A Novel Photosensitizer 31,131-phenylhydrazine -Mppa (BPHM) and Its in Vitro Photodynamic Therapy against HeLa Cells
Abstract
:1. Introduction
2. Results and Discussion
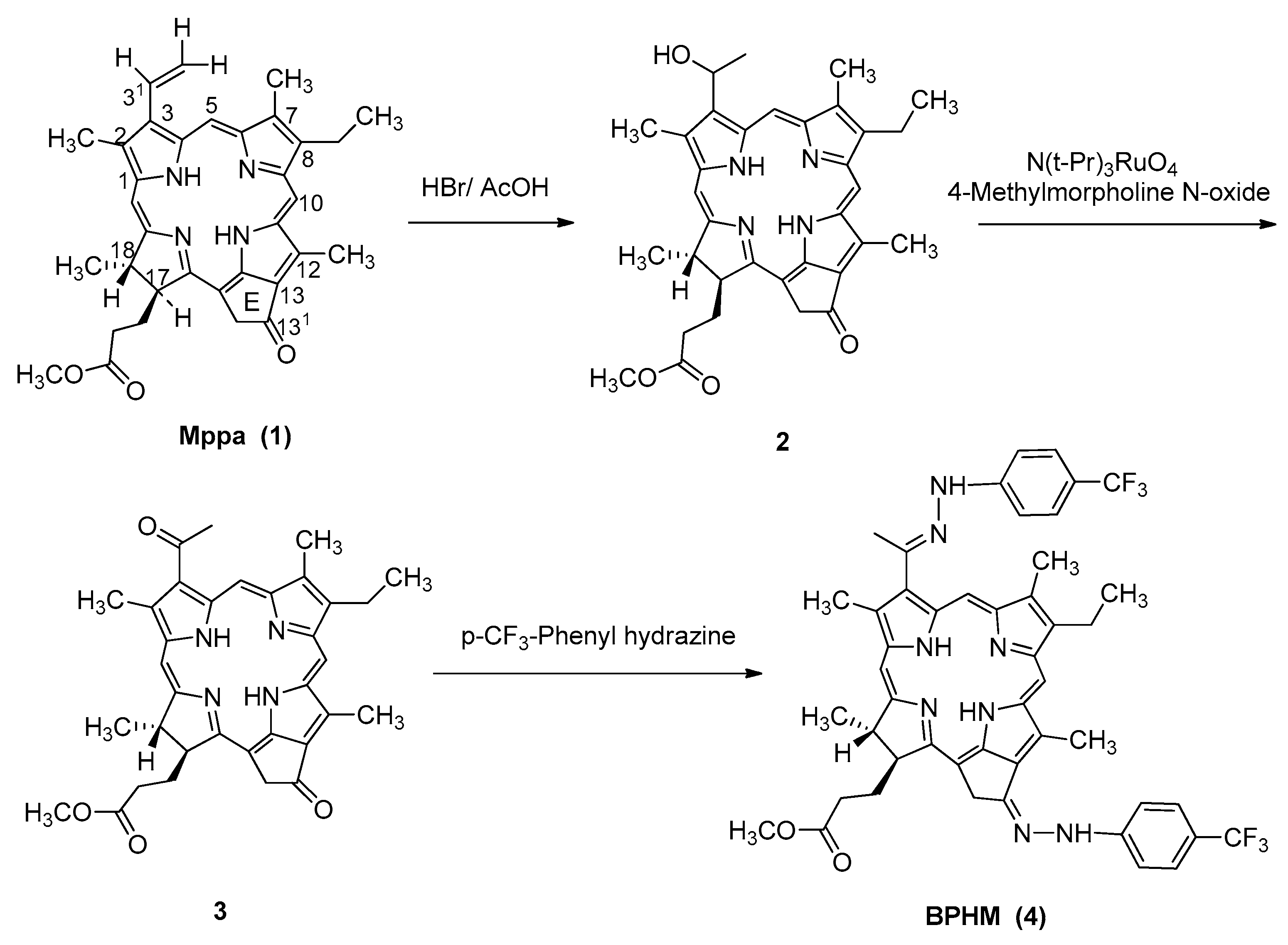
2.1. Chemistry
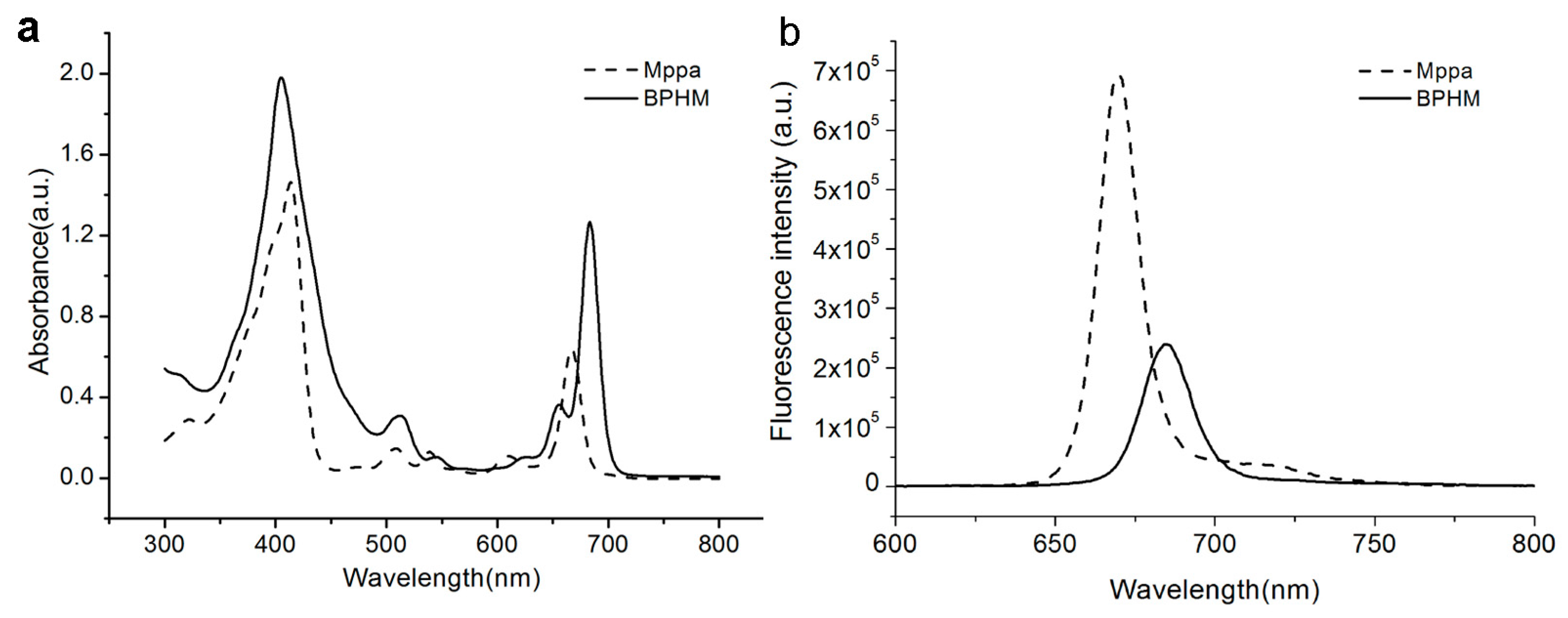
2.2. Optical Properties
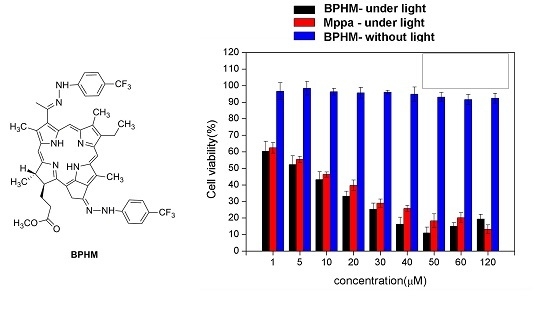
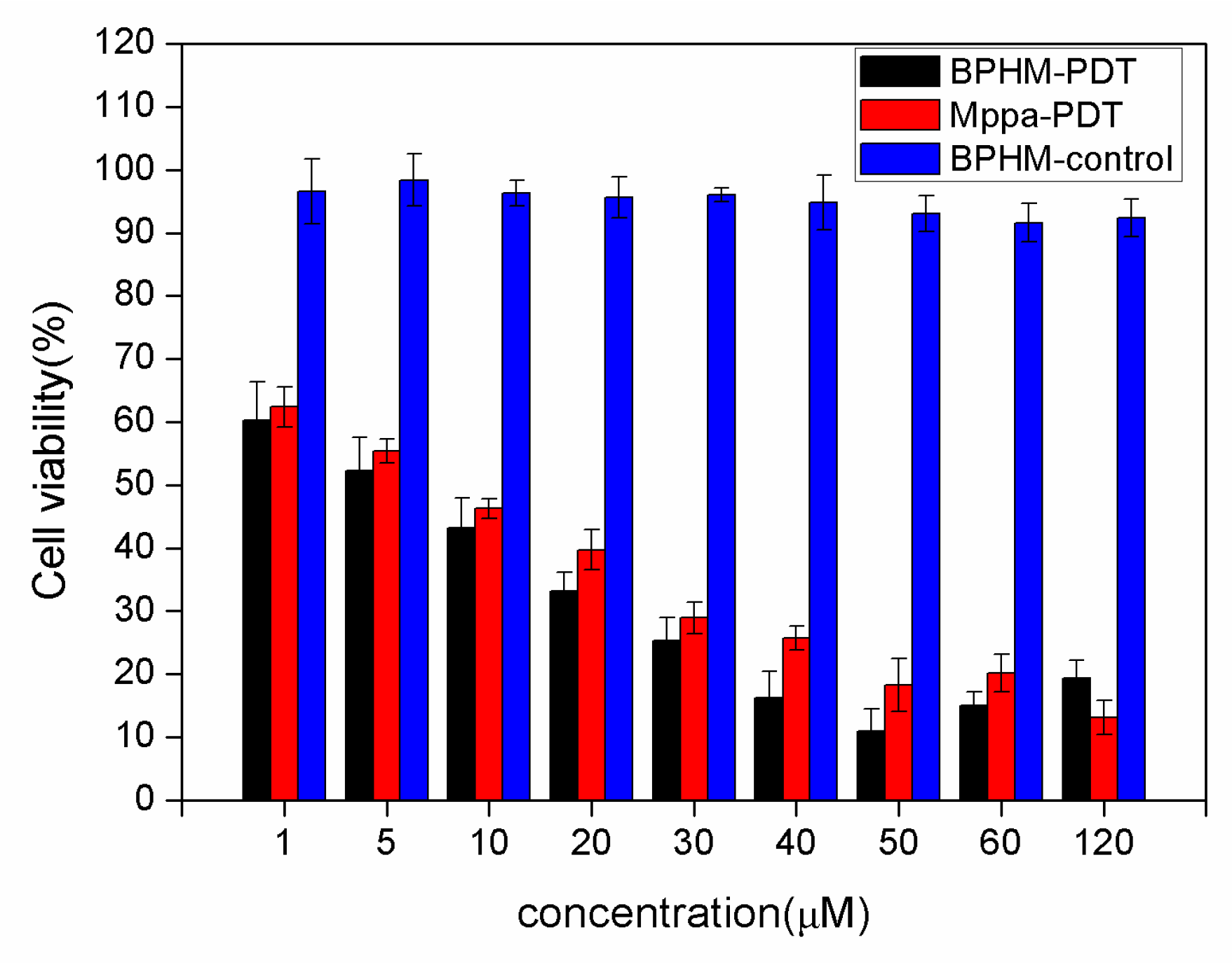
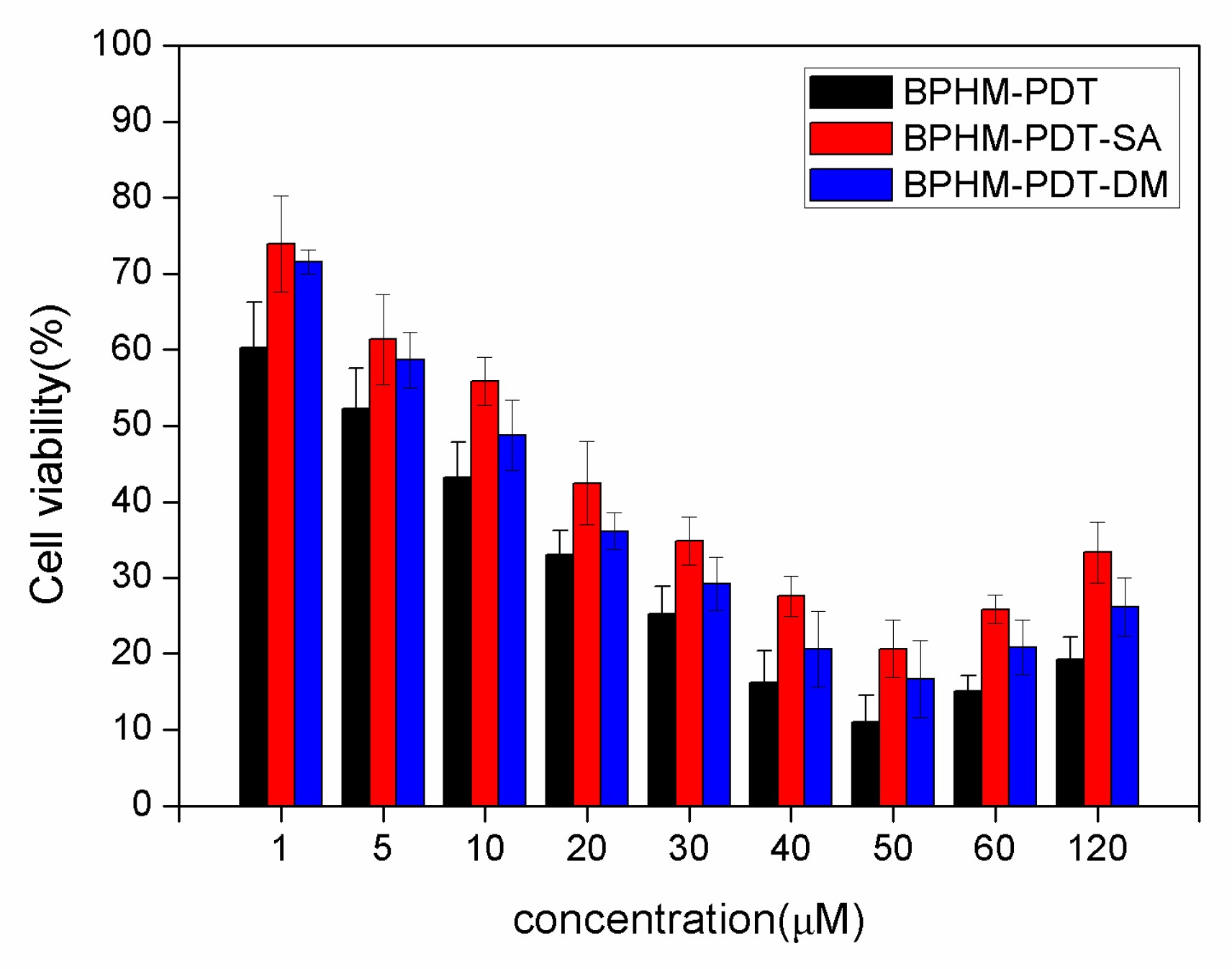
2.3. Photodynamic Activities
2.4. Formation of Reactive Oxygen Species in PDT
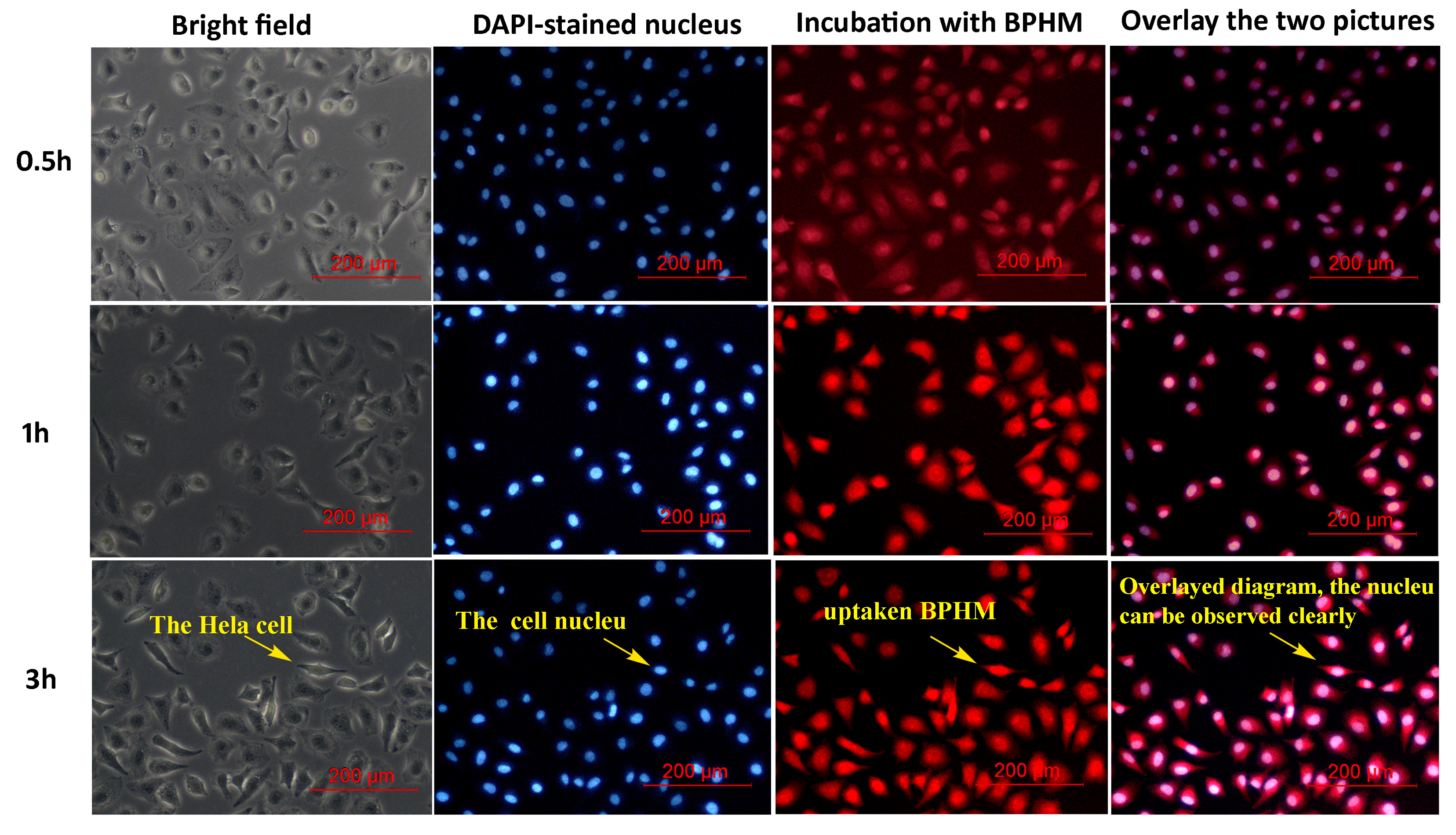
2.5. Cellular Uptake
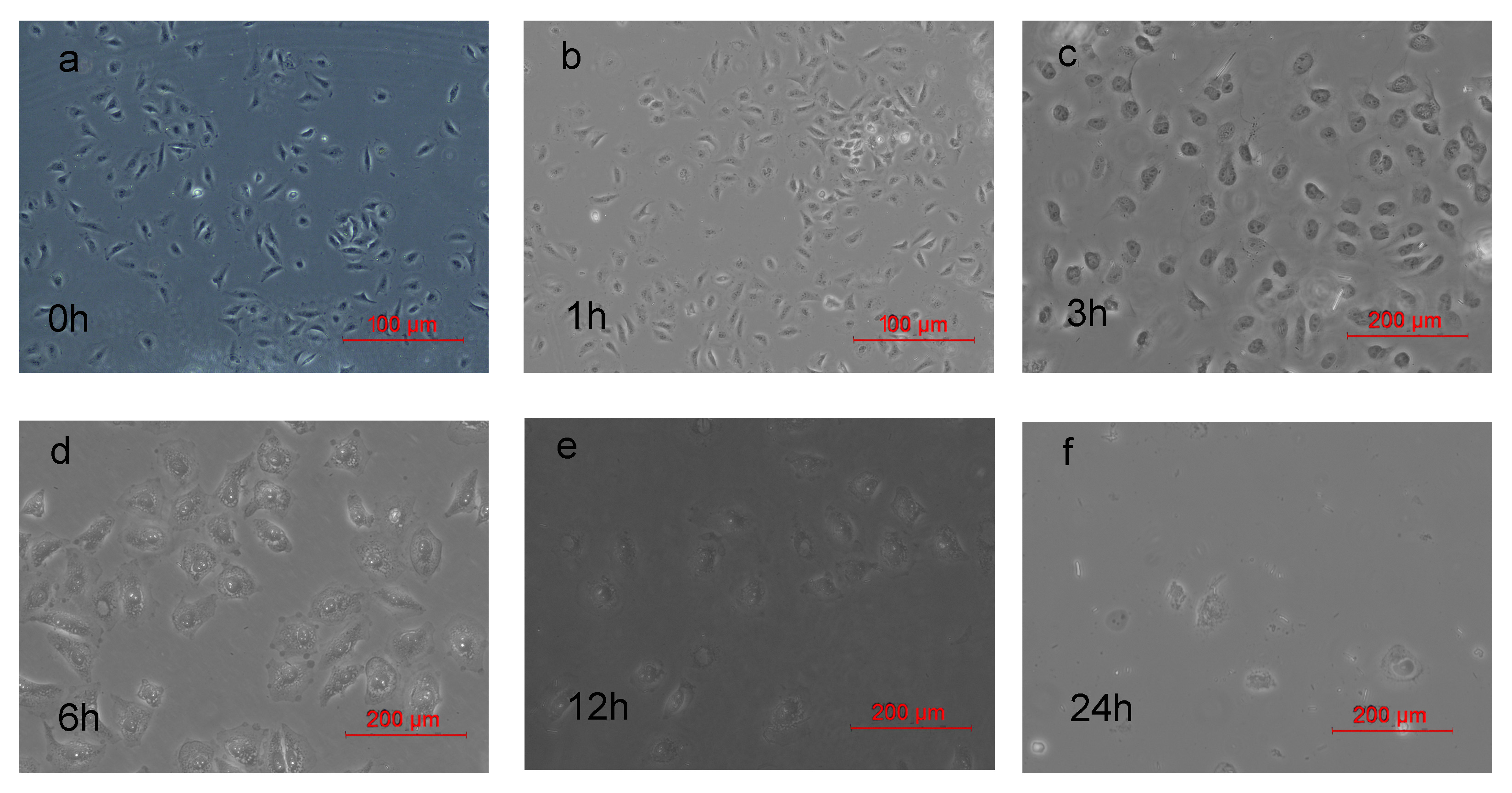
2.6. Morphological Changes of HeLa Cells after PDT
3. Experimental Section
3.1. General Remarks
3.2. Synthesis of 31-hydroxyethylMppa (2)
3.3. Synthesis of 3-ethoxy-Mppa (3)
3.4. Synthesis of 31,131-bisphenylhydrazone-Mppa (BPHM, 4)
3.5. Cell Culture and MTT Colorimetric Assay
3.6. In Vitro Cytotoxicity
3.7. Cellular Uptake of BPHM
3.8. Morphological Changes of HeLa Cells after PDT
3.9. Type I and Type II Mechanism of PDT
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Braathen, L.R.; Szeimies, R.M.; Basset-Seguin, N.; Bissonnette, R.; Foley, P.; Pariser, D.; Roelandts, R.; Wennberg, A.M.; Morton, C.A. Guidelines on the use of photodynamic therapy for nonmelanoma skin cancer: An international consensus. J. Am. Acad. Dermatol. 2007, 56, 125–143. [Google Scholar] [CrossRef] [PubMed]
- Kato, H.; Okunaka, T.; Shimatani, H. Photodynamic therapy for early stage bronchogenic carcinoma. J. Clin. Laser. Med. Surg. 1996, 14, 235–238. [Google Scholar] [PubMed]
- Nathan, T.R.; Whitelaw, D.E.; Chang, S.C.; Lees, W.R.; Ripley, P.M.; Payne, H.; Jones, L.; Parkinson, M.C.; Emberton, M.; Gillams, A.R.; Mundy, A.R.; Bown, S.G. Photodynamic Therapy for Prostate Cancer Recurrence After Radiotherapy: A Phase I Study. J. Urol. 2002, 168, 1427–1432. [Google Scholar] [CrossRef]
- Ahn, T.-G.; Lee, B.-R.; Choi, E.-Y.; Kim, D.W.; Han, S.-J. Photodynamic therapy for breast cancer in a BALB/c mouse model. J. Gynecol. Oncol. 2012, 23, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Bredell, M.G.; Besic, E.; Maake, C.; Walt, H. The application and challenges of clinical PD-PDT in the head and neck region: A short review. J. Photochem. Photobiol. 2010, 101, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Wolfsen, H.C. Carpe luz-seize the light: Endoprevention of esophageal adenocarcinoma when using photodynamic therapy with porfimer sodium. Gastrointest Endosc. 2005, 62, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.S.; Chen, J.; Keltner, L.; Christophersen, J.; Zheng, F.; Krouse, M.; Singhal, A. New technology for deep light distribution in tissue for phototherapy. Cancer J. 2002, 8, 154–163. [Google Scholar]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Hisazumi, H.; Miyoshi, N.; Ueki, O.; Nakajima, K. Cellular binding of hematoporphyrin derivative (HpD) in human bladder cancer cell line: KK-47. Prog. Clin. Biol. Res. 1984, 170, 443–457. [Google Scholar] [PubMed]
- Dougherty, T.J.; Kaufman, J.E.; Goldfarb, A.; Weishaupt, K.R.; Boyle, D.; Mittleman, A. Photoradiation therapy for the treatment of malignant tumors. Cancer Res. 1978, 38, 2628–2634. [Google Scholar] [PubMed]
- Lei, T.C.; Glazner, G.F.; Duffy, M.; Scherrer, L.; Pendyala, S.; Li, B.; Wang, X.; Wang, H.; Huang, Z. Optical properties of hematoporphyrin monomethyl ether (HMME), a PDT photosensitizer Photodiagnosis. Photodyn. Ther. 2012, 9, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Shaker, M.N.; Ramadan, H.S.; Mohamed, M.M.; Ei khatib, A.M.; Roston, G.D. Enhanced photodynamic efficacy of PLGA-encapsulated 5-ALA nanoparticles in mice bearing Ehrlich ascites carcinoma. Appl. Nanosci. 2014, 4, 777–789. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Couleaud, P.; Morosini, V.; Frochot, C.; Richeter, S.; Raehm, L.; Durand, J.O. Silica-based nanoparticles for photodynamic therapy applications. Nanoscale 2010, 7, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.M.; Sharman, W.M.; Lier, J.E.V. Current status of phthalocyanines in the photodynamic therapy of cancer. J. Porphyr. Phthalocyanines 2001, 5, 161–169. [Google Scholar] [CrossRef]
- Cakir, V.; Cakir, D.; Biyikhoglu, Z.; Kantekin, H. Synthesis, characterization and aggregation behavior of novel peripherally tetra-substituted octacationic water soluble metal-free and metallophthalocyanines. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 61–70. [Google Scholar] [CrossRef]
- Zhang, X.; Choi, E.J.; Zheng, Z.; Zhu, L.; Cho, S.B.; Kim, K.Y.; Kim, J.; Cha, I.H. Apoptotic effect of pheophorbide a-mediated photodynamic therapy on DMBA/TPA-induced mouse papillomas. Laser Med. Sci. 2015, 30, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Waruna, J.R.G.; Hu, X.; Vicente, M.G.H.; Smith, K.M. Syntheses and cellular investigations of 17(3)-, 15(2)-, and 13(1)-amino acid derivatives of chlorin e(6). J. Med. Chem. 2011, 54, 7464–7476. [Google Scholar]
- Ethirajian, M.; Chen, Y.H.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.T.; Xiong, L.; Liu, Z.P.; Miao, X.Y.; Lin, L.W.; Wen, Y. In vivo and in vitro evaluation of the cytotoxic effects of Photosan-loaded hollow silica nanoparticles on liver cancer. Nanoscale Res. Lett. 2014, 9, 319–327. [Google Scholar] [PubMed]
- Wang, F.; Chen, X.; Zhao, Z.; Tang, S.; Huang, X.; Lin, C.; Cai, C.; Zheng, N. Synthesis of magnetic, fluorescent and mesoporous core-shell-structured nanoparticles for imaging, targeting and photodynamic therapy. J. Mater. Chem. 2011, 30, 11244–11252. [Google Scholar] [CrossRef]
- Gary-Bobo, M.; Mir, Y.; Rouxel, C.; Brevet, D.; Basile, I.; Maynadier, M.; Vaillant, O.; Mongin, O.; Blanchard-Desce, M.; Morere, A.; et al. Mannose-functionalized mesoporous silica nanoparticles for efficient two-photon photodynamic therapy of solid tumors. Angew Chem. Int. Ed. 2011, 50, 11425–11429. [Google Scholar] [CrossRef] [PubMed]
- Eichwurzel, I.; Stielb, H.; Roder, B. Photophysical studies of the pheophorbide a dimer. J. Photochem. Photobiol. B 2000, 54, 194–200. [Google Scholar] [CrossRef]
- Vail, S.A.; Evans, D.R.; Pan, W. Long Wavelength Absorbing Porphyrin Photosensitizers for Dye-Sensitized Solar Cells. US Patent US8907081 B2, 9 December 2014. [Google Scholar]
- Kalka, K.; Merk, H.; Mukhtar, H. Photodynamic therapy in dermatology. J. Am. Acad. Dermatol. 2000, 42, 389–413. [Google Scholar] [CrossRef]
- Simpson, C.R.; Kohl, M.; Essenpreis, M.; Cope, M. Near-infrared optical properties of ex vivo human skin and subcutaneous tissues measured using the Monte Carlo inversion technique. Phys. Med. Biol. 1998, 43, 2465–2478. [Google Scholar] [CrossRef] [PubMed]
- Sparrow, J.R.; Zbou, J.; Cai, B. DNA is a target of the photodynamic effects elicited in A2E-Laden RPE by bule-light illumination. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2245–2251. [Google Scholar] [CrossRef]
- Ashikaga, T.; Wada, M.; Kobayashi, H.; Moria, M.; Katsumura, Y.; Fukui, H.; Kato, S.; Yamaguchi, M.; Takamatsu, T. Effect of the photocatalytic activity of TiO2 on plasmid DNA. Mutat. Res. 2000, 466, 1–7. [Google Scholar] [CrossRef]
- Yang, X.; Tan, G.; Qu, F.; Jin, Y.; Wang, J. Synthesis of phenylhydrazone and amino acid schiff base of pyropheophorbide-a. Chin. J. Org. Chem. 2014, 34, 1206–1211. [Google Scholar] [CrossRef]
- Liu, K.; Xing, R.R.; Zou, Q.L.; Ma, G.H.; Möhwald, H.; Yan, X.H. Simple peptide-tuned self-assembly of photosensitizers towards anticancer photodynamic therapy. Angew. Chem. Int. Ed. 2016, 55, 3036–3039. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Fei, J.B.; Yan, X.H.; Wang, A.H.; Li, J.B. Enzyme-responsive release of doxorubicin from monodisperse dipeptide-based nanocarriers for highly efficient cancer treatment in vitro. Adv. Funct. Mater. 2015, 25, 1193–1204. [Google Scholar] [CrossRef]
- Zhao, F.F.; Shen, G.Z.; Chen, C.J.; Xing, R.R.; Zou, Q.L.; Ma, G.H.; Yan, X.H. Nanoengineering of stimuli-responsive protein-based biomimetic protocells as versatile drug delivery tools. Chem. Eur. J. 2014, 20, 6880–6887. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compound BPHM are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Tan, G.; Cheng, J.; Zhao, L.; Wang, Z.; Jin, Y. A Novel Photosensitizer 31,131-phenylhydrazine -Mppa (BPHM) and Its in Vitro Photodynamic Therapy against HeLa Cells. Molecules 2016, 21, 558. https://doi.org/10.3390/molecules21050558
Li W, Tan G, Cheng J, Zhao L, Wang Z, Jin Y. A Novel Photosensitizer 31,131-phenylhydrazine -Mppa (BPHM) and Its in Vitro Photodynamic Therapy against HeLa Cells. Molecules. 2016; 21(5):558. https://doi.org/10.3390/molecules21050558
Chicago/Turabian StyleLi, Wenting, Guanghui Tan, Jianjun Cheng, Lishuang Zhao, Zhiqiang Wang, and Yingxue Jin. 2016. "A Novel Photosensitizer 31,131-phenylhydrazine -Mppa (BPHM) and Its in Vitro Photodynamic Therapy against HeLa Cells" Molecules 21, no. 5: 558. https://doi.org/10.3390/molecules21050558
APA StyleLi, W., Tan, G., Cheng, J., Zhao, L., Wang, Z., & Jin, Y. (2016). A Novel Photosensitizer 31,131-phenylhydrazine -Mppa (BPHM) and Its in Vitro Photodynamic Therapy against HeLa Cells. Molecules, 21(5), 558. https://doi.org/10.3390/molecules21050558