistry of the genus Crataegus. Phytochemistry 2012, 79, 5–26. [Google Scholar] [CrossRef] [PubMed]
- Denev, P.N.; Kratchanov, C.G.; Ciz, M.; Lojek, A.; Kratchanova, M.G. Bioavailability and antioxidant activity of black chokeberry (Aronia melanocarpa) polyphenols: In vitro and in vivo evidences and possible mechanisms of action: A review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 471–489. [Google Scholar] [CrossRef]
- Verma, R.; Gangrade, T.; Punasiya, R.; Ghulaxe, C. Rubus fruticosus (blackberry) use as an herbal medicine. Pharmacogn. Rev. 2014, 8, 101–104. [Google Scholar] [PubMed]
- Liaudanskas, M.; Viškelis, P.; Raudonis, R.; Kviklys, D.; Uselis, N.; Janulis, V. Phenolic composition and antioxidant activity of Malus domestica leaves. Sci. World J. 2014, 2014, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Dickoré, W.B.; Kasperek, G. Species of Cotoneaster (Rosaceae, Maloideae) indigenous to, naturalising or commonly cultivated in Central Europe. Willdenowia 2010, 40, 13–45. [Google Scholar] [CrossRef]
- Jerzak, E. Cotoneaster Species Cultivated in Poland (in Polish); Officina Botanica: Krakow, Poland, 2007. [Google Scholar]
- Slabaugh, P.E.; Shaw, N.L. Cotoneaster Medik. In The Woody Plant Seed Manual; Bonner, F.T., Karrfalt, R.P., Eds.; U.S. Department of Agriculture, Forest Service: Washington, DC, USA, 2008; pp. 442–446. [Google Scholar]
- Holzer, V.M.D.; Lower-Nedza, A.D.; Nandintsetseg, M.; Batkhuu, J.; Brantner, A.H. Antioxidant constituents of Cotoneaster melanocarpus Lodd. Antioxidants 2013, 2, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, S.; Ghiaee, A.; Naghibi, F.; Mosaddegh, M. Antiplasmodial activity and cytotoxicity of plants used in traditional medicine of Iran for the treatment of fever. Iran J. Pharm. Res. 2015, 14, 103–107. [Google Scholar] [PubMed]
- Azadbakht, M.; Pishva, N.; Mohammadi-Samani, S.; Alinejad, F. The effect of purgative manna on the infant jaundice. Iran. J. Pharm. Sci. 2005, 1, 95–100. [Google Scholar]
- Yaghooti, F.; Sani, A.M. Antibacterial activity of methanolic extracts from Cotoneaster nummularioides, Cynodon dactylon and Cardaria draba on typical food-borne pathogens. Inter. J. Biosci. 2015, 6, 349–356. [Google Scholar]
- Zengin, G.; Uysal, A.; Gunes, E.; Aktumsek, A. Survey of phytochemical composition and biological effects of three extracts from a wild plant (Cotoneaster nummularia Fisch. et Mey.): A potential source for functional food ingredients and drug formulations. PLoS ONE 2014, 9, e113527. [Google Scholar] [CrossRef] [PubMed]
- Sokkar, N.; El-Gindi, O.; Sayed, S.; Mohamed, S.; Ali, Z.; Alfishawy, I. Antioxidant, anticancer and hepatoprotective activities of Cotoneaster horizontalis Decne extract as well as α-tocopherol and amygdalin production from in vitro culture. Acta. Physiol. Plant 2013, 35, 2421–2428. [Google Scholar] [CrossRef]
- Mohamed, S.A.; Sokkar, N.M.; El-Gindi, O.; Ali, Z.Y.; Alfishawy, I.A. Phytoconstituents investigation, anti-diabetic and anti-dyslipidemic activities of Cotoneaster horizontalis Decne cultivated in Egypt. Life Sci. J. 2012, 9, 394–403. [Google Scholar]
- El-Mousallamy, A.M.; Hussein, S.A.; Merfort, I.; Nawwar, M.A. Unusual phenolic glycosides from Cotoneaster orbicularis. Phytochemistry 2000, 53, 699–704. [Google Scholar] [CrossRef]
- Palme, E.; Bilia, A.R.; Morelli, I. Flavonols and isoflavones from Cotoneaster simonsii. Phytochemistry 1996, 42, 903–905. [Google Scholar] [CrossRef]
- Palme, E.; Bilia, A.R.; de Feo, V.; Morelli, I. Flavonoid glycosides from Cotoneaster thymaefolia. Phytochemistry 1994, 35, 1381–1382. [Google Scholar] [CrossRef]
- Khan, S.; Riaz, N.; Afza, N.; Malik, A.; Aziz-ur-Rehman; Iqbal, L.; Lateef, M. Antioxidant constituents from Cotoneaster racemiflora. J. Asian Nat. Prod. Res. 2009, 11, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Sati, S.C.; Sati, M.; Sharma, A.; Joshi, M. Isolation and characterisation of phenolics from the roots of Cotoneaster acuminatus and determination of their antimicrobial activity. Int. J. Pharm. Pharm. Sci. 2010, 2, 58–60. [Google Scholar]
- Kokubun, T.; Harborne, J.B. Phytoalexin induction in the sapwood of plants of the Maloideae (Rosaceae): Biphenyls or dibenzofurans. Phytochemistry 1995, 40, 1649–1654. [Google Scholar] [CrossRef]
- Kokubun, T.; Harborne, J.B.; Eagles, J.; Waterman, P.G. Dibenzofuran phytoalexins from the sapwood of Cotoneaster acutifolius and five related species. Phytochemistry 1995, 38, 57–60. [Google Scholar] [CrossRef]
- Pashinina, L.T.; Chumbalov, T.K.; Sheichenko, V.I.; Shukenova, R.Z. Dimeric proanthocyanidins of Cotoneaster oligantha. Chem. Nat. Comp. 1978, 14, 166–172. [Google Scholar] [CrossRef]
- Khan, S.; Yasmeen, S.; Afza, N.; Malik, A.; Iqbal, L.; Lateef, M. Cotonoates A and B, new aromatic esters from Cotoneaster racemiflora. Z. Naturforsch. 2008, 63b, 1219–1222. [Google Scholar]
- Khan, S.; Wang, Z.; Wang, R.; Zhang, L. Horizontoates A–C: New cholinesterase inhibitors from Cotoneaster horizontalis. Phytochem. Lett. 2014, 10, 204–208. [Google Scholar] [CrossRef]
- Clifford, M.N.; Knight, S.; Kuhnert, N. Discriminating between the six isomers of dicaffeoylquinic acid by LC-MSn. J. Agric. Food Chem. 2005, 53, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Nandutu, A.M.; Clifford, M.; Howell, N.K. Analysis of phenolic compounds in Ugandan sweet potato varieties (NSP, SPK AND TZ). Afr. J. Biochem. Res. 2007, 1, 29–36. [Google Scholar]
- Hamed, A.I.; Al-Ayed, A.S.; Moldoch, J.; Piacenta, S.; Oleszek, W.; Stochmal, A. Profiles analysis of proanthocyanidins in the argun nut (Medemia argun—An ancien Egyptian palm) by LC-ESI-MS/MS. J. Mass Spectrom. 2014, 49, 306–315. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Demirata, B.; Ozyürek, M.; Celik, S.E.; Bektaşoğlu, B.; Berker, K.I.; Ozyurt, D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Molecules 2007, 12, 1496–1547. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Gawlik-Dziki, U. Polyphenols of Rosa L. leaves extracts and their radical scavenging activity. Z. Naturforsch. C. 2007, 62, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Olszewska, M.A.; Nowak, S.; Michel, P.; Banaszczak, P.; Kicel, A. Assessment of the content of phenolics and antioxidant action of inflorescences and leaves of selected species from the genus Sorbus sensu stricto. Molecules 2010, 15, 8769–8783. [Google Scholar] [CrossRef] [PubMed]
- Thi, N.D.; Hwang, E.S. Bioactive compound contents and antioxidant activity in Aronia (Aronia melanocarpa) leaves collected at different growth stages. Prev. Nutr. Food Sci. 2014, 19, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Nour, V.; Trandafir, I.; Cosmulescu, S. Antioxidant capacity, phenolic compounds and minerals content of blackcurrant (Ribes nigrum L.) leaves as influenced by harvesting date and extraction method. Ind. Crops Prod. 2014, 53, 133–139. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Beecher, G.R. Proanthocyanidins: Biological activities associated with human health. Pharm. Biol. 2004, 42, 2–20. [Google Scholar] [CrossRef]
- Zhou, H.C.; Tam, N.F.; Lin, Y.M.; Ding, Z.H.; Chai, W.M.; Wei, S.D. Relationships between degree of polymerization and antioxidant activities: A study on proanthocyanidins from the leaves of a medicinal mangrove plant Ceriops tagal. PLoS ONE 2014, 9, e107606. [Google Scholar]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive compounds. Phytother. Res. 2009, 23, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Leigh, M.J. Health benefits of grape seed proanthocyanidin extract (GSPE). Nutr. Noteworthy 2003, 6, 1–5. [Google Scholar]
- Nagels, L.; van Dongen, W.; de Brucker, J.; de Pooter, H. High-performance liquid chromatographic separation of naturally occurring esters of phenolic acids. J. Chromatogr. A 1980, 187, 181–187. [Google Scholar] [CrossRef]
- Kicel, A.; Wolbiś, M. Phenolic content and DPPH radical scavenging activity of the flowers and leaves of Trifolium repens. Nat. Prod. Commun. 2013, 1, 99–102. [Google Scholar]
- Olszewska, M.A.; Michel, P. Antioxidant activity of inflorescences, leaves and fruits of three Sorbus species in relation to their polyphenolic composition. Nat. Prod. Res. 2009, 23, 1507–1521. [Google Scholar] [CrossRef] [PubMed]
- Porter, L.J.; Hrstich, L.N.; Chan, B.G. The conversion of procyanidins and prodelphinidins to cyanidin and delphinidin. Phytochemistry 1986, 25, 223–230. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kicel, A.; Olszewska, M.A. Evaluation of antioxidant activity, and quantitative estimation of flavonoids, saponins and phenols in crude extract and dry fractions of Medicago lupulina aerial parts. Nat. Prod. Commun. 2015, 10, 483–486. [Google Scholar] [PubMed]
- Granica, S.; Czerwińska, M.E.; Piwowarski, J.P.; Ziaja, M.; Kiss, A.K. Chemical composition, antioxidative and anti-inflammatory activity of extracts prepared from aerial parts of Oenothera biennis L. and Oenothera paradoxa Hudziok obtained after seeds cultivation. J. Agric. Food Chem. 2013, 61, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Plant samples are available from the authors.
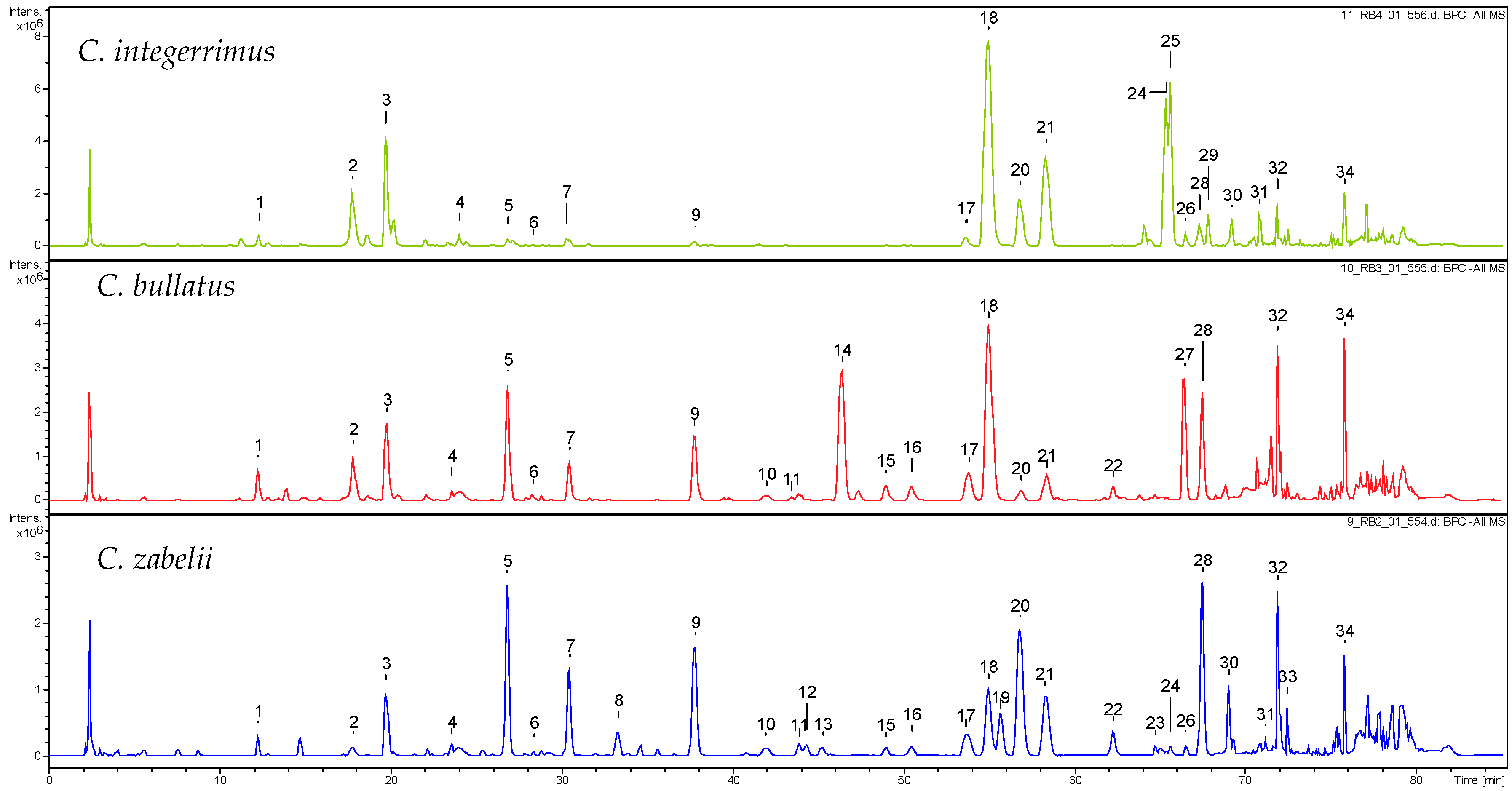
| No. a | Compound | tR (min) | UV (nm) | [M − H]− m/z | C. integerrimus | C. tomentosus | C. melanocarpus | C. lucidus | C. divaricatus | C. horizontalis | C. nanshan | C. hjelmqvistii | C. dielsianus | C. splendens | C. bullatus | C. zabelii |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3-O-caffeoylquinic acid (NCHA) b,c | 12.2 | 294, 325 | 353.1 | + | + | + | + | + | + | + | + | + | + | + | + |
| 2 | dicaffeoylquinic acid isomer d | 17.8 | 295, 325 | 515.1 | + | + | + | + | + | + | + | + | + | + | + | + |
| 3 | 5-O-caffeoylquinic acid (CHA) b,c | 19.7 | 294, 325 | 353.1 | + | + | + | + | + | + | + | + | + | + | + | + |
| 4 | 4-O-caffeoylquinic acid (CCHA) b,c | 23.6 | 294, 325 | 353.1 | + | + | + | + | + | + | + | + | + | + | + | + |
| 5 | procyanidin dimer B-2 c | 26.8 | 280 | 577.2 | + | + | + | + | + | + | + | + | + | + | + | + |
| 6 | 5-p-coumaroylquinic acid b | 27.9 | 289, 310 | 337.1 | + | + | + | + | + | + | + | + | + | + | + | + |
| 7 | (−)-epicatechin c | 30.5 | 280 | 289.1 | + | + | + | + | + | + | + | + | + | + | + | |
| 8 | caffeic acid derivative d | 33.3 | 290, 328 | 613.1 e | + | + | + | + | ||||||||
| 9 | procyanidin trimer C-1 c | 37.8 | 280 | 865.2 | + | + | + | + | + | + | + | + | + | + | + | + |
| 10 | procyanidin B-type tetramer d | 41.9 | 280 | 1153.1 | + | + | + | + | + | + | + | + | + | |||
| 11 | procyanidin B-type trimer d | 43.9 | 280 | 865.2 | + | + | + | + | + | + | ||||||
| 12 | quercetin 3-O-β-glucoside-7-O-α-rhamnoside c | 44.3 | 265, 350 | 609.2 | + | + | ||||||||||
| 13 | procyanidin B-type tetramer d | 45.6 | 280 | 1153.2 | + | + | + | + | ||||||||
| 14 | quercetin 3-O-β-(2′′-O-β-xylosyl)galactoside c | 46.3 | 268, 355 | 595.1 | + | + | + | + | + | + | ||||||
| 15 | epicatechin derivative d | 48.9 | 280 | 739.2 | + | + | + | + | + | + | + | + | + | + | + | |
| 16 | epicatechin derivative d | 50.5 | 280 | 739.2 | + | + | + | + | + | + | + | + | + | + | + | |
| 17 | quercetin rhamnoside-hexoside d | 53.7 | 265, 350 | 609.1 | + | + | + | + | + | + | + | + | + | + | + | + |
| 18 | hyperoside c | 55.0 | 265, 355 | 463.1 | + | + | + | + | + | + | + | + | + | + | + | + |
| 19 | quercetin dirhamnoside d | 55.7 | 275, 345 | 593.1 | + | + | + | |||||||||
| 20 | rutin c | 56.8 | 260, 355 | 609.1 | + | + | + | + | + | + | + | + | + | + | + | + |
| 21 | isoquercitrin c | 58.4 | 275, 350 | 463.1 | + | + | + | + | + | + | + | + | + | + | + | + |
| 22 | procyanidin B-type dimer d | 62.2 | 280 | 577.1 | + | + | + | + | + | + | ||||||
| 23 | procyanidin B-type trimer d | 64.7 | 280 | 865.2 | + | + | + | + | + | |||||||
| 24 | quercetin hexoside derivative d | 65.4 | 256, 355 | 505.1 | + | + | + | + | + | + | ||||||
| 25 | quercetin hexoside derivative d | 65.6 | 256, 355 | 505.1 | + | + | + | + | ||||||||
| 26 | kaempferol rhamnoside-hexoside d | 66.5 | 273, 345 | 593.1 | + | + | + | + | + | + | + | + | + | |||
| 27 | quercetin rhamnoside-hexoside d | 66.6 | 276, 350 | 609.1 | + | + | + | + | + | + | + | + | ||||
| 28 | quercitrin c | 67.3 | 276, 350 | 447.1 | + | + | + | + | + | + | + | + | + | + | + | |
| 29 | dicaffeoylquinic acid isomer d | 67.8 | 285, 325 | 515.1 | + | + | + | + | + | + | + | + | + | + | ||
| 30 | quercetin hexoside derivative d | 69.2 | 255, 355 | 505.1 | + | + | + | |||||||||
| 31 | dicaffeoylquinic acid isomer d | 70.9 | 286, 325 | 515.1 | + | + | + | + | + | + | + | + | + | + | ||
| 32 | unknown compound | 71.8 | 280 | 451.1 | + | + | + | + | + | + | + | + | + | + | + | + |
| 33 | kaempferol rhamnoside-hexoside d | 72.5 | 275, 345 | 593.1 | + | + | + | + | + | |||||||
| 34 | unknown compound | 75.8 | 316 | 487.3 | + | + | + | + | + | + | + | + | + | + | + | + |
| No. | Leaf Sample | TPC (% GAE) | TPAC (% CYE) | TFC (%) | |
|---|---|---|---|---|---|
| QU | KA | ||||
| 1 | C. integerrimus | 8.74 ± 0.38 C | 5.59 ± 0.05 A | 1.32 ± 0.04 H | 0.073 ± 0.003 G |
| 2 | C. tomentosus | 5.17 ± 0.12 A | 2.60 ± 0.01 D | 0.36 ± 0.01 B | 0.097 ± 0.004 H |
| 3 | C. melanocarpus | 5.48 ± 0.07 A | 2.14 ± 0.03 C | 0.26 ± 0.01 A | 0.025 ± 0.001 B |
| 4 | C. lucidus | 10.68 ± 0.10 D | 6.34 ± 0.12 F | 0.40 ± 0.01 B,C | 0.027 ± 0.001 B,C |
| 5 | C. divaricatus | 11.97 ± 0.07 E | 9.08 ± 0.04 B | 0.70 ± 0.03 D | 0.049 ± 0.001 E |
| 6 | C. horizontalis | 7.30 ± 0.20 B | 5.36 ± 0.04 A | 0.27 ± 0.01 A | 0.127 ± 0.003 D |
| 7 | C. nanshan | 8.43 ± 0.17 C | 4.13 ± 0.05 E | 0.97 ± 0.02 G | nd |
| 8 | C. hjelmqvistii | 12.49 ± 0.41 E,F | 9.40 ± 0.36 B | 0.43 ± 0.03 C | 0.035 ± 0.001 C |
| 9 | C. dielsianus | 6.99 ± 0.09 B | 5.69 ± 0.05 A | 0.52 ± 0.02 E | 0.259 ± 0.006 I |
| 10 | C. splendens | 9.92 ± 0.33 D | 9.13 ± 0.12 B | 0.73 ± 0.02 D | 0.126 ± 0.005 D |
| 11 | C. bullatus | 15.43 ± 0.51 G | 14.98 ± 0.08 H | 0.61 ± 0.01 F | nd |
| 12 | C. zabelii | 12.94 ± 0.28 F | 10.86 ± 0.09 G | 0.28 ± 0.01 A | 0.063 ± 0.001 F |
| No. | Leaf Sample | CHAC (%) | ||
|---|---|---|---|---|
| NCHA | CHA | CCHA | ||
| 1 | C. integerrimus | 0.125 ± 0.001 A | 1.58 ± 0.01 B | 0.058 ± 0.001 A |
| 2 | C. tomentosus | 0.069 ± 0.003 C,D | 0.68 ± 0.03 A | 0.037 ± 0.002 B |
| 3 | C. melanocarpus | 0.081 ± 0.002 D | 0.65 ± 0.02 A | 0.031 ± 0.001 E |
| 4 | C. lucidus | 0.170 ± 0.008 B | 1.19 ± 0.05 E | 0.063 ± 0.002 C,D |
| 5 | C. divaricatus | 0.327 ± 0.005 E | 1.70 ± 0.04 C | 0.099 ± 0.004 F |
| 6 | C. horizontalis | 0.108 ± 0.003 A | 1.57 ± 0.04 B | 0.047 ± 0.001 A |
| 7 | C. nanshan | 0.116 ± 0.001 A | 1.75 ± 0.01 C | 0.053 ± 0.001 A |
| 8 | C. hjelmqvistii | 0.166 ± 0.005 B | 2.70 ± 0.01 G | 0.067 ± 0.003 C |
| 9 | C. dielsianus | 0.103 ± 0.003 A | 0.94 ± 0.02 D | 0.039 ± 0.001 B |
| 10 | C. splendens | 0.167 ± 0.008 B | 1.39 ± 0.06 F | 0.040 ± 0.002 B |
| 11 | C. bullatus | 0.161 ± 0.003 B | 0.63 ± 0.01 A | 0.068 ± 0.001 C |
| 12 | C. zabelii | 0.066 ± 0.001 C | 0.57 ± 0.01 A | 0.051 ± 0.001 A |
| r (R2) | DPPH EC50 (µg/mL) | FRAP (mmolFe2+/g) | O2•− SC50 (µg/mL) | H2O2 SC50 (µg/mL) |
|---|---|---|---|---|
| TPC (% GAE) | −0.8298 (0.6885) * | 0.9491 (0.9008) * | −0.4227 (0.1787) | −0.8676 (0.7528) * |
| TPAC (% CYE) | −0.7706 (0.5938) ** | 0.9719 (0.9447) * | −0.2854 (0.0815) | −0.7639(0.5836) ** |
| TFC (%; QU + KA) | −0.1907 (0.0364) | 0.0355 (0.0013) | −0.0976 (0.0095) | −0.0529 (0.0028) |
| CHAC (%; NCHA + CHA + CCHA) | −0.5366 (0.2879) | 0.1392 (0.0194) | −0.4062 (0.1650) | −0.3148 (0.0991) |
| DPPH EC50 (µg/mL) | – | −0.8364 (0.6996) * | 0.4218 (0.1779) | 0.7580 (0.5746)** |
| FRAP (mmol Fe2+/g) | −0.8364 (0.6996) * | – | −0.3490 (0.1218) | −0.7746 (0.6000) ** |
| O2•− SC50 (µg/mL) | 0.4218 (0.1779) | −0.3490 (0.1218) | – | 0.7127 (0.5079) ** |
| H2O2 SC50 (µg/mL) | 0.7580 (0.5746) ** | −0.7746 (0.6000) ** | 0.7127 (0.5079) ** | – |
| No. | Species | Herbarium Code | Collection Site |
|---|---|---|---|
| 1 | C. integerrimus Medik. | KFG/12/CIN | Forestry Experimental Station of Warsaw University of Life Sciences (Rogow, Poland) |
| 2 | C. tomentosus Lindl. | KFG/12/CTM | |
| 3 | C. melanocarpus Lodd. ex. C.K. Schneid. | KFG/12/CMA | |
| 4 | C. lucidus Schltdl. | KFG/12/CLC | |
| 5 | C. divaricatus Rehder et E.H. Wilson | KFG/12/CDV | Botanical Garden (Lodz, Poland) |
| 6 | C. horizontalis Decne. | KFG/12/CHR | |
| 7 | C. nanshan Mottet | KFG/12/CNA | |
| 8 | C. hjelmqvistii Flinck et B. Hylmö | KFG/12/CHQ | |
| 9 | C. dielsianus E. Pritz. | KFG/12/CDL | |
| 10 | C. splendens Flinck et B. Hylmö | KFG/12/CSP | |
| 11 | C. bullatus Bois | KFG/12/CBL | |
| 12 | C. zabelii C.K. Schneid | KFG/12/CZB |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kicel, A.; Michel, P.; Owczarek, A.; Marchelak, A.; Żyżelewicz, D.; Budryn, G.; Oracz, J.; Olszewska, M.A. Phenolic Profile and Antioxidant Potential of Leaves from Selected Cotoneaster Medik. Species. Molecules 2016, 21, 688. https://doi.org/10.3390/molecules21060688
Kicel A, Michel P, Owczarek A, Marchelak A, Żyżelewicz D, Budryn G, Oracz J, Olszewska MA. Phenolic Profile and Antioxidant Potential of Leaves from Selected Cotoneaster Medik. Species. Molecules. 2016; 21(6):688. https://doi.org/10.3390/molecules21060688
Chicago/Turabian StyleKicel, Agnieszka, Piotr Michel, Aleksandra Owczarek, Anna Marchelak, Dorota Żyżelewicz, Grażyna Budryn, Joanna Oracz, and Monika Anna Olszewska. 2016. "Phenolic Profile and Antioxidant Potential of Leaves from Selected Cotoneaster Medik. Species" Molecules 21, no. 6: 688. https://doi.org/10.3390/molecules21060688
APA StyleKicel, A., Michel, P., Owczarek, A., Marchelak, A., Żyżelewicz, D., Budryn, G., Oracz, J., & Olszewska, M. A. (2016). Phenolic Profile and Antioxidant Potential of Leaves from Selected Cotoneaster Medik. Species. Molecules, 21(6), 688. https://doi.org/10.3390/molecules21060688










