Enantioselective Separation of 4,8-DHT and Phytotoxicity of the Enantiomers on Various Plant Species
Abstract
:1. Introduction
2. Results and Discussion
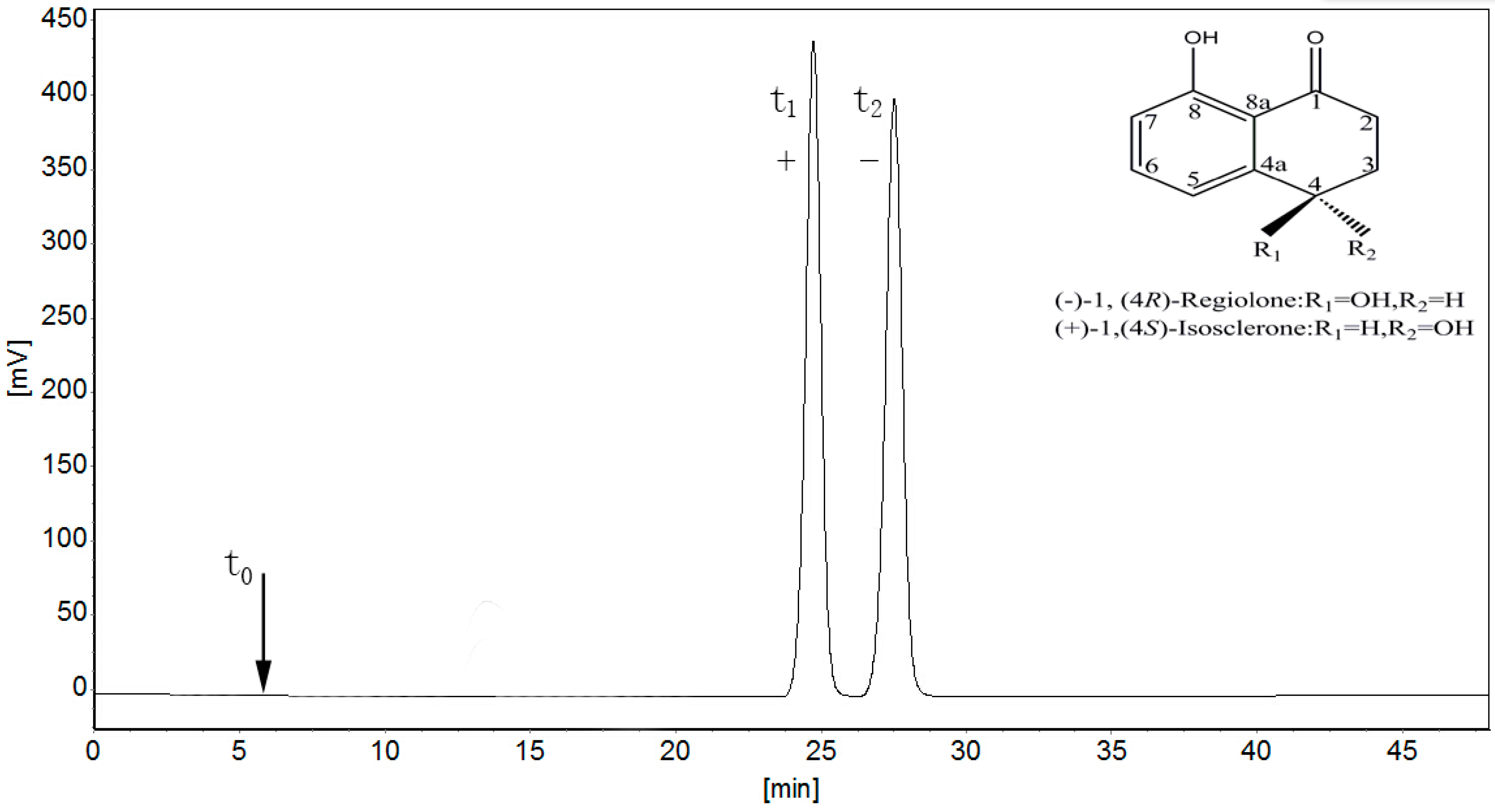
2.1. Separation of 4,8-DHT Enantiomers
2.2. Effects of Rac-4,8-DHT, S-(+)-Isosclerone, and R-(−)-Regiolone on Germination of the Tested Plants
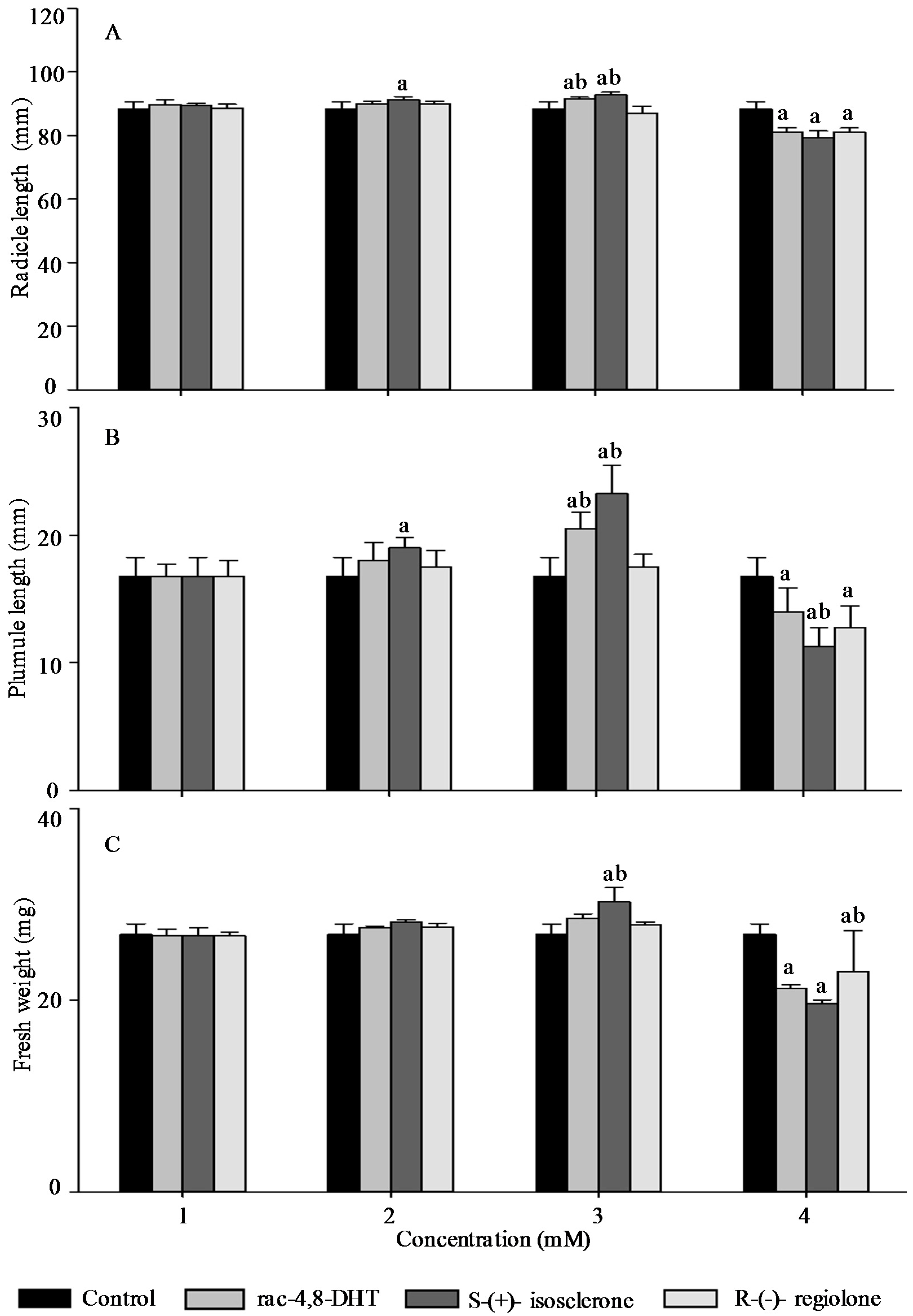
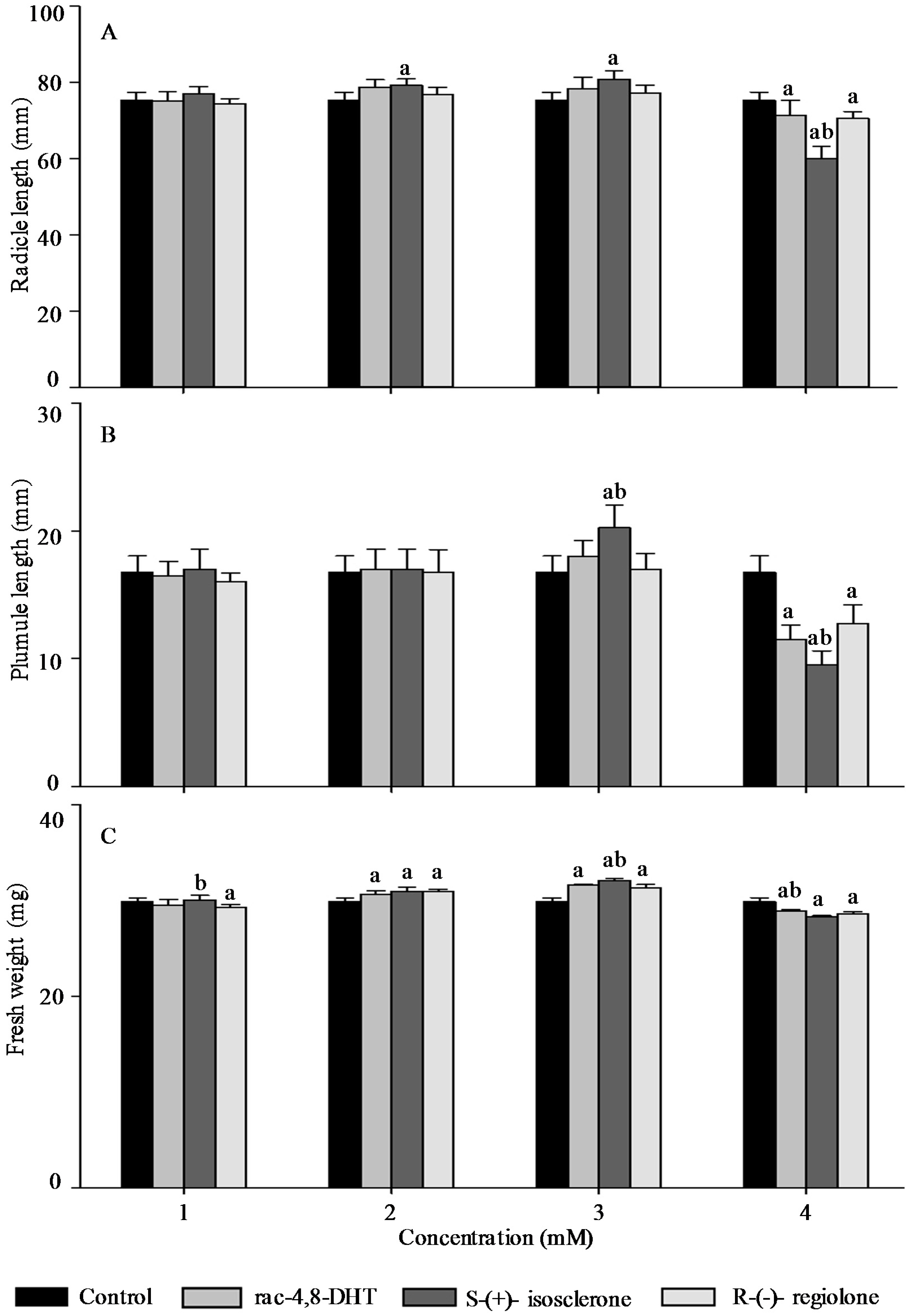
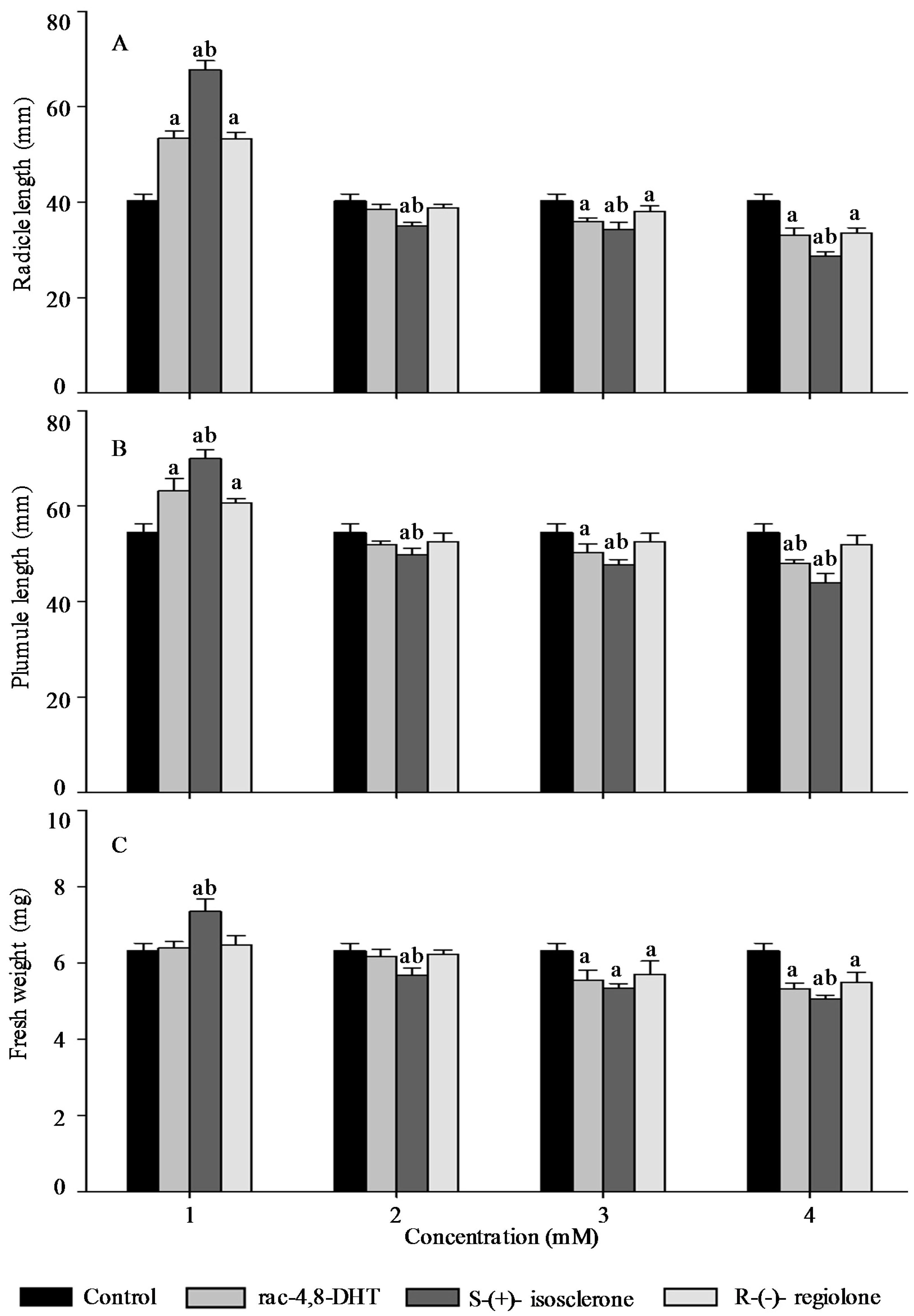
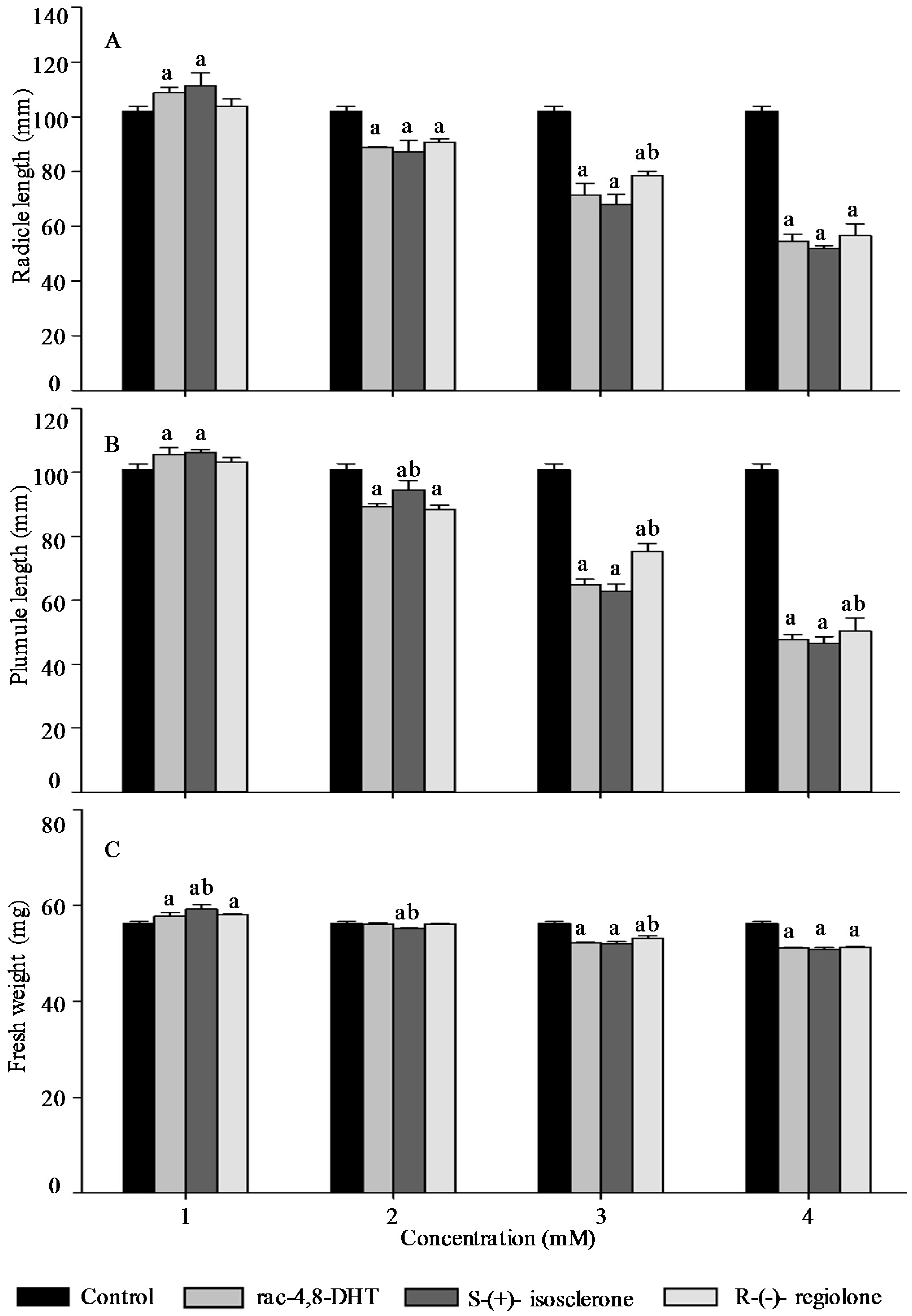
2.3. Effects of Rac-4,8-DHT, S-(+)-Isosclerone, and R-(−)-Regiolone on Seedlings Growth of the Tested Plants
3. Materials and Methods
3.1. Chemicals, Plant Materials and Reagents
3.2. Chromatographic Separation and Analysis
3.3. Effects of 4,8-DHT on Seed Germination
3.4. Effects of 4,8-DHT on Seedling Growth
3.5. Statistic Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| 4,8-DHT | 4,8-dihydroxy-1-tetralone |
| HPLC | high-performance liquid chromatography |
| CSP | chiral stationary phase |
| AA | acetic acid |
| SD | standard deviation |
References
- Williams, A. Opportunities for chiral agrochemicals. Pestic. Sci. 1996, 46, 3–9. [Google Scholar] [CrossRef]
- Liu, W.; Gan, J.; Schlenk, D.; Jury, W.A. Enantioselectivity in environmental safety of current chiral insecticides. PNAS 2005, 102, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.W. Chiral toxicology: It’s the same thing only different. Toxicol. Sci. 2009. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, A.R.; Castro, P.M.L.; Tiritan, M.E. Chiral pharmaceuticals in the environment. Environ. Chem. Lett. 2012, 10, 239–253. [Google Scholar] [CrossRef]
- Zhang, Q.; Hua, X.D.; Shi, H.Y.; Liu, J.S.; Tian, M.M.; Wang, M.H. Enantioselective bioactivity, acute toxicity and dissipation in vegetables of the chiral triazole fungicide flutriafol. J. Hazard. Mater. 2015, 284, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Magrans, J.O.; Alonso-Prados, J.L.; Garcı́a-Baudı́n, J.M. Importance of considering pesticide stereoisomerism-proposal of a scheme to apply Directive 91/414/EEC framework to pesticide active substances manufactured as isomeric mixtures. Chemosphere 2002, 49, 461–469. [Google Scholar] [CrossRef]
- Ye, J.; Zhao, M.; Liu, J.; Liu, W. Enantioselectivity in environmental risk assessment of modern chiral pesticides. Environ. Pollut. 2010, 158, 2371–2383. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Xie, X.; Liu, W. Enantioselective separation and phytotoxicity on rice seedlings of paclobutrazol. J. Agric. Food Chem. 2011, 59, 4300–4305. [Google Scholar] [CrossRef] [PubMed]
- Yen, J.H.; Tsai, C.C.; Wang, Y.S. Separation and toxicity of enantiomers of organophosphorus insecticide leptophos. Ecotoxicol. Environ. Saf. 2003, 55, 236–242. [Google Scholar] [CrossRef]
- Wang, Y.S.; Tai, K.T.; Yen, J.H. Separation, bioactivity, and dissipation of enantiomers of the organophosphorus insecticide fenamiphos. Ecotoxicol. Environ. Saf. 2004, 57, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, D.; Lei, X.; Jiang, S.; Zhou, Z. Enantiomeric separation of chiral pesticides by high-performance liquid chromatography on an amylose tris-(S)-1-phenylethylcarbamate chiral stationary phase. J. Sep. Sci. 2006, 29, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Lu, D.; Diao, J.; Zhou, Z. Enantioselective toxic effects and biodegradation of benalaxyl in Scenedesmus obliquus. Chemosphere 2012, 87, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.L.; Wang, Y.S.; Yen, J.H. Enantioselective effects of herbicide imazapyr on arabidopsis thaliana. J. Environ. Sci. Health. Part B 2014, 49, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Yashima, E. Polysaccharide-based chiral stationary phases for high-performance liquid chromatographic enantioseparation. J. Chromatogr. A 2001, 906, 105–125. [Google Scholar] [CrossRef]
- Gasparrini, F.; Misiti, D.; Pierini, M.; Villani, C. Enantioselective chromatography on brush-type chiral stationary phases containing totally synthetic selectors theoretical aspects and practical applications. J. Chromatogr. A 1996, 724, 79–90. [Google Scholar] [CrossRef]
- Hutta, M.; Rybár, I.; Chalányová, M. Liquid chromatographic method development for determination of fungicide epoxiconazole enantiomers by achiral and chiral column switching technique in water and soil. J. Chromatogr. A 2002, 959, 143–152. [Google Scholar] [CrossRef]
- Qi, Y.L.; Liu, D.H.; Sun, M.J.; Di, S.S.; Wang, P.; Zhou, Z.Q. The chiral separation and enantioselective degradation of the chiral herbicide napropamide. Chirality 2014, 26, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Findlay, J.A.; Kwan, D. Metabolites from a Scytalidium species. Canadian. J. Chem. 1973, 51, 3299–3301. [Google Scholar] [CrossRef]
- Machida, K.; Matsuoka, E.; Kasahara, T.; Kikuchi, M. Studies on the constituents of Juglans Species. I. structural determination of (4S)- and (4R)-4-hydroxy-α-tetralone derivatives from the fruit of Juglans mandshurica Maxim. var. sieboldiana MAKINO. Chem. Pharm. Bull. 2005, 53, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.L.; Chen, S.Y.; Liu, J.S.; Jin, C.S.; Xu, F.Q. Chemical constituents of Carya cathayensis and their antitumor bioactivity. J. Chin. Med. Mater. 2011, 34, 1055–1057. [Google Scholar]
- Li, X.X.; Yu, M.F.; Ruan, X.; Zhang, Y.Z.; Wang, Q. Phytotoxicity of 4,8-dihydroxy-1-tetralone isolated from Caryacathayensis Sarg. to various plant species. Molecules 2014, 19, 15452–15467. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Kang, K.H.; Kim, H.J.; Kim, S.K. In vitro induction of apoptosis by isosclerone from marine-derived fungus Aspergillus fumigatus. Bioorg. Med. Chem. Lett. 2014, 24, 3923–3927. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, B.; Jiang, Y.; Liu, Z.; Liu, Y.; Wang, X.; Kuang, H. Studies on cytotoxic activity against HepG-2 cells of naphthoquinones from green walnut husks of Juglans mandshurica Maxim. Molecules 2015, 20, 15572–15588. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.H.; Wang, Q.; Ruan, X.; Pan, C.D.; Jiang, D.A. Phenolics and plant allelopathy. Molecules 2010, 15, 8933–8952. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Kaida, Y. Resolution by high-performance liquid chromatography using polysaccharide carbamates and benzoates as chiral stationary phases. J. Chromatogr. A 1994, 666, 403–419. [Google Scholar] [CrossRef]
- Wainer, I.W.; Alembik, M.C.; Smith, E. Resolution of enantiomeric amides on a cellulose tribenzoate chiral stationary phase: Mobile phase modifier effects on retention and stero-selectivity. J. Chromatogr. A 1987, 388, 65–74. [Google Scholar] [CrossRef]
- Lao, W.; Gan, J. High-performance liquid chromatographic separation of imidazolinone herbicide enantiomers and their methyl derivatives on polysaccharide-coated chiral stationary phases. J. Chromatogr. A 2006, 1117, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Xu, C.; Zhou, S.; Liu, W.; Gan, J. Enantiomeric separation of imidazolinone herbicides using chiral high-performance liquid chromatography. Chirality 2007, 19, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Shamsipur, M.; Abdollahpour, A.; Heydari, R. Development and validation of a new high performance liquid chromatographic method for enantioseparation of dorzolamide hydrochloride on a coated cellulose phenylcarbamate chiral stationary phase. J. Liq. Chromatogr. Relat. Technol. 2011, 34, 1367–1380. [Google Scholar] [CrossRef]
- Evidente, A.; Superchi, S.; Cimmino, A.; Mazzeo, G.; Mugnai, L.; Rubiales, D.; Andolfi, A.; Villegas-Fernández, A.M. Regiolone and isosclerone, two enantiomeric phytotoxic naphthalenone pentaketides: Computational assignment of absolute configuration and its relationship with phytotoxic activity. Eur. J. Org. Chem. 2011, 28, 5564–5570. [Google Scholar] [CrossRef]
- Ruan, X.; Li, Z.H.; Wang, Q.; Pan, C.D.; Jiang, D.A.; Wang, G.G. Autotoxicity and allelopathy of 3,4-dihydroxyacetophenone isolated from Picea schrenkiana needles. Molecules 2011, 16, 8874–8893. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Liu, W.; Sheng, G. Enantioselective degradation and ecotoxicity of the chiral herbicide diclofop in three freshwater alga cultures. J. Agric. Food Chem. 2008, 56, 2139–2146. [Google Scholar] [CrossRef] [PubMed]
- Lenton, J.R.; Appleford, N.E.J.; Temple-Smith, K.E. Growth retardant activity of paclobutrazol enantiomers in wheat seedlings. Plant Growth Regul. 1994, 15, 281–291. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, Q.; Zhang, A.; Wen, Y.; Liu, W. Enantioselective effects of chiral herbicide diclofop acid on rice Xiushui 63 seedlings. Bull. Environ. Contam. Toxicol. 2009, 83, 85–91. [Google Scholar] [CrossRef] [PubMed]
- International Seed Testing Association (ISTA). ISTA International Rules for Seed Testing; International Seed Testing Association: Zurich, Switzerland, 2010. [Google Scholar]
- Sample Availability: Samples of the compounds rac-4,8-DHT, S-(+)-isosclerone, and R-(−)-regiolone are available from the authors.

| Plant Species | Forms | Germination Vigor | Germination Rate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 1 mM | 2 mM | 3 mM | 4 mM | Control | 1 mM | 2 mM | 3 mM | 4 mM | ||
| Lettuce | rac | 75 ± 2 | 76 ± 1 | 83 ± 1 a | 69 ± 2 a | 60 ± 1 a | 85 ± 1 | 86 ± 1 | 94 ± 1 a | 83 ± 2 a | 79 ± 1 a |
| S | 76 ± 2 | 85 ± 2 a | 65 ± 2 a,b | 57 ± 2 a,b | 87 ± 1 | 96 ± 1 a | 82 ± 2 a | 62 ± 1 a,b | |||
| R | 76 ± 2 | 82 ± 2 a | 69 ± 2 a | 63 ± 2 a | 86 ± 2 | 92 ± 1 a | 83 ± 2 a | 80 ± 1 a | |||
| Cucumber | rac | 83 ± 1 | 85 ± 1 a | 86 ± 2 a | 90 ± 2 a | 81 ± 2 | 87 ± 2 | 88 ± 2 | 90 ± 1 a | 93 ± 2 a | 82 ± 2 a |
| S | 86 ± 2 a | 87 ± 2 a | 90 ± 2 a | 79 ± 2 a | 89 ± 1 | 90 ± 1 a | 93 ± 2 a | 80 ± 2 a,b | |||
| R | 84 ± 2 | 86 ± 1 a | 89 ± 1 a | 81 ± 1 | 88 ± 2 | 88 ± 1 | 91 ± 1 a,b | 83 ± 1 a | |||
| Radish | rac | 82 ± 1 | 83 ± 1 | 83 ± 1 | 86 ± 2 a | 79 ± 1 a | 88 ± 2 | 87 ± 2 | 89 ± 1 | 91 ± 1 | 83 ± 2 a |
| S | 84 ± 1 a | 84 ± 2 a | 89 ± 1 a,b | 74 ± 1 a,b | 88 ± 2 | 90 ± 1 | 93 ± 1 a,b | 77 ± 2 a,b | |||
| R | 82 ± 1 | 83 ± 1 | 85 ± 1 a | 80 ± 2 a | 88 ± 1 | 90 ± 1 | 90 ± 2 | 82 ± 2 a | |||
| Onion | rac | 40 ± 4 | 63 ± 3 a,b | 36 ± 3 | 34 ± 2 a | 32 ± 1 a | 45 ± 4 | 68 ± 1 a,b | 43 ± 1 | 42 ± 1 a | 40 ± 1 a |
| S | 67 ± 4 a,b | 33 ± 2 a | 32 ± 2 a | 30 ± 1 a | 69 ± 3 a,b | 40 ± 1 a,b | 39 ± 1 a | 33 ± 1 a,b | |||
| R | 59 ± 2 ab | 36 ± 2 a | 34 ± 2 a | 33 ± 2 a | 63 ± 1 a | 43 ± 1 | 42 ± 1 a | 40 ± 1 a | |||
| Wheat | rac | 68 ± 2 | 71 ± 2 | 64 ± 2 | 38 ± 5 a | 27 ± 2 a | 80 ± 2 | 83 ± 2 | 75 ± 4 a | 43 ± 5 a | 27 ± 2 a |
| S | 74 ± 3a | 58 ± 3 a,b | 35 ± 4 a | 24 ± 3 a | 85 ± 1 a | 65 ± 1 a,b | 40 ± 4 a | 25 ± 1 a | |||
| R | 70 ± 1 | 65 ± 1 | 39 ± 3 a | 28 ± 3 a | 83 ± 1 | 79 ± 3 | 50 ± 4 a,b | 29 ± 1 a | |||
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.; Ma, X.-Y.; Ruan, X.; Jiang, D.-A.; Pan, C.-D.; Wang, Q. Enantioselective Separation of 4,8-DHT and Phytotoxicity of the Enantiomers on Various Plant Species. Molecules 2016, 21, 528. https://doi.org/10.3390/molecules21040528
Yang L, Ma X-Y, Ruan X, Jiang D-A, Pan C-D, Wang Q. Enantioselective Separation of 4,8-DHT and Phytotoxicity of the Enantiomers on Various Plant Species. Molecules. 2016; 21(4):528. https://doi.org/10.3390/molecules21040528
Chicago/Turabian StyleYang, Li, Xiao-Yan Ma, Xiao Ruan, De-An Jiang, Cun-De Pan, and Qiang Wang. 2016. "Enantioselective Separation of 4,8-DHT and Phytotoxicity of the Enantiomers on Various Plant Species" Molecules 21, no. 4: 528. https://doi.org/10.3390/molecules21040528
APA StyleYang, L., Ma, X.-Y., Ruan, X., Jiang, D.-A., Pan, C.-D., & Wang, Q. (2016). Enantioselective Separation of 4,8-DHT and Phytotoxicity of the Enantiomers on Various Plant Species. Molecules, 21(4), 528. https://doi.org/10.3390/molecules21040528






