A Near Infrared Spectroscopy (NIRS) and Chemometric Approach to Improve Apple Fruit Quality Management: A Case Study on the Cultivars “Cripps Pink” and “Braeburn”
Abstract
:1. Introduction
2. Results and Discussion
2.1. Diversity of the Sample Set: Relevant Quality Parameters of “Braeburn” and “Cripps Pink” Apples Considering Three Different Harvest Time-Points and Their Evolution during Long-Term CA Storage
| Cultivar | “Braeburn” | “Cripps Pink” | ||||
|---|---|---|---|---|---|---|
| Harvest Time Point | HT1 | HT2 | HT3 | HT1 | HT2 | HT3 |
| sample number | 30 | 30 | 30 | 30 | 30 | 30 |
| starch index * | 2.7 ± 0.4 b | 3.5 ± 0.7 a | 3.7 ± 0.6 a | 2.8 ± 0.3 a | 3.0 ± 0.2 a | 3.5 ± 0.2 b |
| weight [g] * | 206.6 ± 40.1 | 209.5 ± 31.7 | 208.8 ± 33.2 | 208.3 ± 33.4 | 211.1 ± 33.7 | 215.4 ± 23.4 |
| pH # | 3.55 ± 0.06 | 3.54 ± 0.08 | 3.58 ± 0.08 | 3.51 ± 0.05 | 3.49 ± 0.06 | 3.49 ± 0.04 |
| TA [g/L malic acid] # | 5.6 ± 0.5 a | 5.3 ± 0.9 a | 4.6 ± 0.7 b | 5.6 ± 0.6 a | 5.1 ± 0.5 b | 5.4 ± 0.4 a |
| TSS [°Brix] * | 10.6 ± 3.0 a | 9.9 ± 2.6 a | 12.2 ± 1.5 b | 13.4 ± 0.4 | 13.3 ± 0.5 | 13.2 ± 0.5 |
| Ff [N] * | 92.5 ± 9.4 | 86.7 ± 11.1 | 87.2 ± 12.3 | 110.0 ± 10.4 a | 105.0 ± 7.5 a | 94.4 ± 7.9 b |
| D [mm] * | 3.91 ± 0.47 a | 3.55 ± 0.26 b | 3.68 ± 0.53 a,b | 5.15 ± 0.73 | 4.90 ± 0.68 | 5.01 ± 0.89 |
| Wf [J] * | 0.21 ± 0.04 b | 0.18 ± 0.03 a | 0.18 ± 0.04 a | 0.32 ± 0.07 a | 0.29 ± 0.06 a,b | 0.28 ± 0.06 b |
| FLC [N] * | 70.8 ± 6.2 b | 63.2 ± 7.1 a | 63.8 ± 9.8 a | 95.4 ± 6.8 a | 92.2 ± 5.1 a | 84.2 ± 6.0 b |
| S [N/mm] * | 37.6 ± 4.8 a,b | 39.6 ± 13.0 a | 33.5 ± 5.7 b | 37.8 ± 3.6 | 37.0 ± 4.2 | 36.9 ± 4.9 |
| Cultivar | “Braeburn” | “Cripps Pink” | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CA storage [weeks] | 0 | 7 | 15 | 21 | 28 | 32 | 0 | 6 | 15 | 20 | 27 | 30 |
| sample number | 30 | 30 | 26 | 26 | 26 | 28 | 30 | 30 | 30 | 30 | 30 | 28 |
| weight [g] * | 206.6 | 208.7 | 196.9 | 197.3 | 199.7 | 204.0 | 208.3 | 212.3 | 197.9 | 202.3 | 199.6 | 201.3 |
| total juice [mL] * | 125.7 a | 122.7 a | 102.2 b | 110.0 a,b | 120.5 a,b | 130.0 a | 116.9 a,b | 103.5 b,c | 98.9 c | 111.3 b,c | ||
| pH # | 3.55 a | 3.49 c | 3.57 a | 3.66 b | 3.68 b | 3.51 a | 3.60 b | 3.74 c | 3.70 d | 3.79 e | 3.84 f | |
| TA [g/L malic acid] # | 5.6 a | 5.6 a | 5.1 b | 4.6 c | 4.5 c | 5.6 a | 4.8 b | 4.0 c | 4.0 c | 3.6 d | 3.6 d | |
| TSS [°Brix] * | 10.6 b | 13.0 a | 12.9 a | 12.9 a | 13.4 a | 12.6 a,b,c | 12.9 b,c | 12.4 c | 13.0 a,b,c | 13.2 a,b | ||
| Ff [N] * | 92.5 a | 93.4 a | 84.0 b | 78.5 b,c | 76.2 c | 75.9 c | 110.0 a | 91.6 b | 85.9 b,c | 87.1 b,c | 81.2 c | |
| D [mm] * | 3.91 a | 3.62 a,b,c | 3.48 b,c | 3.39 b,c | 3.26 c | 3.67 a,b | 5.15 a | 4.65 b | 4.52 b,c | 4.45 b,c | 4.22 c | |
| Wf [J] * | 0.21 a | 0.19 a,b | 0.17 b,c | 0.16 c | 0.14 c | 0.16 b,c | 0.32 a | 0.24 b | 0.22 b,c | 0.22 b,c | 0.19 c | |
| FLC [N] * | 70.8 a | 67.5 a,b | 65.1 b,c | 60.0 c,d | 58.1 d | 57.7 d | 95.4 a | 74.2 b | 63.5 c | 63.0 c,d | 58.3 d | |
| S [N/mm] * | 37.6 a,b | 38.4 a,b | 35.9 a | 38.5 a,b | 42.4 b | 36.3 a,b | 37.8 a,b | 32.4 a | 49.5 b | 31.1 a | 30.4 a | |
| glucose [g/100 g] # | 1.1 | 1.2 | 1.1 | 1.0 | 1.2 | 0.5 a | 0.5 a | 0.7 a,b | 0.9 b | 0.6 a,b | ||
| xylose [g/100 g] * | 0.03 b | 0.05 a | 0.06 a | 0.05 a | 0.06 a | 0.03 a | 0.03 a | 0.05 a,b | 0.06 b | 0.06 b | ||
| sucrose [g/100 g] # | 2.9 a | 3.2 a | 1.7 b | 1.2 b,c | 0.9 c | 3.5 a | 3.0 a,b | 2.4 b | 2.6 b | 2.4 b | ||
| fructose [g/100 g] * | 2.6 | 2.3 | 2.2 | 2.2 | 2.1 | 3.1 | 2.7 | 2.5 | 3.2 | 2.8 | ||
2.2. Qualitative Analysis by Means of Near Infrared Spectroscopy
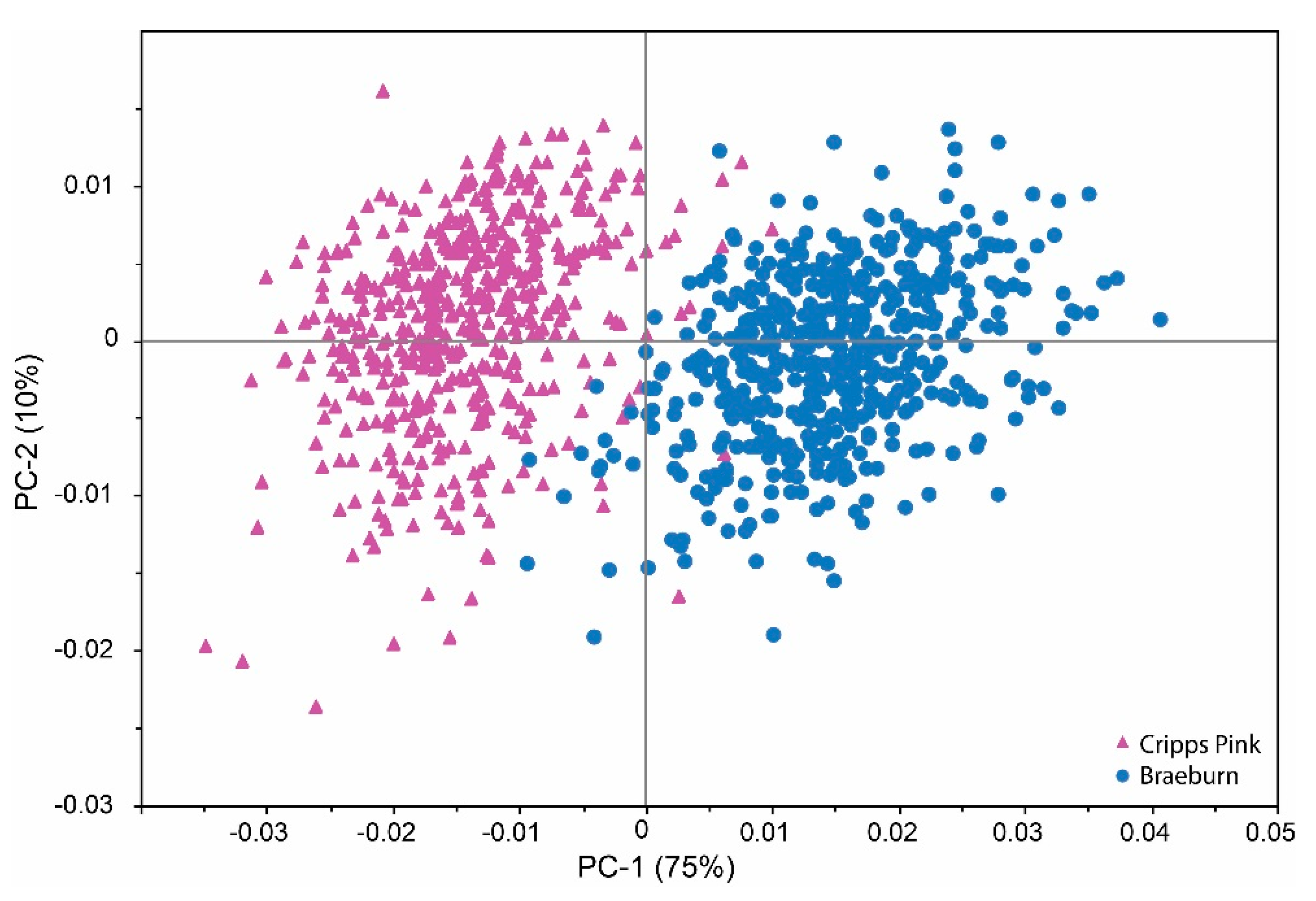
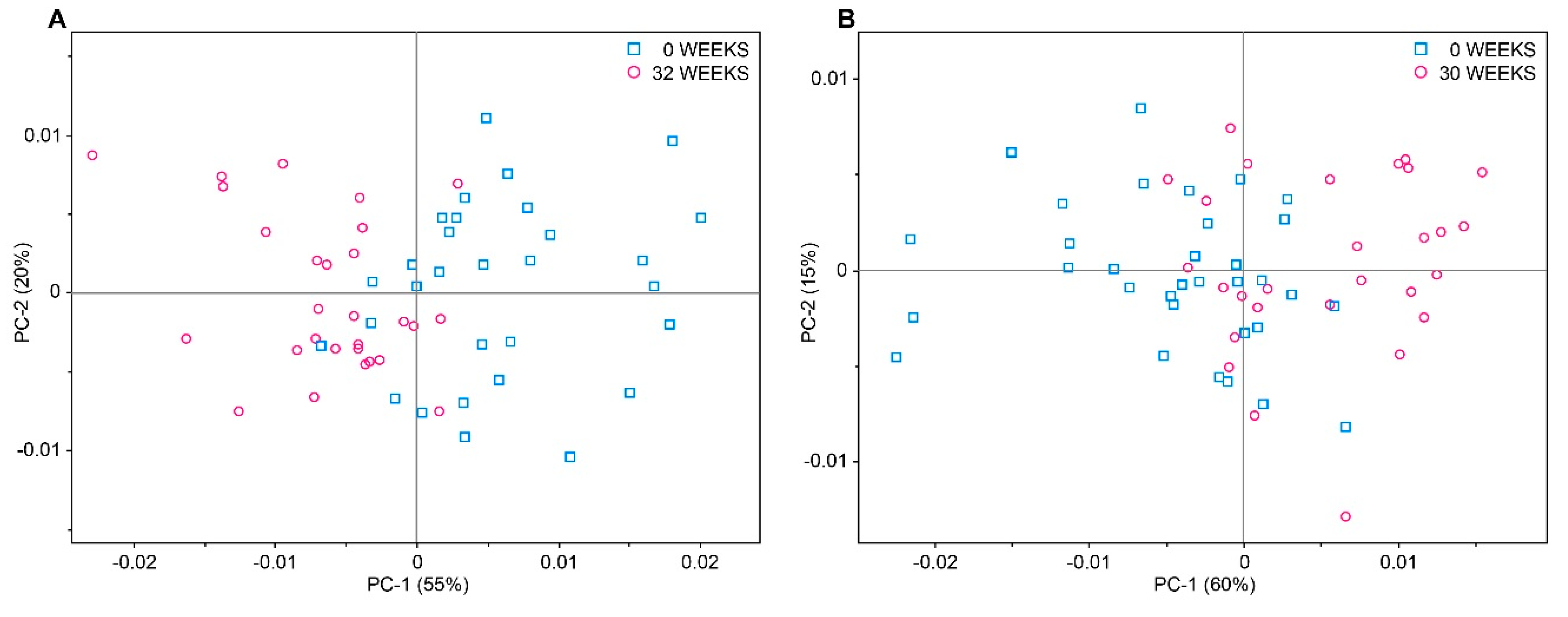
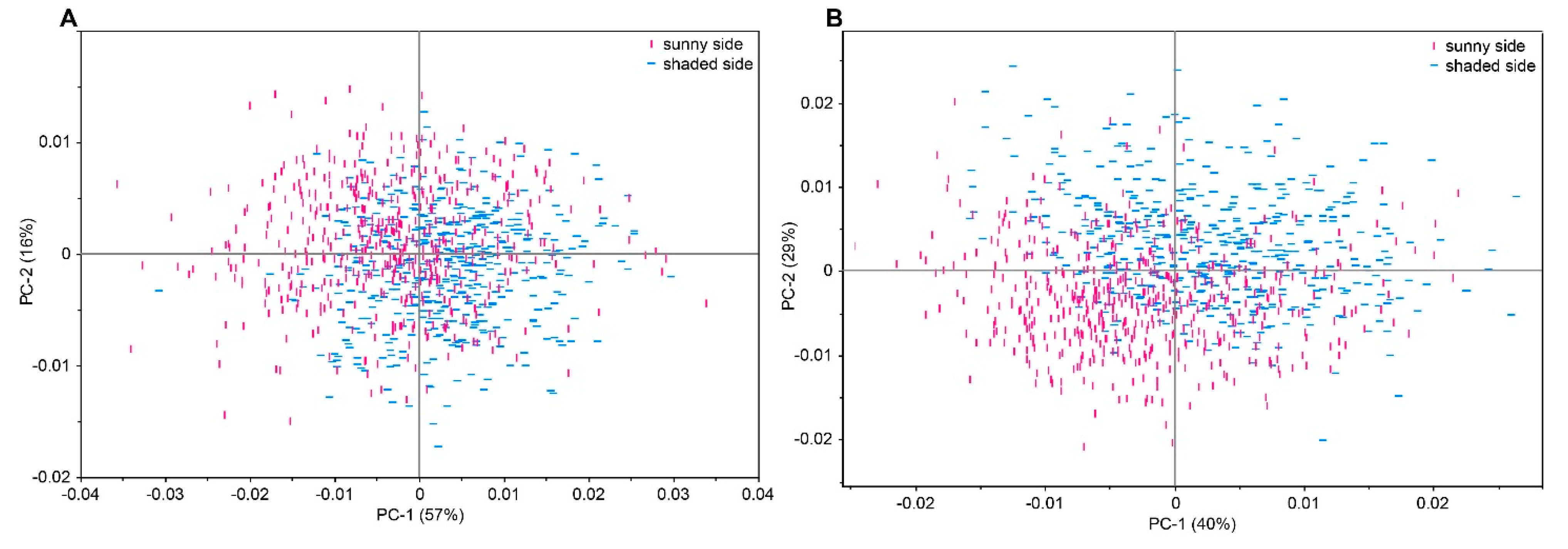
2.3. Development of Multivariate Calibration Models
| Parameters # | Cultivar | Range | Wavelength Selection [nm] | Data Treatment | LV | N | Calibration | Validation | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SEC | r2 | SEP | r2 | Bias | |||||||
| TSS [°Brix] | CP | 11.3–14.9 | 1041–2325 | n01, 1st derivative BCAP | 3 | 510 | 0.57 | 0.03 | 0.56 | 0.02 | –0.00 |
| BB | 10.0–14.7 | 1388–2083 | 1st derivative BCAP, SNV | 6 | 388 | 0.52 | 0.49 | 0.52 | 0.38 | –0.08 | |
| both | 10.7–14.6 | 1111–1351, 1408–2000 | 1st derivative BCAP, ncl | 5 | 866 | 0.58 | 0.15 | 0.59 | 0.14 | –0.00 | |
| TA [g/L malic acid] | CP | 2.7–6.4 | 1041–2380 | ncl, 1st derivative BCAP | 8 | 533 | 0.32 | 0.85 | 0.44 | 0.69 | –0.04 |
| BB | 3.2–6.5 | 1136–2272 | 1st derivative BCAP, MSC full | 6 | 428 | 0.43 | 0.52 | 0.45 | 0.50 | –0.04 | |
| both | 2.7–6.8 | 1000–2000 | SNV, 1st derivative BCAP | 8 | 959 | 0.41 | 0.74 | 0.48 | 0.67 | 0.06 | |
| pH | CP | 3.39–4.00 | 1000–2439 | ncl | 12 | 533 | 0.06 | 0.81 | 0.06 | 0.81 | 0.00 |
| BB | 3.37–3.84 | 1000–1282, 1515–1851, 2083–2272 | ncl | 12 | 428 | 0.05 | 0.62 | 0.05 | 0.62 | 0.01 | |
| both | 3.37–4.00 | 1111–2439 | SNV | 10 | 959 | 0.09 | 0.49 | 0.09 | 0.50 | –0.00 | |
| Ff [N] | CP | 60.8–109.8 | 1000–2495 | none | 9 | 346 | 9.4 | 0.11 | 9.4 | 0.14 | 0.05 |
| BB | 49.0–110.8 | 1086–2325 | none | 12 | 494 | 7.8 | 0.56 | 7.9 | 0.55 | –0.03 | |
| both | 49.0–124.5 | 1111–2272 | none | 9 | 867 | 10.8 | 0.31 | 9.8 | 0.29 | 0.03 | |
| D [mm] | CP | 3.04–7.85 | 1086–2380 | none | 14 | 357 | 0.69 | 0.30 | 0.75 | 0.29 | 0.08 |
| BB | 2.59–4.79 | 1111–2439 | none | 14 | 495 | 0.39 | 0.18 | 0.40 | 0.15 | 0.00 | |
| both | 2.59–7.85 | 1086–2380 | none | 14 | 868 | 0.66 | 0.46 | 0.68 | 0.45 | 0.01 | |
| Wf [J] | CP | 0.11–0.48 | 1111–1351, 1408–2000 | none | 12 | 358 | 0.06 | 0.18 | 0.06 | 0.08 | 0.00 |
| BB | 0.08–0.27 | 1086–2439 | none | 11 | 491 | 0.03 | 0.35 | 0.03 | 0.38 | –0.00 | |
| both | 0.08–0.48 | 1086–2439 | none | 11 | 867 | 0.05 | 0.32 | 0.05 | 0.36 | 0.00 | |
| FLC [N] | CP | 43.9–101.2 | 1098–2222 | none | 13 | 334 | 8.7 | 0.40 | 9.2 | 0.24 | 0.47 |
| BB | 33.9–93.3 | 1111–2272 | none | 13 | 424 | 6.4 | 0.50 | 6.5 | 0.46 | –0.27 | |
| both | 33.9–101.2 | 1063–2272 | none | 7 | 758 | 9.2 | 0.29 | 8.6 | 0.22 | –0.30 | |
| SECV | r2CV | ||||||||||
| glucose [g/100 g] | CP | 0.3–1.3 | 1111–2252 | SNV. 1st derivative SG 9 points | 12 | 73 | 0.2 | 0.85 | |||
| BB | 0.6–1.8 | 1111–2380 | SNV. 1st derivative SG 9 points | 10 | 77 | 0.3 | 0.79 | ||||
| both | 0.3–1.8 | 1063–2272 | SNV. 1st derivative SG 9 points | 10 | 150 | 0.2 | 0.83 | ||||
| xylose [g/100 g] | CP | 0.02–0.08 | 1111–1351, 1408–2000 | 1st derivative BCAP. SNV | 8 | 73 | 0.02 | 0.81 | |||
| BB | 0.01–0.07 | 1136–2272 | 1st derivative BCAP. SNV | 8 | 77 | 0.01 | 0.76 | ||||
| both | 0.01–0.08 | 1063–2272 | 1st derivative BCAP. SNV | 7 | 150 | 0.01 | 0.59 | ||||
| sucrose [g/100 g] | CP | 1.4–4.1 | 1111–2380 | ncl. 1st derivative BCAP | 10 | 73 | 0.7 | 0.85 | |||
| BB | 0.5–3.9 | 1111–2380 | ncl. 1st derivative BCAP | 10 | 77 | 0.8 | 0.79 | ||||
| both | 0.5–4.1 | 1111–2272 | ncl. 1st derivative BCAP | 10 | 150 | 0.7 | 0.74 | ||||
| fructose [g/100 g] | CP | 1.6–3.8 | 1111–2272 | ncl. 1st derivative SG 9 points | 8 | 73 | 0.6 | 0.62 | |||
| BB | 0.9–4.3 | 1111–2272 | ncl. 1st derivative SG 9 points | 10 | 77 | 0.9 | 0.76 | ||||
| both | 0.9–4.3 | 1111–2272 | ncl. 1st derivative SG 9 points | 10 | 150 | 0.7 | 0.55 | ||||
3. Experimental Section
3.1. Fruit Material
3.2. NIRS
3.3. Physicochemical Parameters
3.3.1. Standards
3.3.2. Starch Index
3.3.3. Firmness
3.3.4. Total Soluble Solids
3.3.5. Titratable Acid and pH
3.3.6. Extraction and Individual Sugar Determination
3.4. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- DeEll, J.R.; Khanizadeh, S.; Saad, F.; Ferree, D.C. Factors Affecting Apple Fruit Firmness—A Review. J. Am. Pomol. Soc. 2001, 55, 8–27. [Google Scholar]
- Zhang, Y.; Li, P.; Cheng, L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in “Honeycrisp” apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Jan, I.; Rab, A.; Sajid, M. Storage performance of apple cultivars harvested at different stages of maturity. J. Anim. Plant Sci. 2012, 22, 438–447. [Google Scholar]
- Jan, I.; Rab, A. Influence of storage duration on physico-chemical changes in fruit of apple cultivars. J. Anim. Plant Sci. 2012, 22, 708–714. [Google Scholar]
- Róth, E.; Berna, A.; Beullens, K.; Yarramraju, S.; Lammertyn, J.; Schenk, A.; Nicolaï, B. Postharvest quality of integrated and organically produced apple fruit. Postharvest Biol. Technol. 2007, 45, 11–19. [Google Scholar] [CrossRef]
- European Union. Commission Regulation (EC) No. 1619/2001 laying down the marketing standard for apples and pears and amending Regulation (EEC) No. 920/89. Off. J. Eur. Communities 2001, L215, 3–16. [Google Scholar]
- Hoehn, E.; Gasser, F.; Guggenbühl, B.; Künsch, U. Efficacy of instrumental measurements for determination of minimum requirements of firmness, soluble solids, and acidity of several apple varieties in comparison to consumer expectations. Postharvest Biol. Technol. 2003, 27, 27–37. [Google Scholar] [CrossRef]
- Mattheis, J.P.; Fellman, J.K. Preharvest factors influencing flavor of fresh fruit and vegetables. Postharvest Biol. Technol. 1999, 15, 227–232. [Google Scholar] [CrossRef]
- Nicolaï, B.M.; Beullens, K.; Bobelyn, E.; Peirs, A.; Saeys, W.; Theron, K.I.; Lammertyn, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol. 2007, 46, 99–118. [Google Scholar] [CrossRef]
- Tothill, I.E. Rapid and On-Line Instrumentation for Food Quality Assurance; CRC Press; Woodhead: Boca Raton, FL, USA; Cambridge, UK, 2003. [Google Scholar]
- Ciesa, F.; Dalla Via, J.; Wisthaler, A.; Zanella, A.; Guerra, W.; Mikoviny, T.; Märk, T.D.; Oberhuber, M. Discrimination of four different postharvest treatments of “Red Delicious” apples based on their volatile organic compound (VOC) emissions during shelf-life measured by proton transfer reaction mass spectrometry (PTR-MS). Postharvest Biol. Technol. 2013, 86, 329–336. [Google Scholar] [CrossRef]
- Clark, C.J.; McGlone, V.A.; Jordan, R.B. Detection of Brownheart in “Braeburn” apple by transmission NIR spectroscopy. Postharvest Biol. Technol. 2003, 28, 87–96. [Google Scholar] [CrossRef]
- Liu, Y.; Ying, Y. Use of FT-NIR spectrometry in non-invasive measurements of internal quality of “Fuji” apples. Postharvest Biol. Technol. 2005, 37, 65–71. [Google Scholar] [CrossRef]
- Lu, R.; Guyer, D.E.; Beaudry, R.M. Determination of Firmness and Sugar Content of Apples Using Near-Infrared Diffuse Reflectance. J. Texture Stud. 2000, 31, 615–630. [Google Scholar] [CrossRef]
- Osborne, B.G.; Fearn, T.; Hindle, P.T. Practical NIR Spectroscopy with Applications in Food and Beverage Analysis, 2nd ed; Longman Scientific & Technical; Wiley: Harlow, Essex, UK; New York, NY; USA, 1993. [Google Scholar]
- Cozzolino, D. Near infrared spectroscopy in natural products analysis. Planta Med. 2009, 75, 746–756. [Google Scholar] [CrossRef] [PubMed]
- McGlone, V.A.; Jordan, R.B.; Martinsen, P.J. Vis/NIR estimation at harvest of pre- and post-storage quality indices for “Royal Gala” apple. Postharvest Biol. Technol. 2002, 25, 135–144. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Shao, Y. Fast Discrimination of Apple Varieties Using Vis/NIR Spectroscopy. Int. J. Food Prop. 2007, 10, 9–18. [Google Scholar] [CrossRef]
- Giovanelli, G.; Sinelli, N.; Beghi, R.; Guidetti, R.; Casiraghi, E. NIR spectroscopy for the optimization of postharvest apple management. Postharvest Biol. Technol. 2014, 87, 13–20. [Google Scholar] [CrossRef]
- Brookfield, P.; Murphy, P.; Harker, R.; MacRae, E. Starch degradation and starch pattern indices; interpretation and relationship to maturity. Postharvest Biol. Technol. 1997, 11, 23–30. [Google Scholar] [CrossRef]
- Lelièvre, J.M.; Latchè, A.; Jones, B.; Bouzayen, M.; Pech, J.C. Ethylene and fruit ripening. Physiol. Plant. 1997, 101, 727–739. [Google Scholar] [CrossRef]
- Johnston, J.W.; Hewett, E.W.; Hertog, M.L.A.T.M. Postharvest softening of apple (Malus domestica) fruit: A review. N. Z. J. Crop Hortic. Sci. 2002, 30, 145–160. [Google Scholar] [CrossRef]
- Berüter, J. Carbohydrate metabolism in two apple genotypes that differ in malate accumulation. J. Plant Physiol. 2004, 161, 1011–1029. [Google Scholar] [CrossRef] [PubMed]
- Shafiq, M.; Singh, Z.; Khan, A.S. Delayed harvest and cold storage period influence ethylene production, fruit firmness and quality of “Cripps Pink” apple. Int. J. Food Sci. Technol. 2011, 46, 2520–2529. [Google Scholar] [CrossRef]
- Echeverrı́a, G.; Fuentes, T.; Graell, J.; Lara, I.; López, M.L. Aroma volatile compounds of “Fuji” apples in relation to harvest date and cold storage technology: A comparison of two seasons. Postharvest Biol. Technol. 2004, 32, 29–44. [Google Scholar] [CrossRef]
- Ghafir, S.A.M.; Gadalla, S.O.; Murajei, B.N.; El-Nady, M.F. Physiological and anatomical comparison between four different apple cultivars under cold-storage conditions. Afr. J. Plant Sci. 2009, 3, 133–138. [Google Scholar]
- Cosgrove, D.J.; Bedinger, P.; Durachko, D.M. Group I allergens of grass pollen as cell wall-loosening agents. Proc. Natl. Acad. Sci. USA 1997, 94, 6559–6564. [Google Scholar] [CrossRef] [PubMed]
- Crouch, I. 1-Methylcyclopropene (Smartfresh™) as an alternative to modified atmosphere and controlled atmosphere storage of apples and pears. Acta Hort. (ISHS) 2003, 600, 433–436. [Google Scholar]
- Ben, J.; Gaweda, M. Changes of pectic compounds in Jonathan apples under various storage conditions. Acta Physiol. Plant. 1985, 7, 45–54. [Google Scholar]
- Savitzky, A.; Golay, M.J.E. Smoothing and differentiation of data by simplified least squares procedures. Anal. Chem. 1964, 36, 1627–1639. [Google Scholar] [CrossRef]
- Workman, J.; Weyer, L. Practical Guide to interpretive Near-Infrared Spectroscopy; CRC Press: Boca Raton, FL, USA, 2008. [Google Scholar]
- Zanella, A.; Vanoli, M.; Rizzolo, A.; Grassi, M.; Eccher Zerbini, P.; Cubeddu, R.; Torricelli, A.; Spinelli, L. Correlating Optical Maturity Indices and Firmness in Stored “Braeburn” and “Cripps Pink” Apples. Acta Hort. (ISHS) 2013, 1012, 1173–1180. [Google Scholar]
- Ng Jovyn, K.T.; Schröder, R.; Sutherland, P.W.; Hallett, I.C.; Hall, M.I.; Prakash, R.; Smith, B.G.; Melton, L.D.; Johnston, J.W. Cell wall structures leading to cultivar differences in softening rates develop early during apple (Malus x domestica) fruit growth. BMC Plant Biol. 2013, 13, 183–198. [Google Scholar]
- Gwanpua, S.G.; van Buggenhout, S.; Verlinden, B.E.; Christiaens, S.; Shpigelman, A.; Vicent, V.; Kermani, Z.J.; Nicolai, B.M.; Hendrickx, M.; Geeraerd, A. Pectin modifications and the role of pectin-degrading enzymes during postharvest softening of Jonagold apples. Food Chem. 2014, 158, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Peirs, A.; Lammertyn, J.; Ooms, K.; Nicolaı̈, B.M. Prediction of the optimal picking date of different apple cultivars by means of VIS/NIR-spectroscopy. Postharvest Biol. Technol. 2001, 21, 189–199. [Google Scholar] [CrossRef]
- Bobelyn, E.; Serban, A.S.; Nicu, M.; Lammertyn, J.; Nicolai, B.M.; Saeys, W. Postharvest quality of apple predicted by NIR-spectroscopy: Study of the effect of biological variability on spectra and model performance. Postharvest Biol. Technol. 2010, 55, 133–143. [Google Scholar] [CrossRef]
- Li, P.; Ma, F.; Cheng, L. Primary and secondary metabolism in the sun-exposed peel and the shaded peel of apple fruit. Physiol. Plant. 2013, 148, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Niu, X.; Yang, D.; Ying, Y.; Li, B.; Zhu, G.; Wu, J. Determination of Amino Acids in Chinese Rice Wine by Fourier Transform Near-Infrared Spectroscopy. J. Agric. Food Chem. 2010, 58, 9809–9816. [Google Scholar] [CrossRef] [PubMed]
- Lammertyn, J.; Peirs, A.; Baerdemaeker, J.; de Nicolaı̈, B. Light penetration properties of NIR radiation in fruit with respect to non-destructive quality assessment. Postharvest Biol. Technol. 2000, 18, 121–132. [Google Scholar] [CrossRef]
- Næs, T.; Isaksson, T.; Fearn, T.; Davies, T. A User-Friendly Guide to Multivariate Calibration and Classification; NIR Publications: Chichester, UK, 2002. [Google Scholar]
- Liu, Y.; Ying, Y.; Yu, H.; Fu, X. Comparison of the HPLC method and FT-NIR analysis for quantification of glucose, fructose, and sucrose in intact apple fruits. J. Agric. Food Chem. 2006, 54, 2810–2815. [Google Scholar] [CrossRef] [PubMed]
- Dalla Via, J.; Mantinger, H. Agricultural Research in the Field of Fruit Growing in South Tyrol. Erwerbs-Obstbau 2012, 54, 83–115. [Google Scholar] [CrossRef]
- DeLong, J.M.; Prange, R.K.; Harrison, P.A. Using the Streif Index as a Final Harvest Window for Controlled-atmosphere Storage of Apples. HortScience 1999, 34, 1251–1255. [Google Scholar]
- Duprat, F.; Grotte, M.; Loonis, D.; Piétri, E. Etude de la possibilité de mesurer simultanément la fermeté de la chair et de l’épiderme des pommes. Sci. Aliments 2000, 20, 253–264. [Google Scholar] [CrossRef]
- R Core Team. A language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2013. [Google Scholar]
- CAMO Software AS. Unscrambler X, CAMO Software: Oslo, Norway, 2014.
- Bossart, R.; Grabinski, J. Automatic Calibration Method. U.S. Patent 6,480,795; filed 18 January 2001, and issued 12 November 2002,
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eisenstecken, D.; Panarese, A.; Robatscher, P.; Huck, C.W.; Zanella, A.; Oberhuber, M. A Near Infrared Spectroscopy (NIRS) and Chemometric Approach to Improve Apple Fruit Quality Management: A Case Study on the Cultivars “Cripps Pink” and “Braeburn”. Molecules 2015, 20, 13603-13619. https://doi.org/10.3390/molecules200813603
Eisenstecken D, Panarese A, Robatscher P, Huck CW, Zanella A, Oberhuber M. A Near Infrared Spectroscopy (NIRS) and Chemometric Approach to Improve Apple Fruit Quality Management: A Case Study on the Cultivars “Cripps Pink” and “Braeburn”. Molecules. 2015; 20(8):13603-13619. https://doi.org/10.3390/molecules200813603
Chicago/Turabian StyleEisenstecken, Daniela, Alessia Panarese, Peter Robatscher, Christian W. Huck, Angelo Zanella, and Michael Oberhuber. 2015. "A Near Infrared Spectroscopy (NIRS) and Chemometric Approach to Improve Apple Fruit Quality Management: A Case Study on the Cultivars “Cripps Pink” and “Braeburn”" Molecules 20, no. 8: 13603-13619. https://doi.org/10.3390/molecules200813603
APA StyleEisenstecken, D., Panarese, A., Robatscher, P., Huck, C. W., Zanella, A., & Oberhuber, M. (2015). A Near Infrared Spectroscopy (NIRS) and Chemometric Approach to Improve Apple Fruit Quality Management: A Case Study on the Cultivars “Cripps Pink” and “Braeburn”. Molecules, 20(8), 13603-13619. https://doi.org/10.3390/molecules200813603







