Inhibition of Nitric Oxide Production in BV2 Microglial Cells by Triterpenes from Tetrapanax papyriferus
Abstract
:1. Introduction
2. Results and Discussion
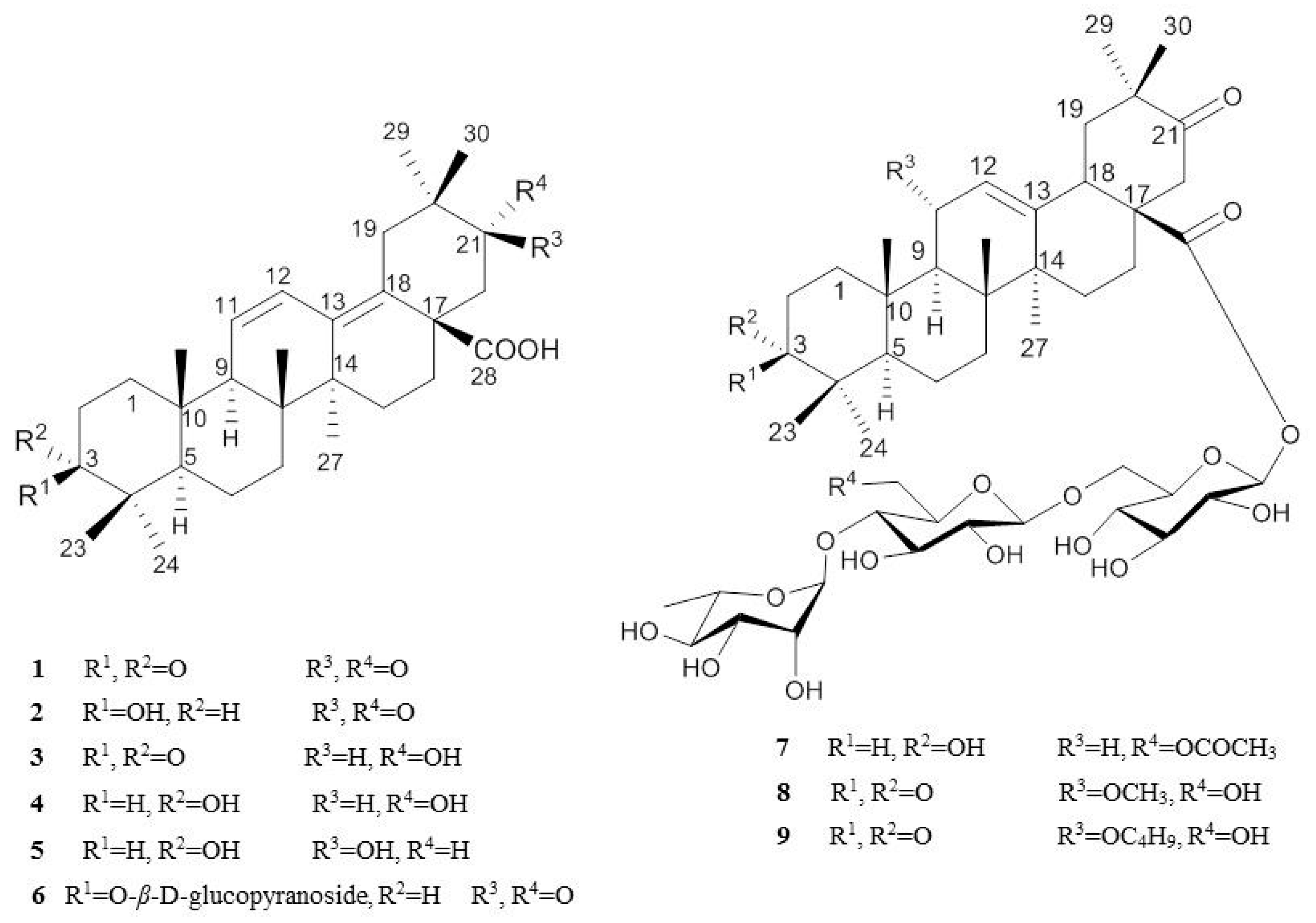
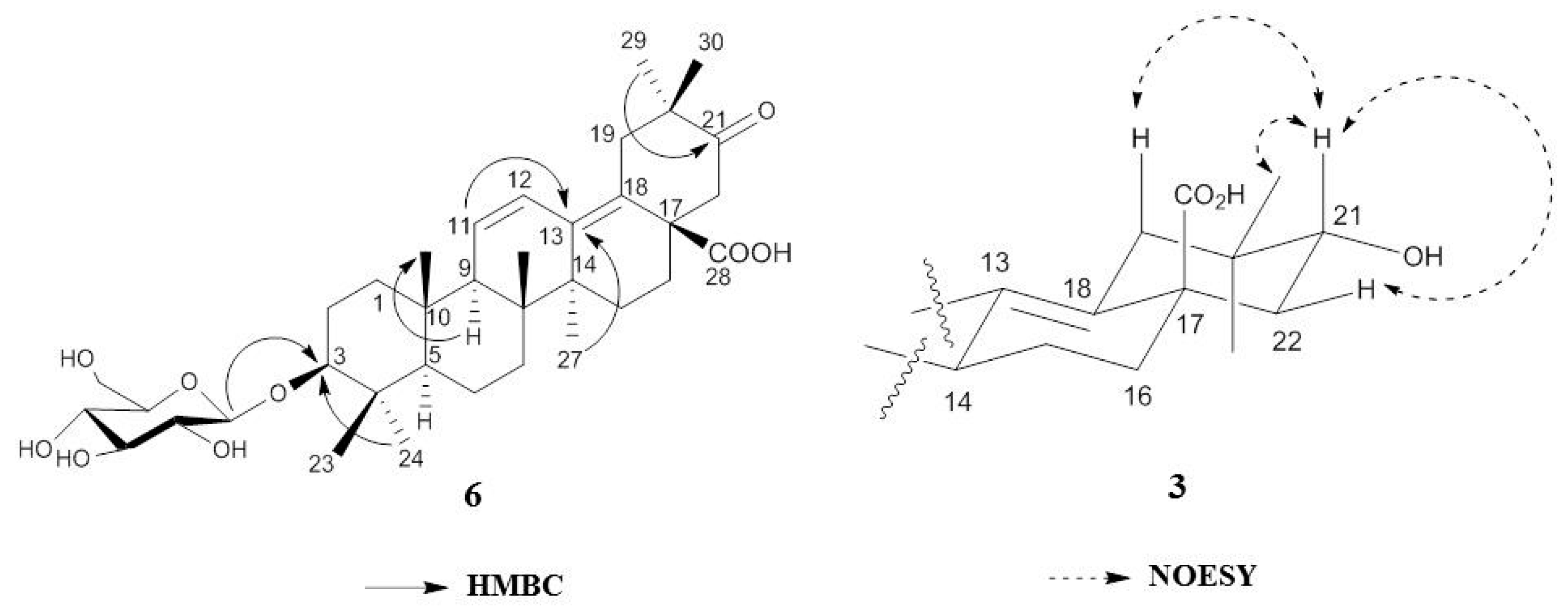
2.1. Isolation and Characterization of Oleanane-Type Triterpenoids
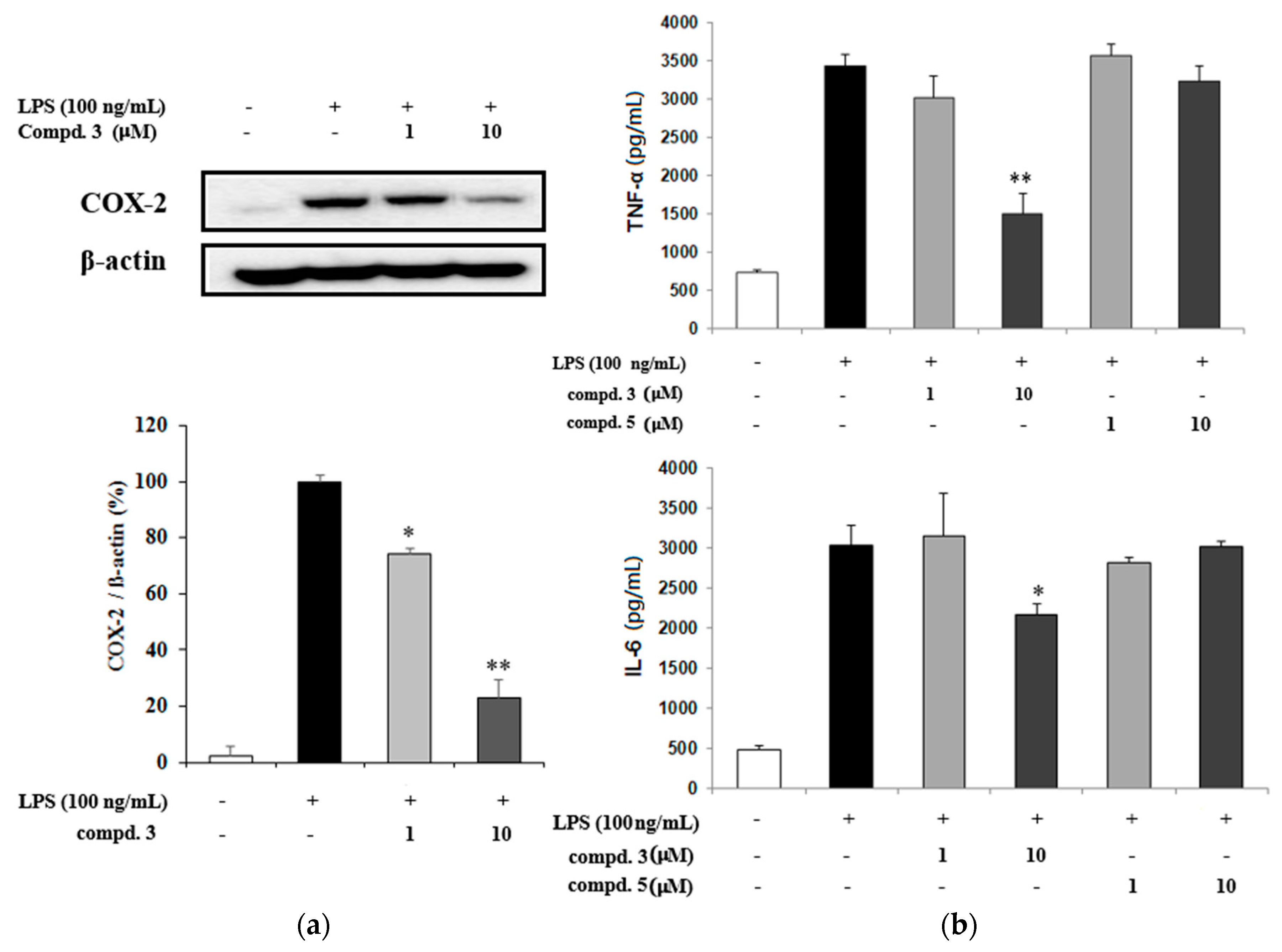
2.2. NO Inhibitory Effects of Oleanane-Type Triterpenoids
3. Materials and Methods
3.1. Plant Materials
3.2. Extraction and Isolation
3.3. Cell Line
3.4. Cell Line Culture
3.5. NO Assay and TNF-α and IL-6 ELISA
3.6. Statistical Analysis
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Eikelenboom, P.; Veerhuis, R. The role of complement and activated microglia in the pathogenesis of Alzheimer’s disease. Neurobiol. Aging 1996, 17, 673–680. [Google Scholar] [CrossRef]
- Boyce, J. Eicosanoids in asthma, allergic inflammation, and host defense. Curr. Mol. Med. 2008, 8, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Bredt, D.S.; Snyder, S.H. Nitric oxide: A physiologic messenger molecule. Annu. Rev. Biochem. 1994, 63, 175–195. [Google Scholar] [CrossRef] [PubMed]
- Dawson, V.; Brahmbhatt, H.; Mong, J.; Dawson, T. Expression of inducible nitric oxide synthase causes delayed neurotoxicity in primary mixed neuronal-glial cortical cultures. Neuropharmacology 1994, 33, 1425–1430. [Google Scholar] [CrossRef]
- Sugishita, E.; Amagaya, S.; Ogihara, Y.J. Studies on the mechanism of anti-inflammatory activities of papyriogenin a and papyriogenin C. J. Pharmacobiodyn. 1983, 6, 287–294. [Google Scholar] [CrossRef]
- Hikino, H.; Kiso, Y.; Amagaya, S.; Ogihara, Y. Antihepatotoxic actions of papyriogenins and papyriosides, triterpenoids of Tetrapanax papyriferum leaves. J. Ethnopharmacol. 1984, 12, 231–235. [Google Scholar] [PubMed]
- Motoh, M.; Keisuke, K.; Iclal, S.; Yukio, O. Minor saponins from Tetrapanax papyriferum. Chem. Pharm. Bull. 1997, 45, 552–554. [Google Scholar]
- Miyakoshi, M.; Ida, Y.; Isoda, S.; Shoji, J. 3α-Hydroxy-oleanane-type triterpene glycosyl esters from leaves of Acanthopanax spinosus. Phytochemistry 1993, 34, 1599–1602. [Google Scholar] [CrossRef]
- Ho, J.C.; Chen, C.M.; Row, L.C. Oleanane-type triterpenes from the flowers, pith, leaves, and fruit of Tetrapanax papyriferus. Phytochemistry 2007, 68, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Perveen, S.; Malik, A.; Tareen, R.B. Structural determination of atricins A and B, new triterpenes from Perovskia atriplicifolia, by 1D and 2D NMR spectroscopy. Maqn. Reson. Chem. 2009, 47, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Thibeault, D.; Gauthier, C.; Legault, J.; Bouchard, J.; Dufour, P.; Pichette, A. Synthesis and structure-activity relationship study of cytotoxic germanicane- and lupane-type 3β-O-monodesmosidic saponins starting from betulin. Bioorg. Med. Chem. 2007, 15, 6144–6157. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Q.; Zhou, L.; Wang, D.; Wu, J.; Li, L.Z.; Huang, X.X.; Liu, Q.B.; Wu, Y.Y.; Lin, S.; Yang, J.Y.; et al. Neuroprotective oleanane triterpenes from the roots of Bupleurum chinense. Bioorg. Med. Chem. Lett. 2016, 26, 1594–1598. [Google Scholar] [CrossRef] [PubMed]
- Sakae, A.; Tadahiro, T.; Yukio, O. Further studies on glycosides from the leaves of Tetrapanax papyriferum. J. Chem. Soc. Perkin Trans. 1 1979, 1, 2044–2047. [Google Scholar]
- Ashour, A.; El-Sharkawy, S.; Amer, M.; Abdel Bar, F.; Katakura, Y.; Miyamoto, T.; Toyota, N.; Bang, T.H.; Kondo, R.; Shimizu, K. Rational design and synthesis of topoisomerase I and II inhibitors based on oleanolic acid moiety for new anti-cancer drugs. Bioorg. Med. Chem. 2014, 22, 211–220. [Google Scholar] [PubMed]
- Şenel, G.; Gülcemal, D.; Masullo, M.; Piacente, S.; Karayıldırım, T. Oleanane-type glycosides from Tremastelma palaestinum (L.) Janchen. Chem. Biodivers. 2014, 11, 408–418. [Google Scholar] [CrossRef] [PubMed]
- Chistokhodova, N.; Nguyen, C.; Calvino, T.; Kachirskaia, I.; Cunningham, G.; Howard Miles, D. Antithrombin activity of medicinal plants from central Florida. J. Ethnopharmacol. 2002, 81, 277–280. [Google Scholar] [CrossRef]
- Ho, J.C.; Chen, C.M.; Row, L.C. Flavonoids and benzene derivatives from the flowers and fruit of Tetrapanax papyriferus. J. Nat. Prod. 2005, 68, 1773–1775. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
| Position | 6 a | 9 a | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) δC | ||
| 1a | 1.86 m | 38.1 | 2.62 m | 34.5 |
| 1b | 2.42 m | |||
| 2a | 2.33 m | 26.3 | 2.19 m | 40.4 |
| 2b | 1.90 m | 1.76 m | ||
| 3 | 3.43 dd (11.94, 4.56) | 88.8 | 216.3 | |
| 4 | 39.5 | 47.7 | ||
| 5 | 0.83 d (11.94) | 55.2 | 1.34 m | 55.5 |
| 6 | 1.56 m, 1.38 m | 18.3 | 1.33 m | 19.6 |
| 7 | 1.24 m | 32.6 | 1.33 m | 32.8 |
| 8 | 41.0 | 38.0 | ||
| 9 | 1.99 s | 54.6 | 1.86 m | 52.6 |
| 10 | 36.6 | 43.0 | ||
| 11 | 5.75 d (10.74) | 127.5 | 4.07 m | 76.3 |
| 12 | 6.58 d (10.56) | 126.0 | 5.83 m | 124.2 |
| 13 | 137.6 | 145.6 | ||
| 14 | 42.2 | 42.0 | ||
| 15a | 2.34 m | 26.5 | 2.12 m | 28.0 |
| 15b | 2.23 m | 1.04 m | ||
| 16a | 2.24 m | 32.1 | 2.10 m | 25.6 |
| 16b | 1.70 m | 1.79 m | ||
| 17 | 50.2 | 45.4 | ||
| 18 | 132.2 | 3.67 m | 40.8 | |
| 19a | 2.80 m | 40.5 | 3.13 m | 46.2 |
| 19b | 2.60 d(14.70) | 2.55 m | ||
| 20 | 46.0 | 50.8 | ||
| 21 | 213.8 | 212.0 | ||
| 22a | 3.30 d (14.22) | 50.4 | 2.21 m | 47.2 |
| 22b | 2.48 d (14.22) | 1.73 m | ||
| 23 | 1.33 s | 27.9 | 1.13 s 26.4 1.04 s 21.4 | |
| 24 | 1.00 s | 16.5 | ||
| 25 | 0.93 s | 18.3 | 1.10 s 16.5 | |
| 26 | 0.95 s | 16.9 | 1.09 s 18.9 | |
| 27 | 1.12 s | 20.2 | 1.04 s | 25.0 |
| 28 | 185.0 | 173.9 | ||
| 29 | 1.15 s | 24.8 | 1.17 s | 24.5 |
| 30 | 1.12 s | 25.7 | 1.22 s | 25.2 |
| 1′ | 4.95 s | 107.0 | 4.95 6.21 m | 96.0 |
| 2′ | 4.06 m | 75.7 | 4.28 m | 73.8 |
| 3′ | 4.26 m | 78.7 | 4.18 m 78.5 | |
| 4′ | 4.24 m | 71.8 | 4.26 m | 70.7 |
| 5′ | 4.04 m | 78.3 | 3.86 m | 75.1 |
| 6′a | 4.61 m | 63.0 | 4.66 m | 69.3 |
| 6′b | 4.43 dd (11.46, 5.04) | 4.26 m | ||
| 1′′ | 4.91 m | 104.9 | ||
| 2′′ | 3.90 m | 74.6 | ||
| 3′′ | 3.61 m | 77.0 | ||
| 4′′ | 4.33 m | 78.2 | ||
| 5′′ | 4.08 m | 77.9 | ||
| 6′′a | 4.14 m | 61.2 | ||
| 6′′b | 4.02 m | |||
| 1′′ | 5.78 m | 102.6 | ||
| 2′′′ | 4.49 m | 72.6 | ||
| 3′′′ | 4.62 m | 72.4 | ||
| 4′′′ | 4.08 m | 73.6 | ||
| 5′′′ | 4.88 m | 70.2 | ||
| 6′′′ | 1.65 d (5.94) | 18.4 | ||
| OBu | 3.56 m, 3.24 m | 66.7 | ||
| 1.47 m, 1,33 m | 32.8 | |||
| 1.32 m | 19.8 | |||
| 0.81 m | 13.9 | |||
| 1.0 μM | 5.0 μM | 10.0 μM | ||||
| Nitrite (%) | Viability (%) | Nitrite (%) | Viability (%) | Nitrite (%) | Viability (%) | |
| 1 | 83.7 ± 9.1 | 83.5 ± 6.1 | 67.2 ± 11.4 | 81.4 ± 4.9 | 6.7 ± 2.3 *** | 75.2 ± 7.2 |
| 2 | 85.9 ± 13.5 | 79.8 ± 6.7 | 48.4 ± 9.3 | 78.1 ± 2.5 | 41.4 ± 8.5 * | 76.1 ± 5.5 |
| 3 | 85.3 ± 8.4 | 83.2 ±7.3 | 9.2 ± 5.3 *** | 66.8 ± 8.1 | 5.4 ± 2.4 *** | 64.0 ±7.1 |
| 4 | 79.6 ± 5.5 | 102.0 ± 0.6 | 56.3 ± 6.8 | 106.0 ± 4.6 | 11.9 ± 4.2 ** | 87.6 ± 1.8 |
| 5 | 92.0 ± 4.4 | 97.7 ± 5.1 | 10.5 ± 3.6 *** | 79.7 ± 10.6 | 6.9 ± 5.0 *** | 77.9 ± 4.2 |
| 6 | 97.2 ± 3.9 | 88.1 ± 1.5 | 83.3 ± 9.3 | 92.0 ± 5.1 | 73.4 ± 9.2 | 88.5 ± 6.3 |
| 10.0 μM | 50.0 μM | 100.0 μM | ||||
| Nitrite (%) | Viability (%) | Nitrite (%) | Viability (%) | Nitrite (%) | Viability (%) | |
| 7 | 69.2 ± 8.9 | 97.9 ± 8.2 | 38.4 ± 1.1 * | 92.7 ± 6.8 | 15.1 ± 6.9 ** | 93.3 ± 5.1 |
| 8 | 95.4 ± 3.8 | 93.1 ± 7.5 | 87.4 ± 7.0 | 96.8 ± 5.6 | 53.9 ± 7.0 | 90.0 ± 4.5 |
| 9 | 103.5 ± 3.8 | 88.2 ± 1.7 | 38.4 ± 9.9 * | 88.5 ± 7.1 | 14.3 ± 6.7 ** | 92.0 ± 5.5 |
| 29.7 ± 2.4 * | 89.9 ± 6.6 | 17.5 ± 3.4 ** | 90.4 ± 7.4 | 14.4 ± 3.4 ** | 95.5 ± 4.3 | |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, N.; Moon, E.H.; Kim, H.W.; Hong, J.; Beutler, J.A.; Sung, S.H. Inhibition of Nitric Oxide Production in BV2 Microglial Cells by Triterpenes from Tetrapanax papyriferus. Molecules 2016, 21, 459. https://doi.org/10.3390/molecules21040459
Cho N, Moon EH, Kim HW, Hong J, Beutler JA, Sung SH. Inhibition of Nitric Oxide Production in BV2 Microglial Cells by Triterpenes from Tetrapanax papyriferus. Molecules. 2016; 21(4):459. https://doi.org/10.3390/molecules21040459
Chicago/Turabian StyleCho, Namki, Eun Hye Moon, Hyun Woo Kim, Jaewoo Hong, John A. Beutler, and Sang Hyun Sung. 2016. "Inhibition of Nitric Oxide Production in BV2 Microglial Cells by Triterpenes from Tetrapanax papyriferus" Molecules 21, no. 4: 459. https://doi.org/10.3390/molecules21040459
APA StyleCho, N., Moon, E. H., Kim, H. W., Hong, J., Beutler, J. A., & Sung, S. H. (2016). Inhibition of Nitric Oxide Production in BV2 Microglial Cells by Triterpenes from Tetrapanax papyriferus. Molecules, 21(4), 459. https://doi.org/10.3390/molecules21040459







