Recent Developments in Solid-Phase Extraction for Near and Attenuated Total Reflection Infrared Spectroscopic Analysis
Abstract
:1. Introduction
2. Basis of Solid-Phase Extraction
3. Principles of Infrared Spectroscopic Analysis Techniques
3.1. Near-Infrared (NIR) SPECTROSCOpy vs. Mid Infrared Spectroscopy
3.1.1. Miniaturization in NIR and MIR
3.1.2. Multivariate Data Analysis in NIR and MIR
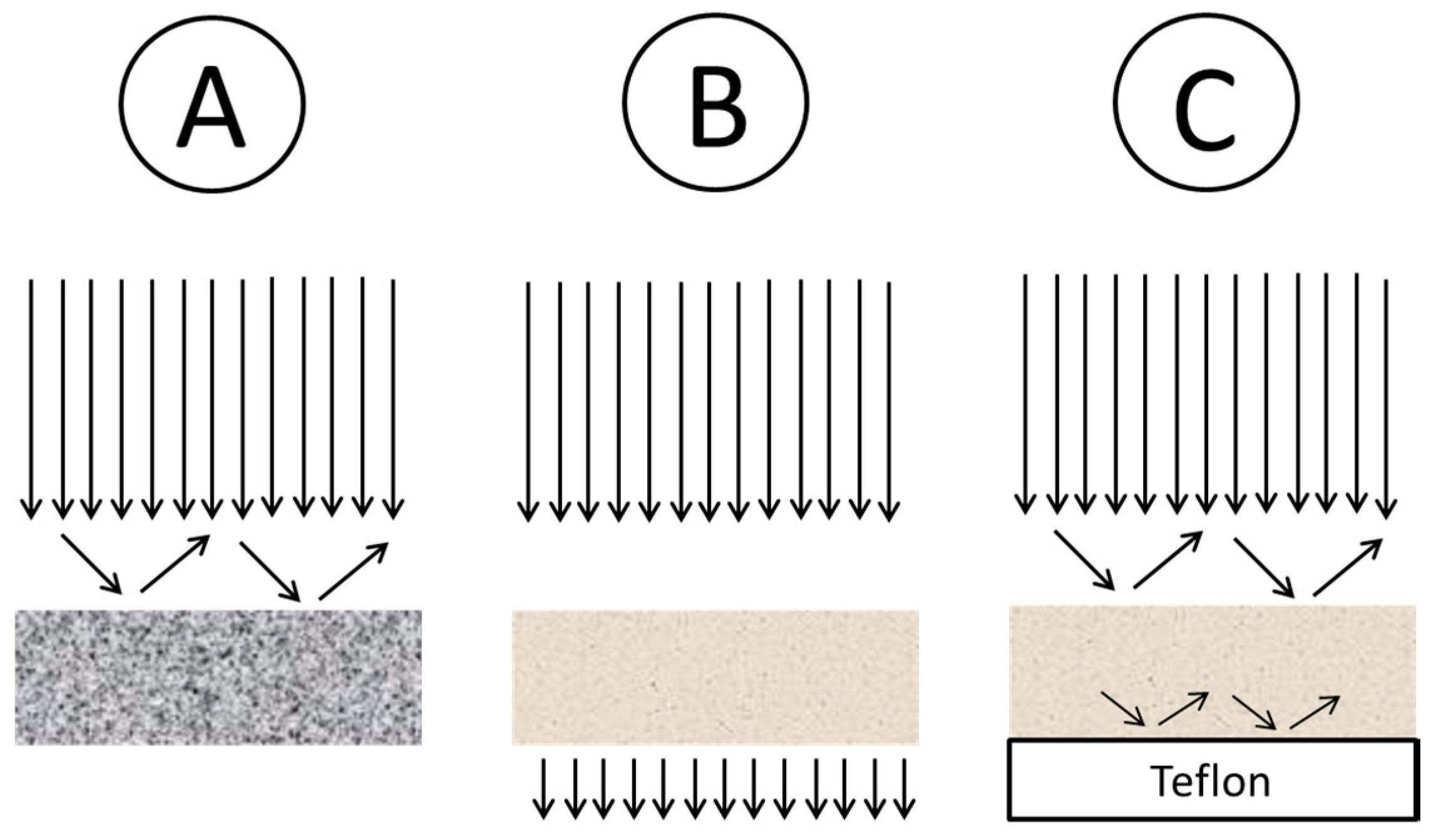
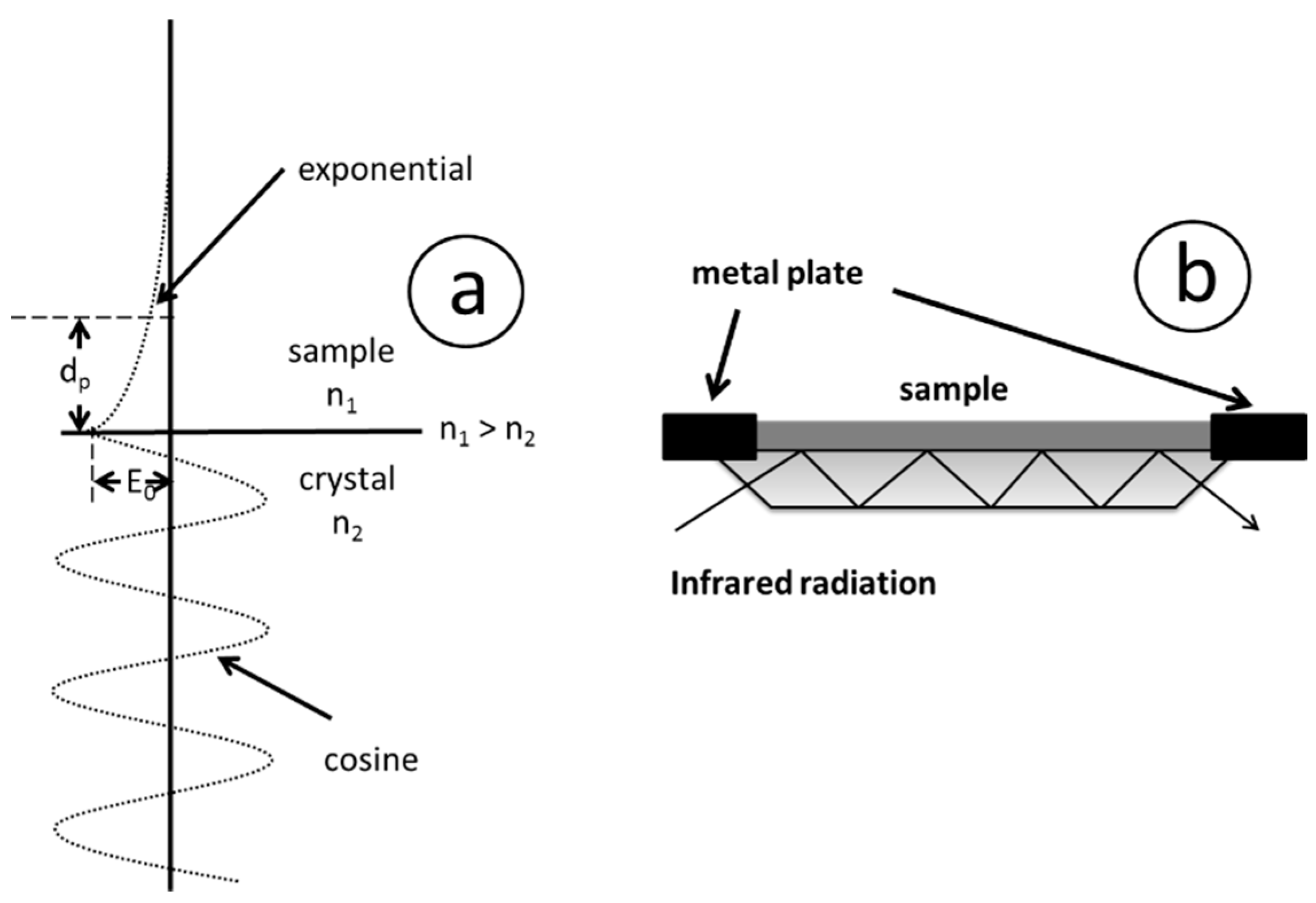
3.2. Attenuated Total Reflection (ATR) Spectroscopy
- Non-soluble materials can be analyzed (e.g., duroplasts)
- No KBr pellet is required
- Relatively high penetration depth for optical transparent materials
- Improved signal to noise ratio compared to measurement using KBr pellet
3.3. Imaging and Mapping Spectroscopy
3.3.1. Instrumental Setup
3.3.2. Measurement Modes
- (1)
- Point mapping: different areas of the sample are analyzed consecutively
- (2)
- Line mapping: defines a series of spectra along one dimension
- (3)
- Area mapping: defines a series of spectra to be collected in two dimensions. Sampling of large areas requires multiple positioning of a sample
3.3.3. Hyper Spectral Cube
3.3.4. Spectral Treatments in Imaging/Mapping Spectroscopy
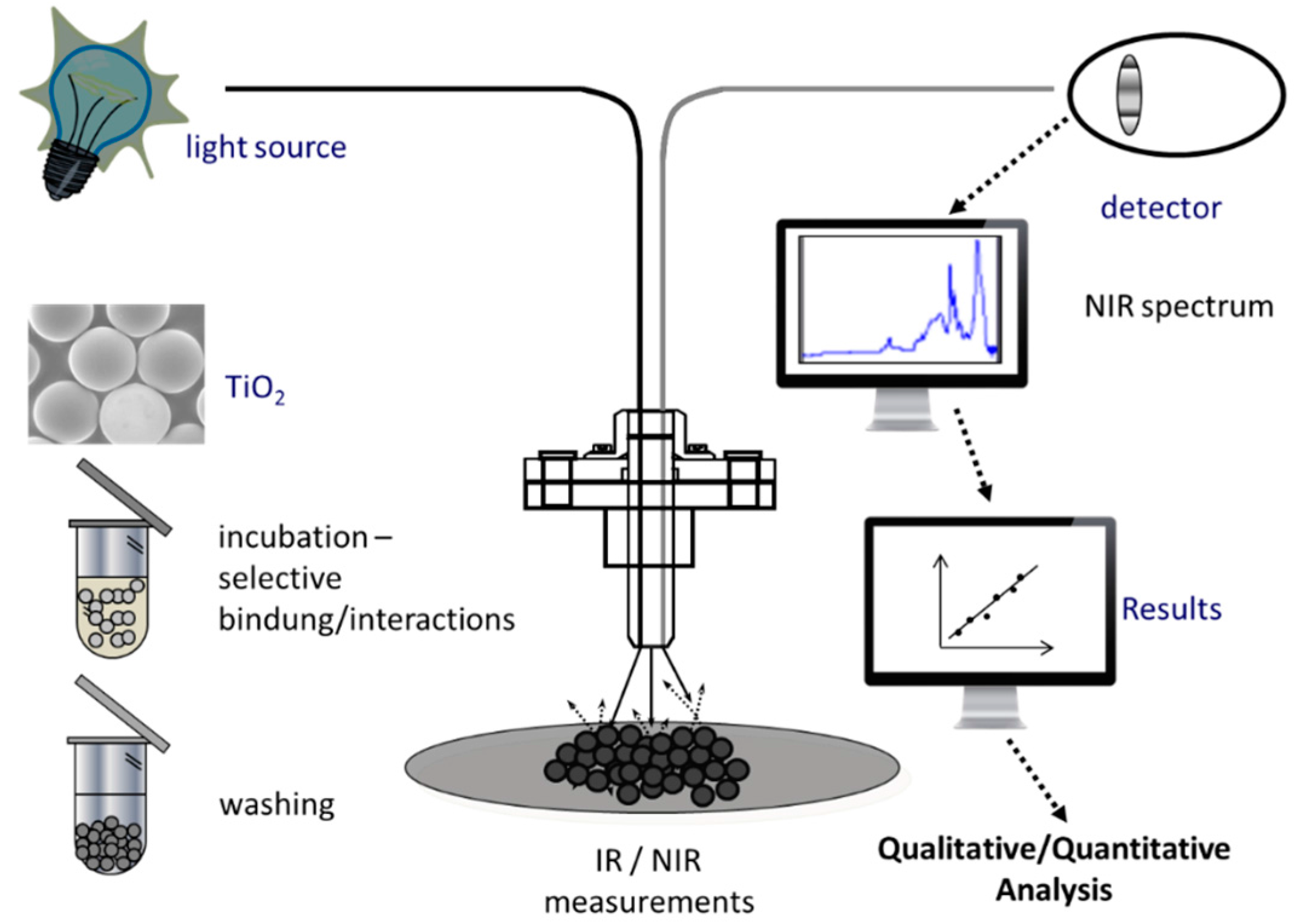
4. Surface-Enhanced Infrared Absorption (SEIRA) Spectroscopy
- The sought sample enrichment along the solid surface reduces the content of water in the observed volume as the strong absorption of liquid water often hampers the IR spectroscopic analysis [75]
- Only those molecules that reside within the optical near-field are probed [73]
- Chip-based technology can be implemented [76]
4.1. Preparation of SEIRA Substrates
4.2. Theoretical Models for SEIRA
4.3. Classical Applications of SEIRA
5. Recent Applications Using Selective Enrichment
5.1. Bioanalysis
5.2. Food Analysis
5.3. Environmental Analysis
6. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR | Attenuated total reflection |
| BTEX | Benzene, toluene and xylene |
| CB | Chlorobenzene |
| DEHP | Diisooctylphthalate |
| DLC | Diamond like carbon |
| DLP | Digital light processing |
| DMD | Digital mirror devices |
| ESI | Electrospray ionisation |
| ESI | Electrospray ionisation |
| FPA | Focal plane array |
| FT-IR | Fourier-transfrom infrared |
| GC | Gas chromatography |
| GMA-DVB | Poly(glycidyl methacrylate-co-divinylbenzene) |
| HC | Hierarchical cluster |
| HDL | High density lipoprotein |
| HPLC | High-performance liquid chromatography |
| KM | K-means |
| LDL | Low density lipoprotein |
| LLE | Liquid-liquid extraction |
| LOD | Limit of detection |
| LVF | Linear variable filter |
| MALDI | Matrix assisted laser desorption ionisation |
| MCT | Mercury cadmium telluride |
| MEMS | Micro-electro-mechanical system |
| MIA | Multivariate imaging analysis |
| MIP | Molecularly imprinted polymers |
| MLR | Multiple linear regression |
| MS | Mass spectrometry |
| MVA | Multivariate analysis |
| PCA | Principal component analysis |
| PDMS | Poly(dimethylsiloxane) |
| PES | Polyethersulfone |
| PIB | Polyisobutylene |
| PLSR | Partial least square regression |
| PS-DVB | Poly(styrene-co-divinylbenzene) |
| PTFE | Polytetrafluoroethylene |
| PVC | Poly(vinylchloride) |
| RE | Relative error |
| SD | Standard deviation |
| SEE | Standard error of estimation |
| SEIRA | Surface enhanced infrared analysis |
| SEP | Standard error of prediction |
| SERS | Surface enhanced Raman spectroscopy |
| SNR | Signal to noise ratio |
| SPE | Solid-phase extraction |
| SPME | Solid-phase micro extraction |
| TEC | Thermoelectric cooling |
| Vis | Visible |
References
- Marvin, C.H.; Brindle, I.D.; Hall, C.D.; Chiba, M. Automated high-performance liquid chromatography for the determination of pesticides in water using solid phase extraction. Anal. Chem. 1990, 62, 1495–1498. [Google Scholar] [CrossRef]
- Junk, G.A.; Richard, J.J.; Grieser, M.D.; Witiak, D.; Witiak, J.L.; Arguello, M.D.; Vick, R.; Svec, H.J.; Fritz, J.S.; Calder, G.V. Use of macroreticular resins in the analysis of water for trace organic contaminants. J. Chromatogr. A 1974, 99, 745–762. [Google Scholar] [CrossRef]
- Przyjazny, A. Evaluation of the suitability of selected porous polymers for preconcentration of organosulphur compounds from water. J. Chromatogr. A 1985, 346, 61–67. [Google Scholar] [CrossRef]
- Wolkoff, A.W.; Creed, C. Use of Sep-Pak R C 18 Cartridges for the collection & concentration of environmental samples. J. Liq. Chromatogr. 2006, 4, 1459–1472. [Google Scholar]
- Huck, C.W.; Bonn, G.K. Evaluation of detection methods for the reversed-phase HPLC determination of 3’,4’,5’-trimethoxyflavone in different phytopharmaceutical products and in human serum. Phytochem. Anal. 2001, 12, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Pirker, R.; Huck, C.; Bonn, G. Simultaneous determination of hypericin and hyperforin in human plasma and serum using liquid-liquid extraction, high-performance liquid chromatography and liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2002, 777, 147–153. [Google Scholar] [CrossRef]
- Valtiner, S.M.; Bonn, G.K.; Huck, C.W. Characterisation of different types of hay by solid-phase micro-extraction-gas chromatography mass spectrometry and multivariate data analysis. Phytochem. Anal. 2008, 19, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Huck, C.W.; Bonn, G.K. Recent developments in polymer-based sorbents for solid-phase extraction. J. Chromatogr. A 2000, 885, 51–72. [Google Scholar] [CrossRef]
- Sun, J.J.; Fritz, J.S. Chemically modified resins for solid-phase extraction. J. Chromatogr. A 1992, 590, 197–202. [Google Scholar] [CrossRef]
- Huck, C.; Buchmeiser, M.; Bonn, G. Fast analysis of flavonoids in plant extracts by liquid chromatography-ultraviolet absorbance detection on poly(carboxylic acid)-coated silica and electrospray ionization tandem mass spectrometric detection. J. Chromatogr. A 2002, 943, 33–38. [Google Scholar] [CrossRef]
- Stecher, G.; Huck, C.W.; Stöggl, W.M.; Bonn, G.K. Phytoanalysis: A challenge in phytomics. TrAC Trends Anal. Chem. 2003, 22, 1–14. [Google Scholar] [CrossRef]
- Pirker, R.; Huck, C.W.; Popp, M.; Bonn, G.K. Simultaneous determination of gentisic, salicyluric and salicylic acid in human plasma using solid-phase extraction, liquid chromatography and electrospray ionization mass spectrometry. J. Chromatogr. B. Anal. Technol. Biomed. Life Sci. 2004, 809, 257–264. [Google Scholar] [CrossRef]
- Sultan, M.; Stecher, G.; Stöggl, W.M.; Bakry, R.; Zaborski, P.; Huck, C.W.; el Kousy, N.M.; Bonn, G.K. Sample pretreatment and determination of non steroidal anti-inflammatory drugs (NSAIDs) in pharmaceutical formulations and biological samples (blood, plasma, erythrocytes) by HPLC-UV-MS and mgr-HPLC. Curr. Med. Chem. 2005, 12, 573–588. [Google Scholar] [PubMed]
- Stecher, G.; Jarukamjorn, K.; Zaborski, P.; Bakry, R.; Huck, C.W.; Bonn, G.K. Evaluation of extraction methods for the simultaneous analysis of simple and macrocyclic trichothecenes. Talanta 2007, 73, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Szabo, Z.; Vallant, R.M.; Takátsy, A.; Bakry, R.; Najam-ul-Haq, M.; Rainer, M.; Huck, C.W.; Bonn, G.K. Laser desorption/ionization mass spectrometric analysis of small molecules using fullerene-derivatized silica as energy-absorbing material. J. Mass Spectrom. 2010, 45, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Sonderegger, H.; Rameshan, C.; Lorenz, H.; Klauser, F.; Klerks, M.; Rainer, M.; Bakry, R.; Huck, C.W.; Bonn, G.K. Surface-assisted laser desorption/ionization-mass spectrometry using TiO2-coated steel targets for the analysis of small molecules. Anal. Bioanal. Chem. 2011, 401, 1963–1974. [Google Scholar] [CrossRef] [PubMed]
- Schönbichler, S.A.; Falser, G.F.; Hussain, S.; Bittner, L.; Abel, G.; Popp, M.; Bonn, G.K.; Huck, C. Comparison of NIR and ATR-IR spectroscopy for the determination of the antioxidant capacity of Primulae flos cum calycibus. Anal. Methods 2014, 6, 6343–6351. [Google Scholar] [CrossRef]
- Hussain, S.; Güzel, Y.; Schönbichler, S.A.; Rainer, M.; Huck, C.W.; Bonn, G.K. Solid-phase extraction method for the isolation of plant thionins from European mistletoe, wheat and barley using zirconium silicate embedded in poly(styrene-co-divinylbenzene) hollow-monoliths. Anal. Bioanal. Chem. 2013, 405, 7509–7521. [Google Scholar] [CrossRef] [PubMed]
- Hussain, S.; Pezzei, C.; Güzel, Y.; Rainer, M.; Huck, C.W.; Bonn, G.K. Zirconium silicate assisted removal of residual proteins after organic solvent deproteinization of human plasma, enhancing the stability of the LC-ESI-MS response for the bioanalysis of small molecules. Anal. Chim. Acta 2014, 852, 284–292. [Google Scholar] [CrossRef] [PubMed]
- Schemeth, D.; Noël, J.-C.; Jakschitz, T.; Rainer, M.; Tessadri, R.; Huck, C.W.; Bonn, G.K. Poly(N-vinylimidazole/ethylene glycol dimethacrylate) for the purification and isolation of phenolic acids. Anal. Chim. Acta 2015, 885, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Uhlschmied, C.; Krieg, C.; Abel, G.; Popp, M.; Huck, C.W.; Bonn, G.K. Evaluation of commercial solid-phase extraction (SPE) carrier materialsfor the selective automated enrichment of monoterpenoides and their analysis in cough drops, mouthwashes and bath additives by gas-chromatography mass spectrometry (GC-MS). Open Anal. Chem. J. 2013, 7, 12–21. [Google Scholar] [CrossRef]
- Feuerstein, I.; Rainer, M.; Bernardo, K.; Stecher, G.; Huck, C.W.; Kofler, K.; Pelzer, A.; Horninger, W.; Klocker, H.; Bartsch, G.; et al. Derivatized cellulose combined with MALDI-TOF MS: A new tool for serum protein profiling. J. Proteome Res. 2005, 4, 2320–2326. [Google Scholar] [CrossRef] [PubMed]
- Aprilita, N.H.; Huck, C.W.; Bakry, R.; Feuerstein, I.; Stecher, G.; Morandell, S.; Huang, H.-L.; Stasyk, T.; Huber, L.A.; Bonn, G.K. Poly(glycidyl methacrylate/divinylbenzene)-IDA-FeIII in phosphoproteomics. J. Proteome Res. 2005, 4, 2312–2319. [Google Scholar] [CrossRef] [PubMed]
- Huck, C.; Bakry, R.; Bonn, G. Sample preparation techniques for mass spectrometry in proteomics using recently developed highly selective materials. Curr. Proteomics 2005, 2, 269–285. [Google Scholar] [CrossRef]
- Rainer, M.; Muhammad, N.-H.; Huck, C.W.; Feuerstein, I.; Bakry, R.; Huber, L.A.; Gjerde, D.T.; Zou, X.; Qian, H.; Du, X.; et al. Ultra-fast mass fingerprinting by high-affinity capture of peptides and proteins on derivatized poly(glycidyl methacrylate/divinylbenzene) for the analysis of serum and cell lysates. Rapid Commun. Mass Spectrom. 2006, 20, 2954–2960. [Google Scholar] [CrossRef] [PubMed]
- Rainer, M.; Najam-ul-Haq, M.; Bakry, R.; Huck, C.W.; Bonn, G.K. Mass spectrometric identification of serum peptides employing derivatized poly(glycidyl methacrylate/divinyl benzene) particles and μ-HPLC. J. Proteome Res. 2007, 6, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Najam-ul-Haq, M.; Rainer, M.; Schwarzenauer, T.; Huck, C.W.; Bonn, G.K. Chemically modified carbon nanotubes as material enhanced laser desorption ionisation (MELDI) material in protein profiling. Anal. Chim. Acta 2006, 561, 32–39. [Google Scholar] [CrossRef]
- Najam-ul-Haq, M. Chemically modified nano crystalline diamond layer as material enhanced laser desorption ionisation (meldi) surface in protein profiling. Curr. Nanosci. 2015, 2, 1–7. [Google Scholar] [CrossRef]
- Kloss, F.R.; Najam-Ul-Haq, M.; Rainer, M.; Gassner, R.; Lepperdinger, G.; Huck, C.W.; Bonn, G.; Klauser, F.; Liu, X.; Memmel, N.; et al. Nanocrystalline diamond—An excellent platform for life science applications. J. Nanosci. Nanotechnol. 2007, 7, 4581–4587. [Google Scholar] [PubMed]
- Vallant, R.M.; Szabo, Z.; Trojer, L.; Najam-ul-Haq, M.; Rainer, M.; Huck, C.W.; Bakry, R.; Bonn, G.K. A new analytical material-enhanced laser desorption ionization (MELDI) based approach for the determination of low-mass serum constituents using fullerene derivatives for selective enrichment. J. Proteome Res. 2007, 6, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Najam-ul-Haq, M.; Rainer, M.; Szabó, Z.; Vallant, R.; Huck, C.W.; Bonn, G.K. Role of carbon nano-materials in the analysis of biological materials by laser desorption/ionization-mass spectrometry. J. Biochem. Biophys. Methods 2007, 70, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Rainer, M.; Najam-ul-Haq, M.; Huck, C.W.; Vallant, R.M.; Heigl, N.; Hahn, H.; Bakry, R.; Bonn, G.K. Carbon based sample supports and matrices for laser desorption/ionization mass spectrometry. Recent Pat. Nanotechnol. 2007, 1, 113–119. [Google Scholar] [CrossRef]
- Vallant, R.M.; Szabo, Z.; Bachmann, S.; Bakry, R.; Najam-ul-Haq, M.; Rainer, M.; Heigl, N.; Petter, C.; Huck, C.W.; Bonn, G.K. Development and application of C60-fullerene bound silica for solid-phase extraction of biomolecules. Anal. Chem. 2007, 79, 8144–8153. [Google Scholar] [CrossRef] [PubMed]
- Najam-ul-Haq, M.; Rainer, M.; Huck, C.W.; Hausberger, P.; Kraushaar, H.; Bonn, G.K. Nanostructured diamond-like carbon on digital versatile disc as a matrix-free target for laser desorption/ionization mass spectrometry. Anal. Chem. 2008, 80, 7467–7472. [Google Scholar] [CrossRef] [PubMed]
- Najam-ul-Haq, M.; Rainer, M.; Huck, C.W.; Ashiq, M.N.; Bonn, G.K. Chemically modified diamond-like carbon (DLC) for protein enrichment and profiling by MALDI-MS. Amino Acids 2012, 43, 823–831. [Google Scholar] [CrossRef] [PubMed]
- Rainer, M.; Sonderegger, H.; Bakry, R.; Huck, C.W.; Morandell, S.; Huber, L.A.; Gjerde, D.T.; Bonn, G.K. Analysis of protein phosphorylation by monolithic extraction columns based on poly(divinylbenzene) containing embedded titanium dioxide and zirconium dioxide nano-powders. Proteomics 2008, 8, 4593–4602. [Google Scholar] [CrossRef] [PubMed]
- Greiderer, A.; Rainer, M.; Najam-ul-Haq, M.; Vallant, R.M.; Huck, C.W.; Bonn, G.K. Derivatized graphitic nanofibres (GNF) as a new support material for mass spectrometric analysis of peptides and proteins. Amino Acids 2009, 37, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Zhong, L.; Liu, D.; Yang, B.; Lou, Y.; Peng, J.; Rainer, M.; Feuerstein, I.; Muhammad, N.-H.; Huck, C.W.; et al. Novel multifunctional chitosan-GMA-IDA-Cu(II) nanospheres for high dynamic range characterization of the human plasma proteome. Anal. Bioanal. Chem. 2011, 400, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, F.; Hussain, D.; Fatima, B.; Musharraf, S.G.; Huck, C.W.; Bonn, G.K.; Najam-ul-Haq, M. Silica-lanthanum oxide: Pioneer composite of rare-earth metal oxide in selective phosphopeptides enrichment. Anal. Chem. 2012, 84, 10180–10185. [Google Scholar] [CrossRef] [PubMed]
- Najam-Ul-Haq, M.; Rainer, M.; Heigl, N.; Szabo, Z.; Vallant, R.; Huck, C.W.; Engelhardt, H.; Bischoff, K.-D.; Bonn, G.K. Nano-structured support materials, their characterisation and serum protein profiling through MALDI/TOF-MS. Amino Acids 2008, 34, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Bakry, R.; Rainer, M.; Huck, C.W.; Bonn, G.K. Protein profiling for cancer biomarker discovery using matrix-assisted laser desorption/ionization time-of-flight mass spectrometry and infrared imaging: A review. Anal. Chim. Acta 2011, 690, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Najam-ul-Haq, M.; Jabeen, F.; Hussain, D.; Saeed, A.; Musharraf, S.G.; Huck, C.W.; Bonn, G.K. Versatile nanocomposites in phosphoproteomics: A review. Anal. Chim. Acta 2012, 747, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Hussain, D.; Najam-ul-Haq, M.; Jabeen, F.; Ashiq, M.N.; Athar, M.; Rainer, M.; Huck, C.W.; Bonn, G.K. Functionalized diamond nanopowder for phosphopeptides enrichment from complex biological fluids. Anal. Chim. Acta 2013, 775, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Szultka, M. Past, present, and future of solid phase extraction: A review. Crit. Rev. Anal. Chem. 2012, 42, 198–213. [Google Scholar] [CrossRef]
- Boonjob, W. An Overview about Recent Advances of Micro-Solid Phase Extraction in Flow Based Techniques. Available online: http://www.austinpublishinggroup.com/analytical-pharmaceutical-chemistry/fulltext/ ajapc-v1-id1006.php (accessed on 10 February 2016).
- Huck, C.W.; Ohmacht, R.; Szabo, Z.; Bonn, G.K. Near infrared spectroscopy, cluster and multivariate analysis : Characterisation of silica materials for liquid chromatography. J. Near Infrared Spectrosc. 2006, 14, 51–57. [Google Scholar] [CrossRef]
- Heigl, N.; Petter, C.H.; Lieb, M.; Bonn, G.K.; Huck, C.W. Near-infrared reflection spectroscopy and partial least squares regression for determining the total carbon coverage of silica packings for liquid chromatography. Vib. Spectrosc. 2009, 49, 155–161. [Google Scholar] [CrossRef]
- Heigl, N.; Greiderer, A.; Petter, C.H.; Kolomiets, O.; Siesler, H.W.; Ulbricht, M.; Bonn, G.K.; Huck, C.W. Simultaneous determination of the micro-, meso-, and macropore size fractions of porous polymers by a combined use of Fourier transform near-infrared diffuse reflection spectroscopy and multivariate techniques. Anal. Chem. 2008, 80, 8493–500. [Google Scholar] [CrossRef] [PubMed]
- Petter, C.H.; Heigl, N.; Bakry, R.; Bonn, G.K.; Ritsch, A.; Huck, C.W. Quantification of low-density and high-density lipoproteins in human serum by material enhanced infrared spectroscopy (MEIRS). Curr. Med. Chem. 2009, 16, 4601–4608. [Google Scholar] [CrossRef] [PubMed]
- Huck, C.W. Advances of infrared spectroscopy in natural product research. Phytochem. Lett. 2015, 11, 384–393. [Google Scholar] [CrossRef]
- Siesler, H.W.; Ozaki, Y.; Kawata, S.; Heise, H.M. (Eds.) Near-Infrared Spectroscopy: Principles, Instruments, Applications (Google Ebook); Wiley: Weinheim, Germany, 2008.
- Arthur, C.L.; Pratt, K.; Motlagh, S.; Pawliszyn, J.; Belardi, R.P. Environmental analysis of organic compounds in water using solid phase micro extraction. J. High Resolut. Chromatogr. 1992, 15, 741–744. [Google Scholar] [CrossRef]
- Mcclure, W.F. 204 years of near infrared technology: 1800–2003. J. Near Infrared Spectrosc. 2003, 11, 487–518. [Google Scholar] [CrossRef]
- Blanco, M.; Villarroya, I. NIR spectroscopy: A rapid-response analytical tool. TrAC Trends Anal. Chem. 2002, 21, 240–250. [Google Scholar] [CrossRef]
- Barton, F. Theory and principles of near infrared spectroscopy. Spectrosc. Eur. 2002, 14, 12–18. [Google Scholar]
- Pallua, J.D.; Pezzei, C.; Huck-Pezzei, V.; A. Schonbichler, S.; K. Bittner, L.; K. Bonn, G.; Saeed, A.; Majeed, S.; Farooq, A.; Najam-ul-Haq, M.; et al. Advances of infrared spectroscopic imaging and mapping technologies of plant material. Curr. Bioact. Compd. 2011, 7, 106–117. [Google Scholar] [CrossRef]
- Lutz, O.M.D.; Bonn, G.K.; Rode, B.M.; Huck, C.W. Reproducible quantification of ethanol in gasoline via a customized mobile near-infrared spectrometer. Anal. Chim. Acta 2014, 826, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Huck-Pezzei, V.A.; Seitz, I.; Karer, R.; Schmutzler, M.; De Benedictis, L.; Wild, B.; Huck, C.W. Alps food authentication, typicality and intrinsic quality by near infrared spectroscopy. Food Res. Int. 2014, 62, 984–990. [Google Scholar] [CrossRef]
- De Benedictis, L.; Schmutzler, M.; Karer, R.; Eisenstecken, D.; Huck-Pezzei, V.; Robatscher, P.; Oberhuber, M.; Mimmo, T.; Capici, C.; Scampicchio, M.; et al. Near infrared spectroscopy as a tool for quality control of food of the Alpine region—A short report about a cross-border project. NIR News 2012, 23, 12–15. [Google Scholar] [CrossRef]
- Kessler, W.; Kessler, R.W. Multivariate curve resolution: A method of evaluating the kinetics of biotechnological reactions. Anal. Bioanal. Chem. 2006, 384, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Huck, C.W.; Bittner, L. Infrared spectroscopy: A novel tool for the physicochemical characterization of particulate, monolithic and coated stationary phases. Chromatographia 2011, 73, 29–34. [Google Scholar] [CrossRef]
- Pemberger, N.; Bittner, L.; Huck, C.W. Using near-infrared spectroscopy to monitor the curing reaction of silicone adhesives. Spectroscopy 2015, 30, 8–19. [Google Scholar]
- Workman, J.; Weyer, L. Practical Guide and Spectral Atlas for Interpretive Near-Infrared Spectroscopy, 2nd ed.; CRC Press Book: Boca Raton, FL, USA, 2012. [Google Scholar]
- Faber, N.K. Multivariate sensitivity for the interpretation of the effect of spectral pretreatment methods on near-infrared calibration model predictions. Anal. Chem. 1999, 71, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Spectroscopic Properties of Inorganic and Organometallic Compounds: Techniques, Materials and Applications; Royal Society of Chemistry: Cambridge, UK, 2014.
- Fahrenfort, J. Attenuated total reflection. Spectrochim. Acta 1961, 17, 698–709. [Google Scholar] [CrossRef]
- Messerschmidt, R.G.; Sting, D.W. Microscope Having Dual Remote Image Masking. U.S. Patent 4877960, 31 October 1989. [Google Scholar]
- Lewis, E.N.; Treado, P.J.; Reeder, R.C.; Story, G.M.; Dowrey, A.E.; Marcott, C.; Levin, I.W. Fourier Transform Spectroscopic Imaging Using an Infrared Focal-Plane Array Detector. Anal. Chem. 1995, 67, 3377–3381. [Google Scholar] [CrossRef] [PubMed]
- Salzer, R.; Siesler, H.W. Infrared and Raman Spectroscopic Imaging; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Gendrin, C.; Roggo, Y.; Collet, C. Pharmaceutical applications of vibrational chemical imaging and chemometrics: A review. J. Pharm. Biomed. Anal. 2008, 48, 533–553. [Google Scholar] [CrossRef] [PubMed]
- Pezzei, C.; Pallua, J.D.; Schaefer, G.; Seifarth, C.; Huck-Pezzei, V.; Bittner, L.K.; Klocker, H.; Bartsch, G.; Bonn, G.K.; Huck, C.W. Characterization of normal and malignant prostate tissue by Fourier transform infrared microspectroscopy. Mol. Biosyst. 2010, 6, 2287–2295. [Google Scholar] [CrossRef] [PubMed]
- Aroca, R.F.; Ross, D.J.; Domingo, C. Surface-enhanced infrared spectroscopy. Appl. Spectrosc. 2004, 58, 324A–338A. [Google Scholar] [CrossRef] [PubMed]
- De La Chapelle, M.L.; Pucci, A. Nanoantenna: Plasmon-Enhanced Spectroscopies for Biotechnological Applications; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Ataka, K.; Stripp, S.T.; Heberle, J. Surface-enhanced infrared absorption spectroscopy (SEIRAS) to probe monolayers of membrane proteins. Biochim. Biophys. Acta 2013, 1828, 2283–2293. [Google Scholar] [CrossRef] [PubMed]
- Adato, R.; Altug, H. In-situ ultra-sensitive infrared absorption spectroscopy of biomolecule interactions in real time with plasmonic nanoantennas. Nat. Commun. 2013, 4, 2154. [Google Scholar] [CrossRef] [PubMed]
- Osawa, M. Dynamic processes in electrochemical reactions studied by surface-enhanced infrared absorption spectroscopy (SEIRAS). Bull. Chem. Soc. Jpn. 1997, 70, 2861–2880. [Google Scholar] [CrossRef]
- Wanzenböck, H.D.; Mizaikoff, B.; Weissenbacher, N.; Kellner, R. Surface enhanced infrared absorption spectroscopy (SEIRA) using external reflection on low-cost substrates. Fresenius. J. Anal. Chem. 1998, 362, 15–20. [Google Scholar] [CrossRef]
- Sánchez-Cortés, S.; Domingo, C.; García-Ramos, J.V.; Aznárez, J.A. Surface-enhanced vibrational study (seir and sers) of dithiocarbamate pesticides on gold films. Langmuir 2001, 17, 1157–1162. [Google Scholar] [CrossRef]
- Waswa, J.; Irudayaraj, J.; DebRoy, C. Direct detection of E. coli O157:H7 in selected food systems by a surface plasmon resonance biosensor. LWT Food Sci. Technol. 2007, 40, 187–192. [Google Scholar] [CrossRef]
- Gonzalvez, A.; Garrigues, S.; de la Guardia, M.; Armenta, S. The ways to the trace level analysis in infrared spectroscopy. Anal. Methods 2011, 3, 43–52. [Google Scholar] [CrossRef]
- Xie, Y.-J.; Wang, Z.; Hu, W.-P.; Xu, S. Fast determination of trace dimethyl fumarate in milk with near infrared spectroscopy following fluidized bed enrichment. Anal. Bioanal. Chem. 2012, 404, 3189–3194. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.P.; Tong, P.J.; Li, X.Y.; Li, X.L.; Du, Y. Determination of diisooctylphthalate in drink by NIR spectroscopy coupled with membrane enrichment technique. J. Instrum. Anal. 2012, 31, 605–608. [Google Scholar]
- Armenta, S.; Lendl, B. Flow through FTIR sensor based on solid phase spectroscopy (SPS) on conventional octadecyl (C18) silica. Vib. Spectrosc. 2009, 51, 60–64. [Google Scholar] [CrossRef]
- Ortega-Barrales, P.; Ayora-Cañada, M.J.; Garrrigues, A.S.; de la Guardia, M. Solid phase Fourier transform near infrared spectroscopy. Analyst 1999, 4, 579–582. [Google Scholar] [CrossRef]
- Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Characterization of an attenuated total reflection-based sensor for integrated solid-phase extraction and infrared detection. Anal. Chem. 2008, 80, 1146–1151. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Yan, Z.; Ye, Z.; Ying, Y. Application of Near-infrared spectroscopy to detection of pesticide phoxim residues. Guang Pu Xue Yu Guang Pu Fen Xi 2009, 29, 2421–2424. [Google Scholar]
- Regan, F.; Meaney, M.; Vos, J.G.; MacCraith, B.D.; Walsh, J.E. Determination of pesticides in water using ATR-FTIR spectroscopy on PVU chloroparaffin coatings. Anal. Chim. Acta 1996, 334, 85–92. [Google Scholar] [CrossRef]
- Li, W.; Zhang, X.; Zheng, K.; Du, Y.; Cap, P.; Sui, T.; Geng, J. Application of near infrared spectroscopy coupled with fluidized bed enrichment and chemometrics to detect low concentration of β-naphthalenesulfonic acid. J. AOAC Int. 2015, 98, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Heglund, D.L.; Tilotta, D.C. Determination of volatile organic compounds in water by solid phase microextraction and infrared spectroscopy. Environ. Sci. Technol. 1996, 30, 1212–1219. [Google Scholar] [CrossRef]
- Ferrer, N.; Romero, M.T. Fourier transform infrared spectroscopy and solid phase extraction applied to the determination of oil and grease in water matrices. Microchim. Acta 2002, 140, 35–39. [Google Scholar] [CrossRef]
- Silva, A.M.S.; Pimentel, M.F.; Raimundo, I.M.; Almeida, Y.M.B. A PVC sensing phase for determination of BTEX in water employing mid-infrared spectroscopy. Vib. Spectrosc. 2008, 46, 39–44. [Google Scholar] [CrossRef]
- Heinrich, P.; Wyzgol, R.; Schrader, B.; Hatzilazaru, A.; Lübbers, D.W. Determination of organic compounds by ir/atr spectroscopy with polymer-coated internal reflection elements. Appl. Spectrosc. 1990, 44, 1641–1646. [Google Scholar] [CrossRef]
- Yang, N.; Ren, G. Application of near-infrared reflectance spectroscopy to the evaluation of rutin and d-chiro-inositol contents in tartary buckwheat. J. Agric. Food Chem. 2008, 56, 761–764. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tsai, S.-S. Cooled internal reflection element for infrared chemical sensing of volatile to semi-volatile organic compounds in the headspace of aqueous solutions. Anal. Chim. Acta 2002, 462, 235–244. [Google Scholar] [CrossRef]
- Karlowatz, M.; Kraft, M.; Mizaikoff, B. Simultaneous quantitative determination of benzene, toluene, and xylenes in water using mid-infrared evanescent field spectroscopy. Anal. Chem. 2004, 76, 2643–2648. [Google Scholar] [CrossRef] [PubMed]
- Jakusch, M.; Mizaikoff, B.; Kellner, R.; Katzir, A. Towards a remote IR fiber-optic sensor system for the determination of chlorinated hydrocarbons in water. Sens. Actuat. B Chem. 1997, 38, 83–87. [Google Scholar] [CrossRef]
- Flavin, K.; Mullowney, J.; Murphy, B.; Owens, E.; Kirwan, P.; Murphy, K.; Hughes, H.; McLoughlin, P. The development of novel organically modified sol-gel media for use with ATR/FTIR sensing. Analyst 2007, 132, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Göbel, R.; Krska, R.; Kellner, R.; Kastner, J.; Lambrecht, A.; Tacke, M.; Katzir, A. Enhancing the sensitivity of chemical sensors for chlorinated hydrocarbons in water by the use of tapered silver halide fibers and tunable diode lasers. Appl. Spectrosc. 1995, 49, 1174–1177. [Google Scholar] [CrossRef]
- Mizaikoff, B. Mid-infrared evanescent wave sensors—A novel approach for subsea monitoring. Meas. Sci. Technol. 1999, 10, 1185–1194. [Google Scholar] [CrossRef]
- Kraft, M.; Karlowatz, M.; Mizaikoff, B.; Stück, R.; Steden, M.; Ulex, M.; Amann, H. Sensor head development for mid-infrared fibre-optic underwater sensors. Meas. Sci. Technol. 2002, 13, 1294–1303. [Google Scholar] [CrossRef]
- Albuquerque, J.S.; Pimentel, M.F.; Silva, V.L.; Raimundo, I.M.; Rohwedder, J.J.R.; Pasquini, C. Silicone sensing phase for detection of aromatic hydrocarbons in water employing near-infrared spectroscopy. Anal. Chem. 2005, 77, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Lima, K.M.G.; Raimundo, I.M.; Pimentel, M.F. Improving the detection limits of near infrared spectroscopy in the determination of aromatic hydrocarbons in water employing a silicone sensing phase. Sens. Actuat. B Chem. 2007, 125, 229–233. [Google Scholar] [CrossRef]
- Pérez-Palacios, D.; Armenta, S.; Lendl, B. Flow-through Fourier transform infrared sensor for total hydrocarbons determination in water. Appl. Spectrosc. 2009, 63, 1015–1021. [Google Scholar] [CrossRef] [PubMed]
- Roy, E.G.; Jiang, C.; Wells, M.L.; Tripp, C. Determining subnanomolar iron concentrations in oceanic seawater using a siderophore-modified film analyzed by infrared spectroscopy. Anal. Chem. 2008, 80, 4689–4695. [Google Scholar] [CrossRef] [PubMed]
- Rose, L.J.; Hodges, L.; O’Connell, H.; Noble-Wang, J. National validation study of a cellulose sponge wipe-processing method for use after sampling Bacillus anthracis spores from surfaces. Appl. Environ. Microbiol. 2011, 77, 8355–8359. [Google Scholar] [CrossRef] [PubMed]
- ASTM D7575—11 Standard Test Method for Solvent-Free Membrane Recoverable Oil and Grease by Infrared Determination. Available online: http://www.astm.org/Standards/D7575.htm (accessed on 25 March 2016).


© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huck, C.W. Recent Developments in Solid-Phase Extraction for Near and Attenuated Total Reflection Infrared Spectroscopic Analysis. Molecules 2016, 21, 633. https://doi.org/10.3390/molecules21050633
Huck CW. Recent Developments in Solid-Phase Extraction for Near and Attenuated Total Reflection Infrared Spectroscopic Analysis. Molecules. 2016; 21(5):633. https://doi.org/10.3390/molecules21050633
Chicago/Turabian StyleHuck, Christian W. 2016. "Recent Developments in Solid-Phase Extraction for Near and Attenuated Total Reflection Infrared Spectroscopic Analysis" Molecules 21, no. 5: 633. https://doi.org/10.3390/molecules21050633
APA StyleHuck, C. W. (2016). Recent Developments in Solid-Phase Extraction for Near and Attenuated Total Reflection Infrared Spectroscopic Analysis. Molecules, 21(5), 633. https://doi.org/10.3390/molecules21050633





