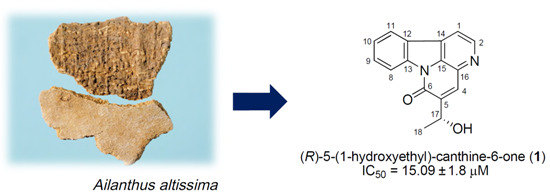
A New Canthinone-Type Alkaloid Isolated from Ailanthus altissima Swingle
Abstract
:1. Introduction
2. Results and Discussion
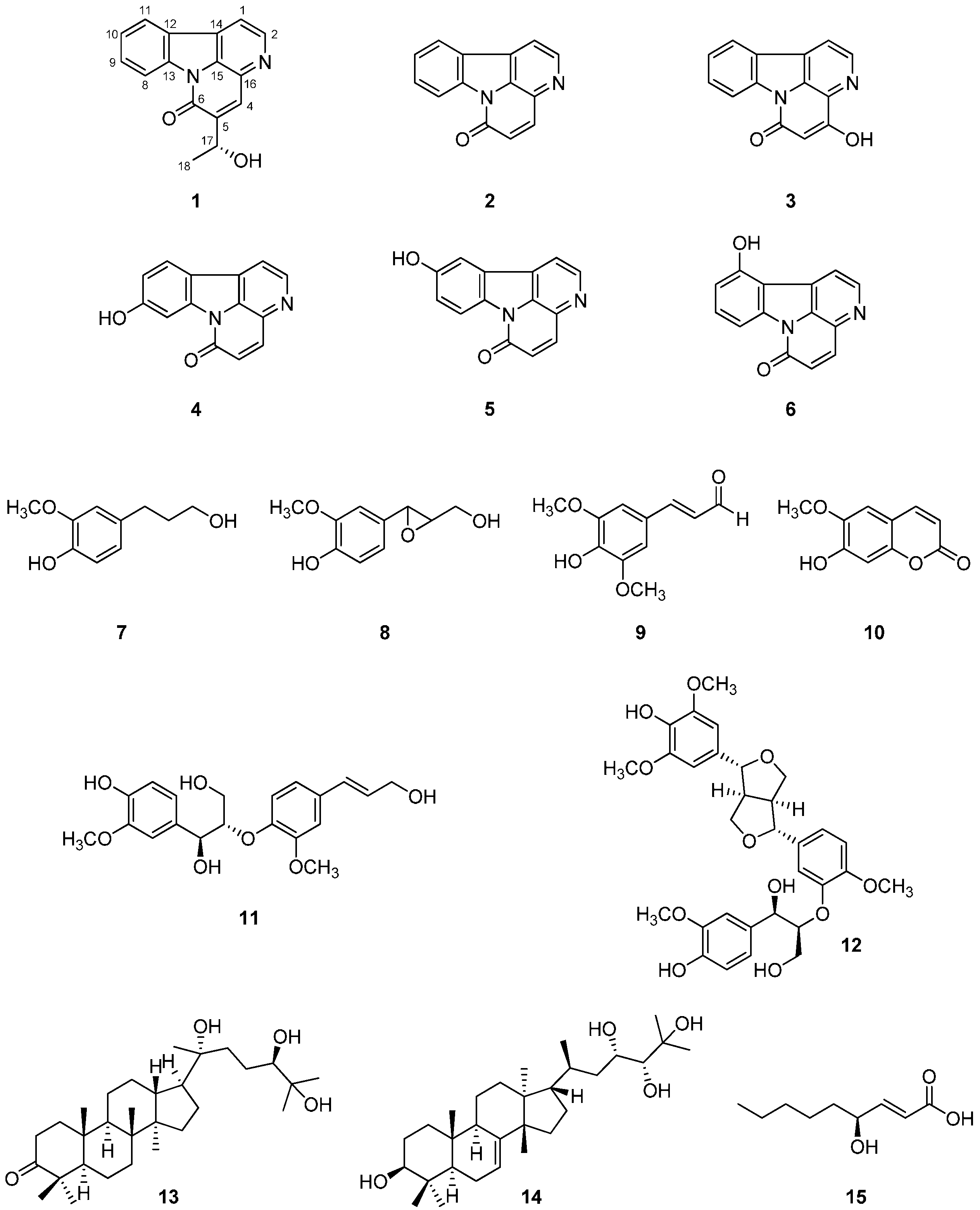
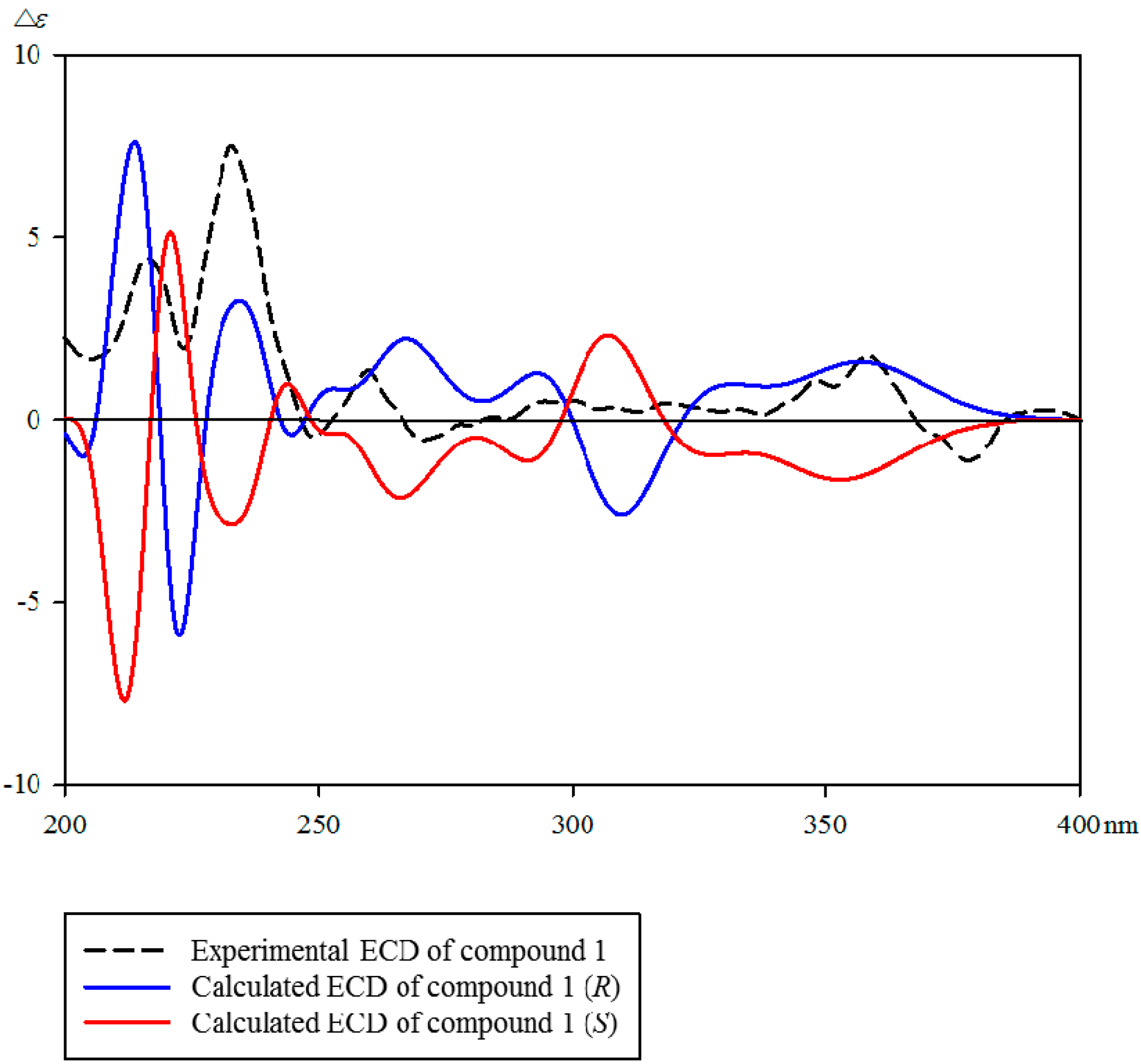
2.1. Identification of Isolated Compounds 1–15 from the Stem Bark of A. altissima
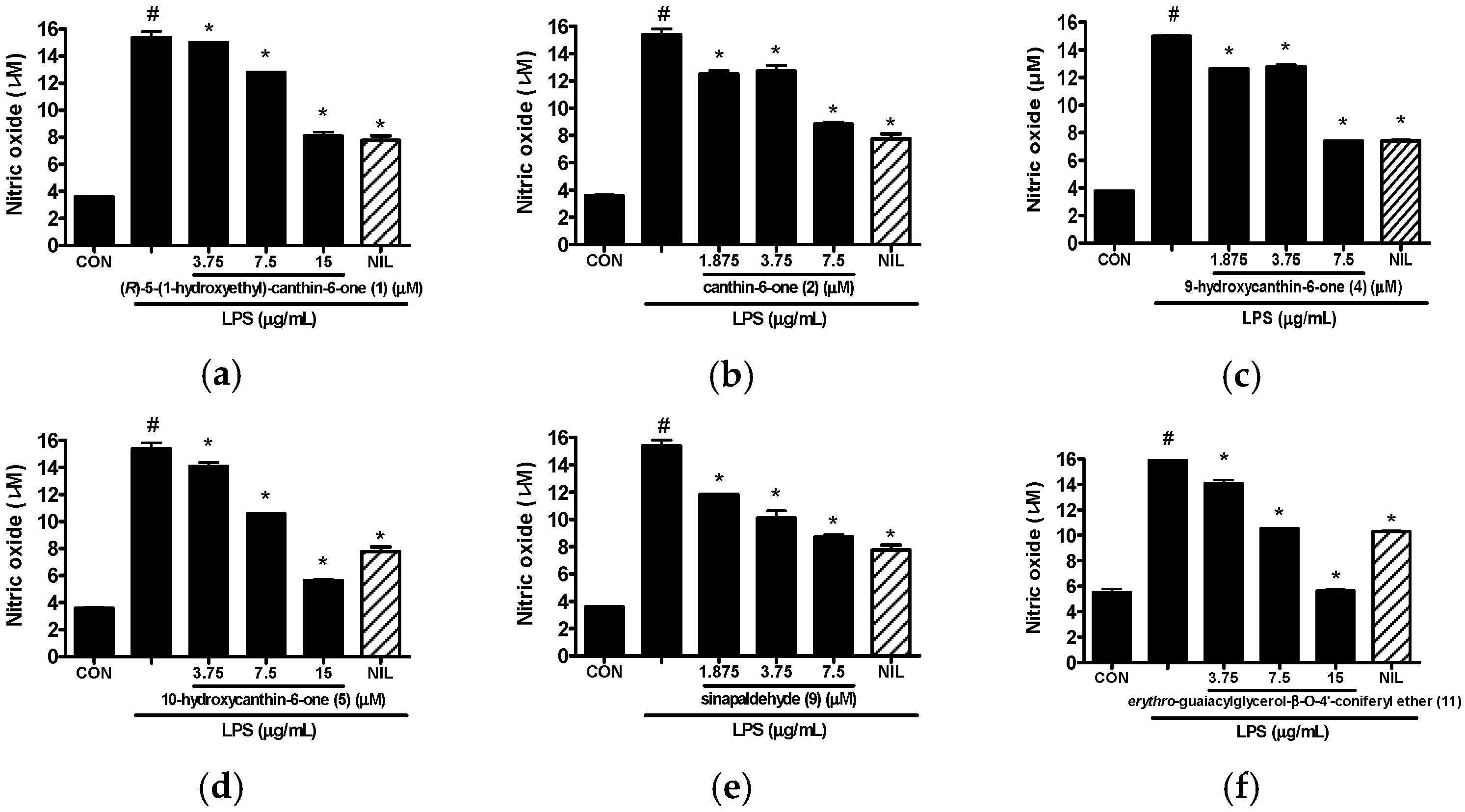
2.2. Anti-Inflammatory Activity of Isolated Compounds 1–15 from the Stem Bark of A. altissima
3. Materials and Methods
3.1. General Procedures
3.2. Plant Meterial
3.3. Extraction and Isolation
3.4. Spectral Data
3.5. Computational Methods
3.6. Materials
3.7. Cell Culture and Sample
3.8. Cell Viability Assay
3.9. Measurment of Nitric Oxide Production
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Howard, J.L. Ailanthus altissima; Fire Effects Information System, U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory: Fort Collins, CO., USA, 2004; Available online: http://www.fs.fed.us/database/feis/ (accessed on 15 June 2014).
- Kowarik, I.; Säumel, I. Biological flora of central Europe: Ailanthus altissima (Mill.) swingle. Perspect. Plant Ecol. 2007, 8, 207–237. [Google Scholar] [CrossRef]
- Jin, M.H.; Yook, J.; Lee, E.; Lin, C.X.; Quan, Z.; Son, K.H.; Bae, K.H.; Kim, H.P.; Kang, S.S.; Chang, H.W. Anti-inflammatory activity of Ailanthus altissima in ovalbumin-induced lung inflammation. Biol. Pharm. Bull. 2006, 29, 884–888. [Google Scholar] [CrossRef] [PubMed]
- Kang, T.-H.; Choi, I.-Y.; Kim, S.-J.; Moon, P.-D.; Seo, J.-U.; Kim, J.-J.; An, N.-H.; Kim, S.-H.; Kim, M.-H.; Um, J.-Y. Ailanthus altissima swingle has anti-anaphylactic effect and inhibits inflammatory cytokine expression via suppression of nuclear factor-κb activation. In Vitro Cell. Dev. Biol. Anim. 2010, 46, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Sladonja, B.; Sušek, M.; Guillermic, J. Review on Invasive Tree of Heaven (Ailanthus altissima (Mill.) Swingle) Conflicting Values: Assessment of Its Ecosystem Services and Potential Biological Threat. J. Environ. Manag. 2015, 56, 1009–1034. [Google Scholar] [CrossRef] [PubMed]
- Kubota, K.; Fukamiya, N.; Hamada, T.; Okano, M.; Tagahara, K.; Lee, K.H. Two new quassinoids, ailantinols A and B, and related compounds from Ailanthus altissima. J. Nat. Prod. 1996, 59, 683–686. [Google Scholar] [CrossRef] [PubMed]
- Ohmoto, T.; Koike, K. Studies on the constituents of Ailanthus altissima SWINGLE. III. The alkaloidal constituents. Chem. Pharm. Bull. 1984, 32, 170–173. [Google Scholar] [CrossRef] [Green Version]
- Souleles, C.; Waigh, R. Indole alkaloids of Ailanthus altissima. J. Nat. Prod. 1984, 47, 741–741. [Google Scholar] [CrossRef]
- Ohmoto, T.; Koike, K. Antiherpes activity of Simarou-baceae alkaloids in vitro. Shoyakugaku Zasshi 1988, 42, 160–162. [Google Scholar]
- Ohmoto, T.; Sung, Y.I. Antimycotic substances in the crudedrugs. II. Shoyakugaku Zasshi 1982, 36, 307–314. [Google Scholar]
- Ohmoto, T.; Sung, Y.I.; Koike, K.; Nikaido, T. Effect of alkaloids of simaroubaceous plants on the local lood flow rate. Shoyakugaku Zasshi 1985, 39, 28–34. [Google Scholar]
- Ohmoto, T.; Nikaido, T.; Koike, K.; Kohda, K.; Sankawa, U. Inhibition of adenosine 3′,5′-cyclic mono-phosphate phosphodiesterase by alkaloids. II. Chem. Pharm. Bull. 1988, 36, 4588–4592. [Google Scholar] [CrossRef] [PubMed]
- Kožuharova, E.; Lebanova, H.; Getov, I.; Benbassat, N.; Kochmarov, V. Ailanthus altissima (Mill.) Swingle—A terrible invasive pest in Bulgaria or potential useful medicinal plant? Rev. Pap. Bothalia 2014, 44, 213–230. [Google Scholar]
- Ferreira, M.E.; De Arias, A.R.; De Ortiz, S.T.; Inchausti, A.; Nakayama, H.; Thouvenel, C.; Hocquemiller, R.; Fournet, A. Leishmanicidal activity of two canthin-6-one alkaloids, two major constituents of Zanthoxylum chiloperone var. angustifolium. J. Ethnopharmacol. 2002, 80, 199–202. [Google Scholar] [CrossRef]
- De Souza Almeida, E.S.; Cechinel Filho, V.; Niero, R.; Clasen, B.K.; Balogun, S.O.; de Oliveira Martins, D.T. Pharmacological mechanisms underlying the anti-ulcer activity of methanol extract and canthin-6-one of Simaba ferruginea A. St-Hil. in animal models. J. Ethnopharmacol. 2011, 134, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Kim, S.J.; Kim, H.Y.; Ryu, B.; Kwak, H.; Hur, J.; Choi, J.-H.; Jang, D.S. Constituents of the stem barks of Ailanthus altissima and their potential to inhibit LPS-induced nitric oxide production. Bioorg. Med. Chem. Lett. 2015, 25, 1017–1020. [Google Scholar] [CrossRef] [PubMed]
- Koike, K.; Ohmoto, T. Carbon-13 Nuclear Magnetic Resonance Study of Canthin-6-one Aalkaloids. Chem. Pharm. Bull. 1985, 33, 5239–5244. [Google Scholar] [CrossRef]
- Crespi-Perellino, N.; Guicciardi, A.; Malyszko, G.; Arlandini, E.; Ballabio, M.; Minghetti, A. Occurrence of indole alkaloids in Ailanthus altissima cell cultures. J. Nat. Prod. 1986, 49, 1010–1014. [Google Scholar] [CrossRef]
- Kardono, L.B.; Angerhofer, C.K.; Tsauri, S.; Padmawinata, K.; Pezzuto, J.M.; Kinghorn, A.D. Cytotoxic and antimalarial constituents of the roots of Eurycoma longifolia. J. Nat. Prod. 1991, 54, 1360–1367. [Google Scholar] [CrossRef] [PubMed]
- Arisawa, M.; Kinghorn, A.D.; Cordell, G.A.; Farnsworth, N.R. Plant anticancer agents. XXIV. Alkaloid constituents of Simaba multiflora. J. Nat. Prod. 1983, 46, 222–225. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Koike, K.; Ohmoto, T. Canthin-6-one alkaloids from Brucea mollis var. tonkinensis. Phytochemistry 1994, 36, 1543–1546. [Google Scholar] [CrossRef]
- Teutonico, R.A.; Dudley, M.W.; Orr, J.D.; Lynn, D.G.; Binns, A.N. Activity and accumulation of cell division-promoting phenolics in tobacco tissue cultures. Plant Physiol. 1991, 97, 288–297. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kostova, I.; Dinchev, D.; Mikhova, B.; Iossifova, T. Epoxyconiferyl alcohol from Fraxinus oxycarpa bark. Phytochemistry 1995, 38, 801–802. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, Z.Y.; Ren, H.; Tan, J.W.; Wan, F.H. Phenolic compounds from Eupatorium adenophorum Spreng. J. Trop. Subtrop. Bot. 2013, 21, 63–68. [Google Scholar]
- Wang, Y.; Zhang, H.; Wang, W.; Zhang, S.; Zhang, X.; Ye, W. Chemical constituents from barks of Ailanthus altissima. Zhong Cao Yao 2012, 43, 649–652. [Google Scholar]
- Matsutomo, T.; Stark, T.D.; Hofmann, T. In vitro activity-guided identification of antioxidants in aged garlic extract. J. Agric. Food Chem. 2013, 61, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Kuo, Y.H. Four new compounds, ficusal, ficusesquilignan A, B, and ficusolide diacetate from the heartwood of Ficus microcarpa. Chem. Pharm. Bull. 2000, 48, 1862–1865. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhao, J.; Tang, W.; Wang, X. A new dammatane-type triterpene from root barks of Ailanthus altissima. Zhong Cao Yao 2014, 45, 161–163. [Google Scholar]
- Jolad, S.D.; Hoffmann, J.J.; Schram, K.H.; Cole, J.R.; Tempesta, M.S.; Bates, R.B. Constituents of Trichilia hispida (Meliaceae). 4. Hispidols A and B, two new tirucallane triterpenoids. J. Org. Chem. 1981, 46, 4085–4088. [Google Scholar] [CrossRef]
- Mu, L.H.; Qiang-Feng, J.; Liu, P.; Hu, Y.; Zhao, H.X. Chemical constituents of the roots of Pottsia laxiflora. Chem. Nat. Compd. 2013, 48, 1004–1007. [Google Scholar] [CrossRef]
- Tran, T.V.A.; Malainer, C.; Schwaiger, S.; Atanasov, A.G.; Heiss, E.H.; Dirsch, V.M.; Stuppner, H. NF-κB Inhibitors from Eurycoma longifolia. J. Nat. Prod. 2014, 77, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.N.; Li, W.; Song, S.B.; Yan, X.T.; Yang, S.Y.; Kim, Y.H. NF-κB Inhibitory Activities of Phenolic and Lignan Components from the Stems of Acanthopanax divaricatus var. albeofructus. Nat. Prod. Sci. 2014, 20, 232–236. [Google Scholar]
- Spartan'14; Wavefunction Inc.: Irvine, CA, USA, 2014.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.01; Gaussian Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- SpecDis; Version 1.64; University of Wuerzburg: Wuerzburg, Germany, 2015.
- Sample Availability: Not available.
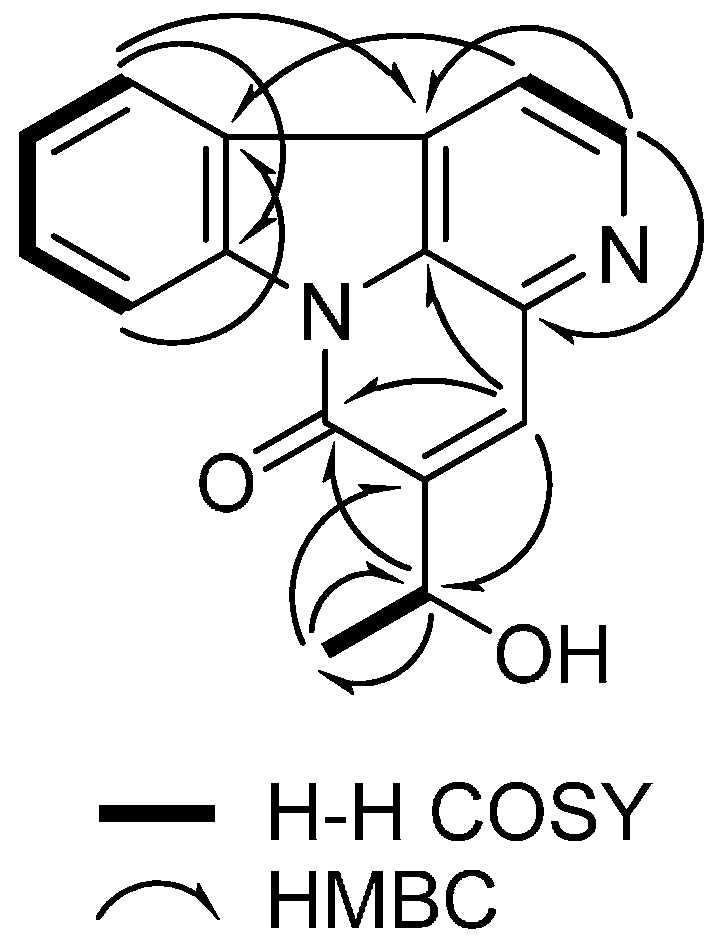
| Position | δH Mult., (J in Hz) | δC | COSY | HMBC |
|---|---|---|---|---|
| 1 | 8.16 d (5.0) | 117.6 | H-2 | H-2 |
| 2 | 8.77 d (5.0) | 146.8 | H-1 | H-1 |
| 4 | 8.18 d (1.0) | 133.5 | H-17 | |
| 5 | 147.8 | H-17, H-18 | ||
| 6 | 160.2 | H-4, H-17 | ||
| 8 | 8.60 d (8.0) | 118.0 | H-9 | H-10 |
| 9 | 7.74 dt (8.0, 1.0) | 132.1 | H-8, H-10 | |
| 10 | 7.58 dt (8.0, 1.0) | 127.1 | H-9, H-11 | |
| 11 | 8.26 d (8.0) | 124.4 | H-10 | H-9 |
| 12 | 126.1 | H-1, H-8, H-10 | ||
| 13 | 140.9 | H-9, H-11 | ||
| 14 | 131.7 | H-2, H-11 | ||
| 15 | 132.5 | H-1, H-4 | ||
| 16 | 137.0 | H-2 | ||
| 17 | 5.16 dq (6.5, 1.0) | 66.1 | H-18 | H-4, H-18 |
| 18 | 1.57 d (6.5) | 23.4 | H-17 | H-17 |
| Compound | IC50 (μM) * | Compound | IC50 (μM) * | Compound | IC50 (μM) * |
|---|---|---|---|---|---|
| 1 | 15.09 ± 1.8 | 6 | >50 | 11 | 10.69 ± 0.4 |
| 2 | 9.09 ± 0.34 | 7 | >50 | 12 | >50 |
| 3 | ND ** | 8 | >50 | 13 | >50 |
| 4 | 7.73 ± 0.3 | 9 | 5.92 ± 0.9 | 14 | >50 |
| 5 | 12.01 ± 0.1 | 10 | >50 | 15 | >50 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.M.; Lee, J.S.; Sezirahiga, J.; Kwon, J.; Jeong, M.; Lee, D.; Choi, J.-H.; Jang, D.S. A New Canthinone-Type Alkaloid Isolated from Ailanthus altissima Swingle. Molecules 2016, 21, 642. https://doi.org/10.3390/molecules21050642
Kim HM, Lee JS, Sezirahiga J, Kwon J, Jeong M, Lee D, Choi J-H, Jang DS. A New Canthinone-Type Alkaloid Isolated from Ailanthus altissima Swingle. Molecules. 2016; 21(5):642. https://doi.org/10.3390/molecules21050642
Chicago/Turabian StyleKim, Hye Mi, Jin Su Lee, Jurdas Sezirahiga, Jaeyoung Kwon, Miran Jeong, Dongho Lee, Jung-Hye Choi, and Dae Sik Jang. 2016. "A New Canthinone-Type Alkaloid Isolated from Ailanthus altissima Swingle" Molecules 21, no. 5: 642. https://doi.org/10.3390/molecules21050642
APA StyleKim, H. M., Lee, J. S., Sezirahiga, J., Kwon, J., Jeong, M., Lee, D., Choi, J.-H., & Jang, D. S. (2016). A New Canthinone-Type Alkaloid Isolated from Ailanthus altissima Swingle. Molecules, 21(5), 642. https://doi.org/10.3390/molecules21050642