Abstract
Herein we describe the synthesis and in vitro biological evaluation of thirteen new, racemic, diversely functionalized imidazo pyranotacrines as non-hepatotoxic, multipotent tacrine analogues. Among these compounds, 1-(5-amino-2-methyl-4-(1-methyl-1H-imidazol-2-yl)-6,7,8,9-tetrahydro-4H-pyrano[2,3-b]quinolin-3-yl)ethan-1-one (4) is non-hepatotoxic (cell viability assay on HepG2 cells), a selective but moderately potent EeAChE inhibitor (IC50 = 38.7 ± 1.7 μM), and a very potent antioxidant agent on the basis of the ORAC test (2.31 ± 0.29 μmol·Trolox/μmol compound).
1. Introduction
Alzheimer′s disease (AD) is an age-related neurodegenerative disorder whose prevalence is expected to rise significantly in the next decades as the average age of the population increases [1]. Worldwide, around 40 million people suffer from AD [2], which is characterized by a progressive memory loss, a decline in language skills and motor capabilities along with other cognitive impairments [3]. Although the etiology of AD is not completely known, common hallmarks such as β-amyloid (Aβ) deposits [4], τ-protein aggregation [5] and oxidative stress [6] play key roles in the physiopathology of the disease [7]. In addition, the selective loss of cholinergic neurons in AD results in a deficit of the neurotransmitter acetylcholine (ACh) in specific brain regions that mediate learning and memory [8]. Alterations in the serotoninergic and dopaminergic systems are also thought to be responsible for the behavioral disturbances observed in AD patients [9]. At present, there are three FDA-approved drugs (donepezil, galantamine and rivastigmine) [10] that ameliorate AD symptoms by inhibiting acetylcholinesterase (AChE), the enzyme responsible for the hydrolysis of ACh, and by rising the ACh content in the synapsis [11]. Apart from the beneficial palliative properties of AChE inhibitors (AChEI) in AD, cholinergic drugs have shown little efficacy to prevent the progression of the disease. Consequently, nowadays there is no efficient therapy to cure, stop or even slow the progression of the disease. Hence, effective therapeutics are urgently sought [12].
The failure to find such a drug or treatment is possibly due to the multifactorial nature of AD. Thus, single drugs acting on specific targets to produce the expected clinical effects might not be effective in interfering with the complex nature of AD. This is the reason why the multi-target-directed ligand (MTDL) approach [13] has received an increasing attention by many research groups that have developed a variety of compounds binding very diverse biochemical targets [14].
Among the AChEIs investigated in the search for a pharmacological treatment for AD, the case of tacrine is particularly relevant [15]. Tacrine (Figure 1) is one of the strongest butyrylcholinesterase (BuChE) inhibitors known to date [16]. This drug, which was withdrawn from the clinical use for AD treatment because of significant liver toxicity [17], has been largely stigmatized instead of being further developed looking for other non-toxic tacrines retaining valuable anti-ChE properties [18]. In this context, we have reconsidered tacrine as a starting scaffold in the design of new Multipotent Non-Toxic Tacrines (MNTTs), [19] by incorporating other pharmacophoric groups capable of binding or inhibiting other molecular targets involved in the onset and/or progression of the disease. With this idea in mind, since 1997 we developed different types of structurally simple and easily available MNTTs [20].
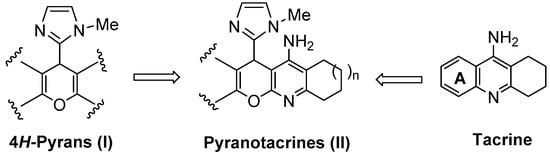
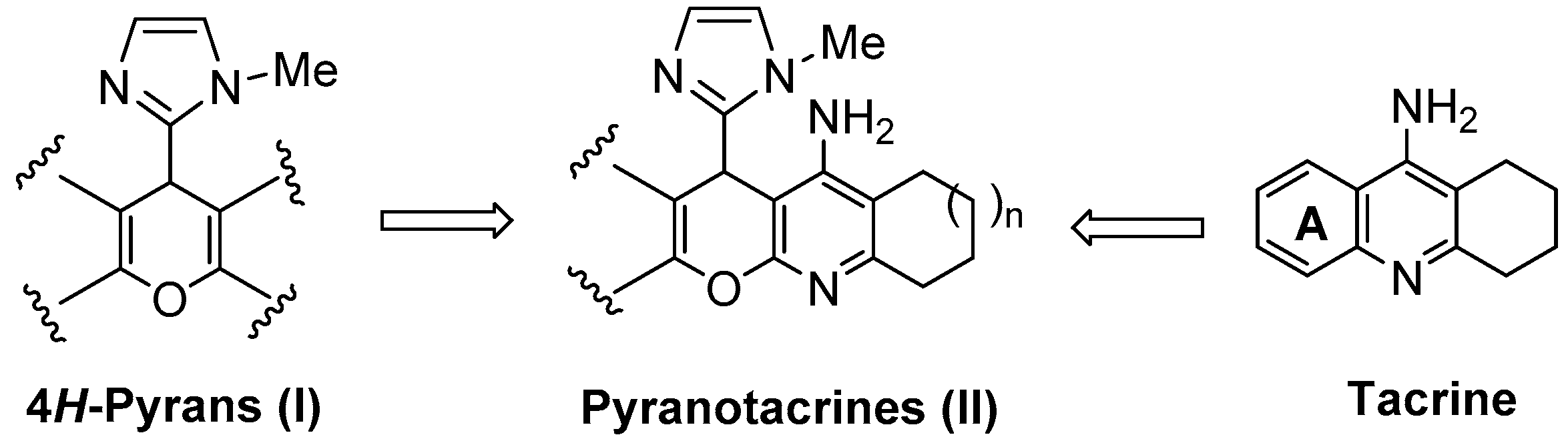
Figure 1.
General structure of tacrine, 4H-pyrans (I), and the new racemic imidazopyranotacrines (II).
Thus, expanding our contribution in this area, in this work we report the synthesis of a number of new imidazopyranotacrines (II), a new family of tacrine derivatives resulting from the substitution of the ring A in tacrine by the 4H-pyrano (I) heterocyclic motif (Figure 1). In this case, we have incorporated, and at the same position, instead of a substituted phenyl ring [21], or a five- (furan [22], thiophen [22], pyrrole [22]) or six-membered heterocyclic ring system [23], the five-membered 1-methyl-1H-imidazol-2-yl motif, in order to improve previous biological results and gain new insights on the structure-activity relationship (SAR) of pyranotacrines. The imizadole ring is a privileged heterocyclic ring system [24] which is present in a number of interesting biologically active molecules such as saripidem, necopidem or zolpidem [25]. This choice was also favored by the ready availability of the imidazole substituted starting derivatives.
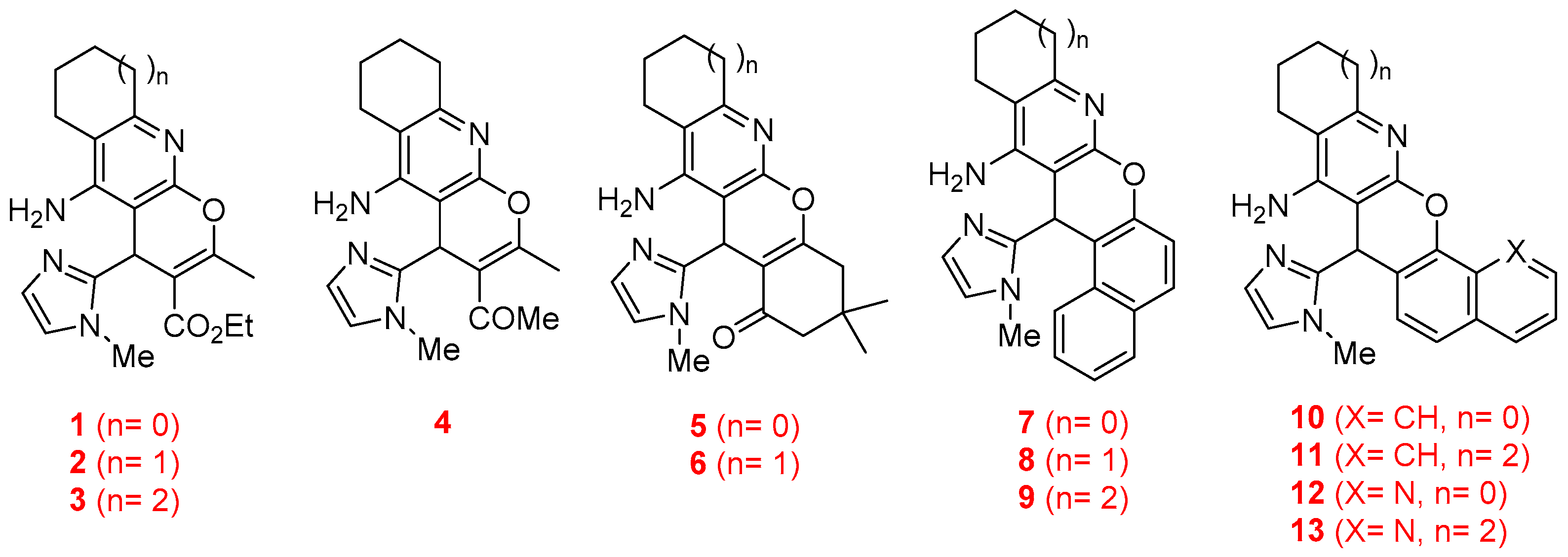
Thus, in this work, we describe the synthesis and biological evaluation of the new imidazopyranotacrines 1–13 (Figure 2), the inhibition of AChE and BuChE enzymes, the antioxidant activities (ORAC-FL method), as well as the evaluation of their hepatotoxicity on HepG2 cells. As a result, we have identified racemic 1-(5-amino-2-methyl-4-(1-methyl-1H-imidazol-2-yl)-6,7,8,9-tetrahydro-4H-pyrano[2,3-b]quinolin-3-yl)ethan-1-one (4) (Figure 2) as a non-hepatotoxic compound on HepG2 cells, endowed with selective but moderate inhibitory activity against AChE from Electrophorus electricus (EeAChE), and very potent antioxidant activity.
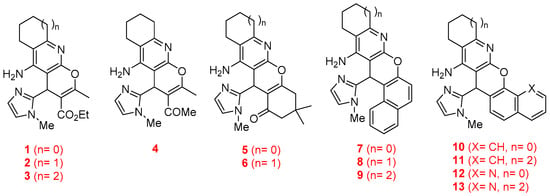
Figure 2.
Structures of the imidazopyranotacrines 1–13.
2. Results and Discussion
2.1. Chemistry
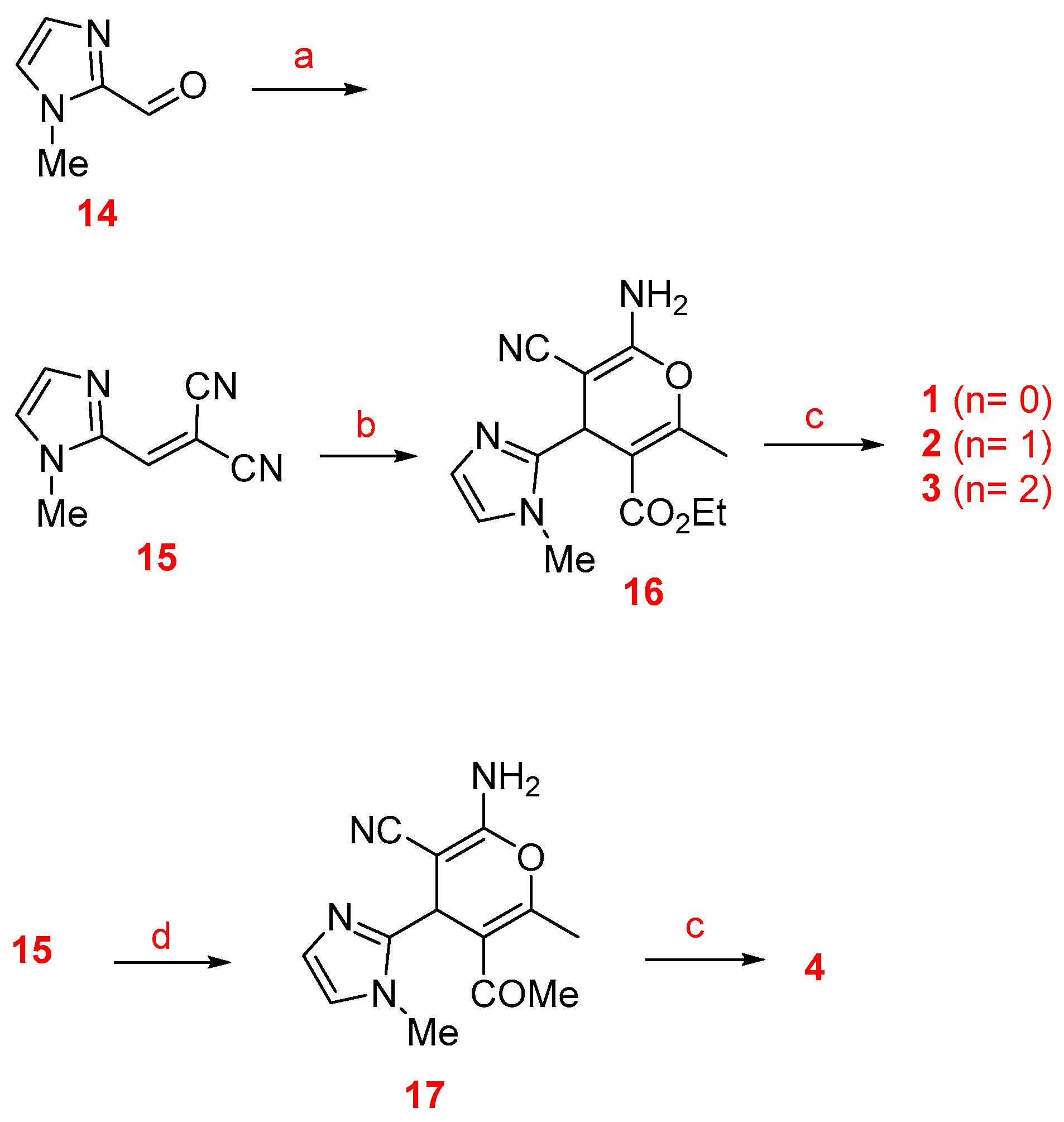
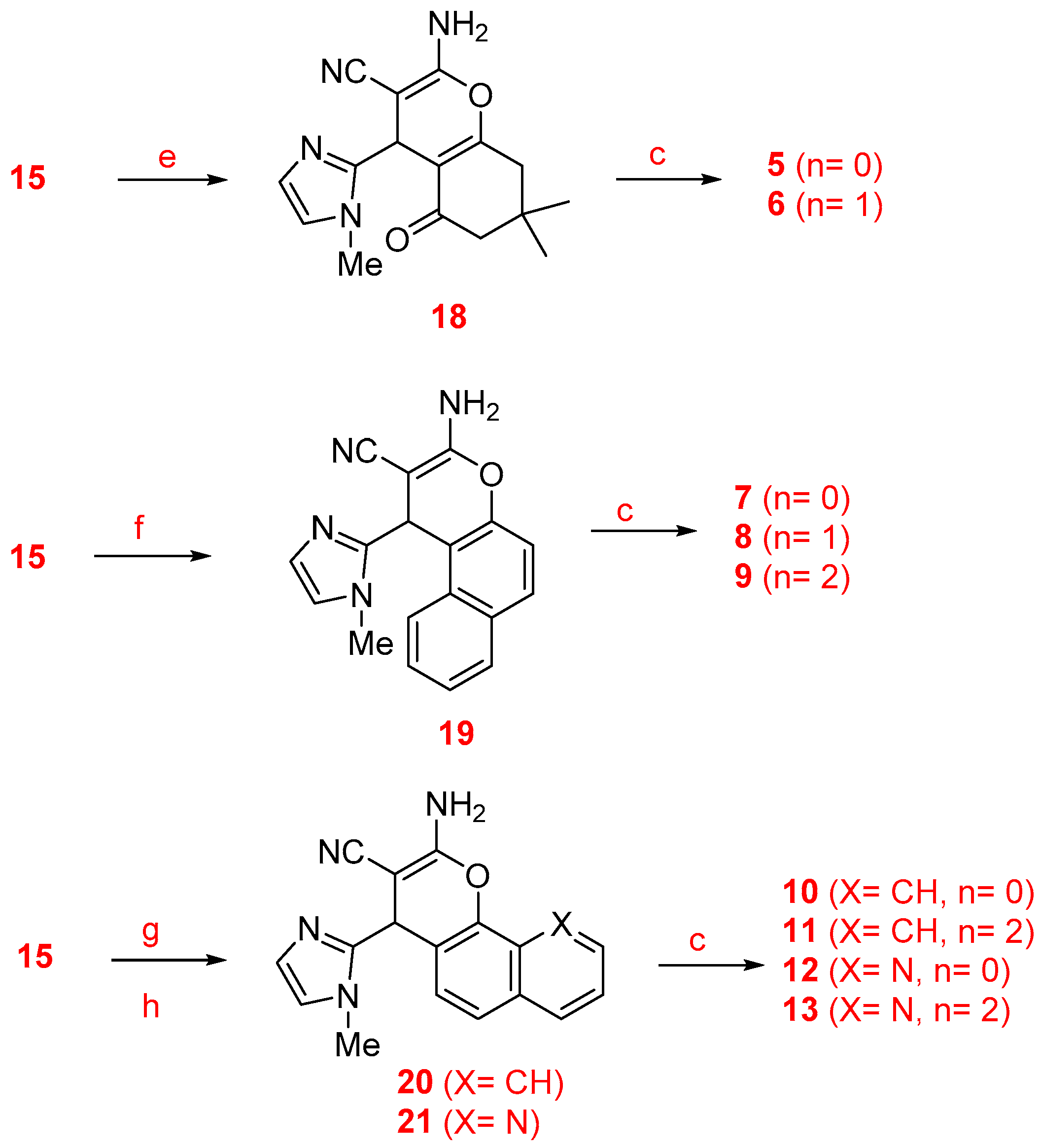
The synthesis of racemic imidazopyranotacrines 1–13 has been carried out by Friedländer-type reactions [26], by treating readily available 4H-pyrans with commercial cycloalkanones, under standard reaction conditions, and in good chemical yields. Cyclopentanone, cyclohexanone and cycloheptanone were chosen in order to evaluate the effect of the size of the cycloalkyl ring on the biological activity of the new pyranotacrines (Scheme 1). Starting from 1-methyl-1H-imidazole-2-carbaldehyde (14), we synthesized 2-(1-methyl-1H-imidazol-2yl)methylene)malononitrile (15), whose reaction with the appropriate 1,3-dicarbonylic compound or naphthol derivative, gave the key 4H-pyrans. For the synthesis of pyranotacrines 1–3, we have used ethyl acetoacetate as starting material, thus leading to imidazopyranotacrines bearing a methyl and ethoxycarbonyl substituents at C-2 and C-3, respectively. Similarly, for the synthesis of the pyranotacrines 4 and 5 or 6, we used 2,4-pentandione, and 5,5-dimethylcyclohexane-1,3-dione, respectively. Finally, for the synthesis of the remaining pyrano-tacrines 19–21 we have prepared some conveniently key functionalized 4H-pyrans from 1-naphthol, 2-naphthol, and 8-hydroxyquinoline. All new compounds showed excellent analytical and spectroscopic data, in good agreement with their structures.
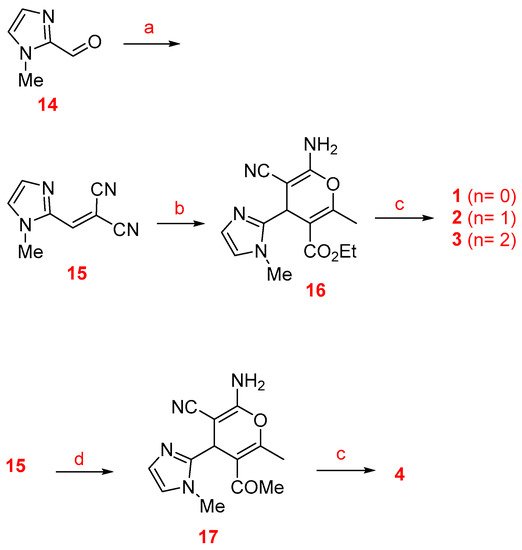
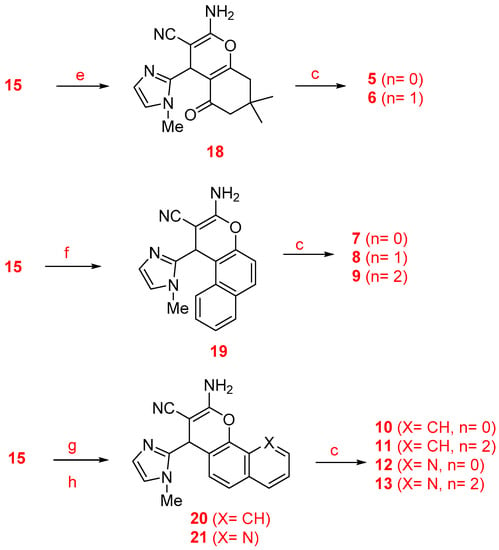
Scheme 1.
Synthesis of the imidazopyranotacrines 1–13. Reagents and conditions: (a) Malononitrile, piperidine, ethanol, rt (93%); (b) ethyl 3-oxobutanoate, piperidine, ethanol, rt (92%); (c) cyclopentanone (n = 0), cyclohexanone (n = 1), cycloheptanone (n = 2), AlCl3, 1,2-dichloroethane, reflux; (d) pentane-2,4-dione, piperidine, ethanol, rt (90%); (e) 5,5-dimethylcyclohexane-1,3-dione, piperidine, ethanol, rt (69%); (f) 2-naphthol, piperidine, ethanol, rt (72%); (g) 1-naphthol, piperidine, ethanol, rt (75%); (h) 8-hydroxyquinoline, piperidine, ethanol, rt (47%).
2.2. Biological Assays
2.2.1. Inhibition of the Cholinesterase Enzymes and Kinetic Analysis
First of all, we addressed the capacity of imidazopyranotacrines 1–13 to inhibit EeAChE and serum horse BuChE (eqBuChE) by using Ellman′s assay [27], and tacrine as reference compound for comparative purposes [28].
As shown in Table 1, racemic imidazopyranotacrines 1–13 were very modest EeAChE inhibitors with potencies in the micromolar range, resulting from two to three orders of magnitude less active than the reference compound tacrine. On the other hand, all derivatives acted as highly selective AChEI, as no or very moderate inhibition of eqBuChE activity was observed. The most potent EeAChEI was the imidazopyranotacrine 8 (IC50 = 6.73 ± 0.52 µM), followed, in decreasing order of activity, by 7, 10, 2 and 1. The three most potent EeAChEIs among the imidazopyranotacrines (i.e., 8, 7 and 10) bear a naphthol ring fused to the central 4H-pyran motif. Furthermore, the 2-naphthol derivatives (7, 8) were more potent than the 1-naphthol ones (10, 11). The 2-naphthol derivative bearing a cyclohexane ring (8) is more potent than the analogues bearing a cyclopentane motif (7) or a cycloheptane motif (9). Note that the incorporation of a heterocyclic nitrogen in the 1-naphthol compounds (compare compound 11 with 13) significantly potentiates the inhibition of EeAChE, affording additional capacity for the inhibition of eqBuChE. For the other imidazopyranotacrines, it is clear that those bearing an ethoxycarbonyl moiety and a methyl group at C3 and C2, respectively, in the 4H-pyran core (1, 2) are more potent than those bearing a fused 3,3-dimethylcyclohexan-1-one (6, 5) or an acetyl and a methyl group (4) at C3 and C2, respectively, in the 4H-pyran core. Note also that imidazopyranotacrines 1 and 2, differing only in the size of the cycloalkyl ring, are almost equipotent EeAChEIs, while 3, bearing a cycloheptane ring, is two orders of magnitude less active. Finally, regarding eqBuChE inhibition, imidazopyranotacrines 1–13 were very weak BuChEIs. Because of the weak inhibitory activity, high concentrations were required for the determination of the IC50 values, exceeding the solubility of some compounds. Thus, imidazopyranotacrines 1–13 were screened at a single concentration, namely 10 µM, giving percentages of inhibition between 12.9 and 75.7 (Table 1).

Table 1.
Inhibitory potencies, expressed as IC50 values (μM), for the inhibition of EeAChE and eqBuChE, and antioxidant power of imidazopyranotacrines 1–13.
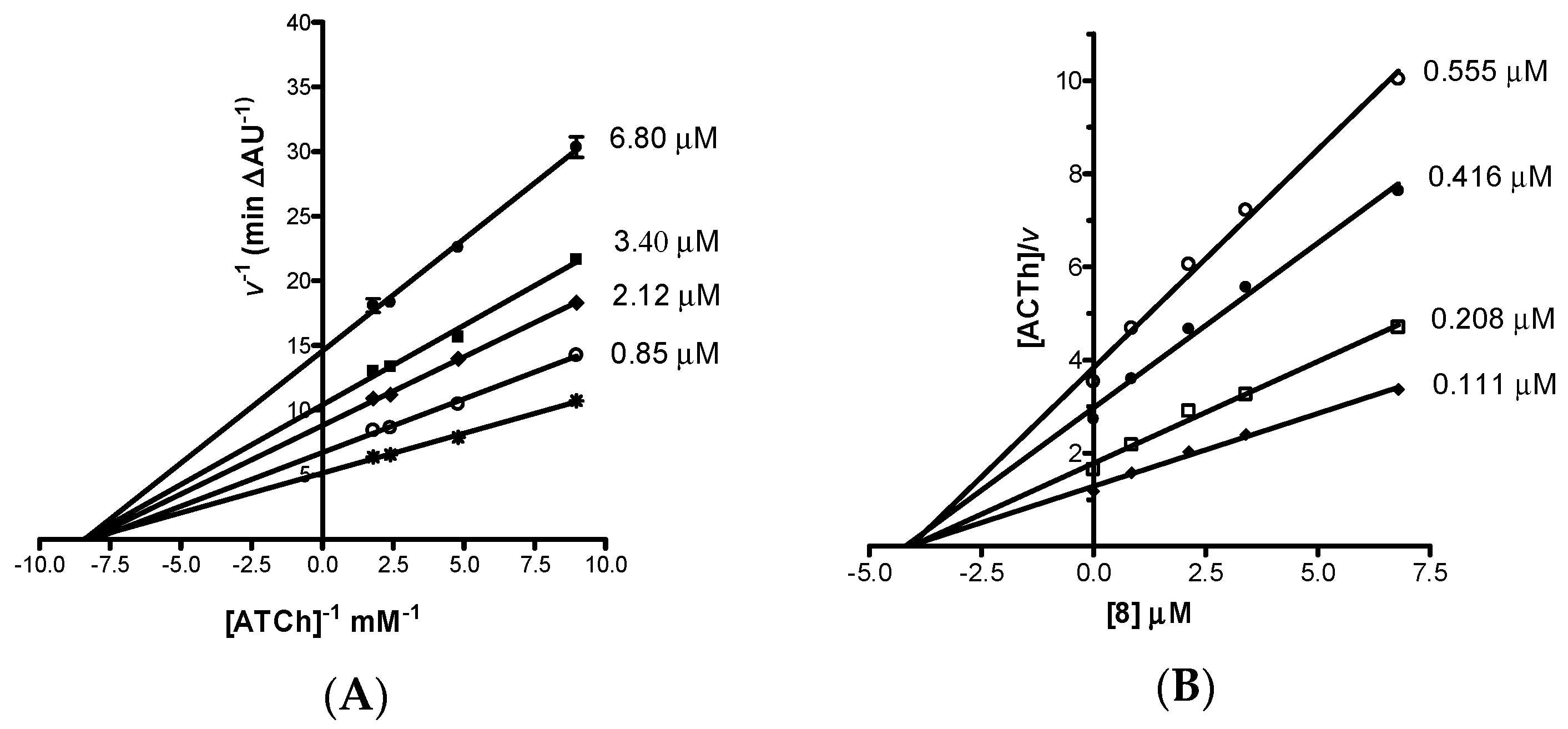
The mechanism of inhibition of EeAChE by the most potent AChEI, imidazopyranotacrine 8, was investigated by building Lineweaver-Burk double reciprocal plots at increasing concentrations of inhibitor and substrate (acetylthiocholine). Analysis of the Lineweaver-Burk reciprocal plots (Figure 3A) showed that Km appeared unaltered while Vmax decreased at increasing concentrations of the inhibitor. This pattern indicated that 8 acts as a non-competitive enzyme inhibitor. The non-competitive mechanism of action was confirmed by building the Cornish-Bowden plot (Figure 3B). Replots of the substrate/velocity versus the concentration of compound 8 provided an estimate of the non-competitive inhibition constant [29], which resulted equal to 4.10 μM. The non-competitive mechanism of action was confirmed by computational chemistry by performing the docking analysis of both enantiomers of compound 8 on AChE (see Supplementary Materials).
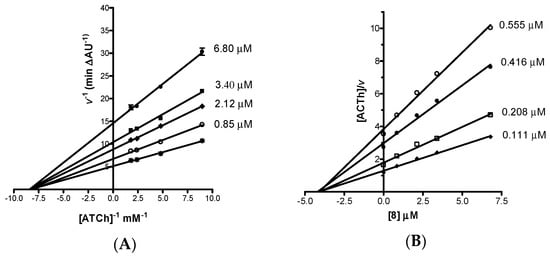
Figure 3.
(A) Lineweaver-Burk plot and (B) Cornish-Bowden plot both illustrating non-competitive inhibition of EeAChE by compound 8. The intersection point in Cornish-Bowden plot gives an estimate of the inhibition constant. Lines were derived from a weighted least-squares analysis of data. ATCh = acetylthiocholine (substrate).
2.2.2. Antioxidant Power
The antioxidant power of the imidazopyranotacrines 1–13 was determined by the oxygen radical absorbance capacity by fluorescence (ORAC-FL) method [30] using fluorescein as fluorescent probe and Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) as a reference compound. Results are expressed as Trolox equivalents (μmol Trolox per μmol test compound). As shown in Table 1, all the imidazopyranotacrines were able to reduce the amount of peroxyl radical, with ORAC values ranging from 1.35 (9) to 2.75 (10). The most potent antioxidant agent was 10 (2.75), followed by 6 (2.34), 11 (2.33), 4 (2.31) and 13 (2.25). Although it was clear that the naphthol derivatives, and particularly the 1-naphthol derivatives 10 and 13, were very potent, we observed that compounds 6 and 4, bearing a fused 3,3-dimethylcyclohexan-1-one, or an acetyl and a methyl groups at C3 and C2, respectively, in the 4H-pyran core, also showed high antioxidant power. Note also that tacrine has a very low ORAC value (0.20 ± 0.04) [28] compared with most of our synthetic imidazopyranotacrines.
Next, and on the basis of the AChE inhibition and ORAC values, imidazopyranotacrines 2, 4, 8 and 10 were evaluated for their potential hepatotoxicity, since tacrine was withdrawn from the market because of its liver toxicity.
2.2.3. Hepatotoxicity Analysis on HepG2 Cells
Drug-induced hepatotoxicity is one of the major reasons for drug [31] withdrawal from the clinic [15], as it happened to tacrine. HepG2 cell is a well-known in vitro system to screen the hepatotoxicity activity [32]. As shown in Table 2, tacrine was safe up to 100 µM, but significantly decreased cell viability at 300 μM and at higher concentrations. Among the selected derivatives, compounds 10 and 8 were more toxic than tacrine (significant reduction of cell viability at 30 and 100 µM), imidazopyranotacrines 2 and 4 resulted significantly less hepatotoxic than tacrine, and very interestingly, were safe even at 300 µM. Imidazopyranotacrine 4 showed the best safety profile, and proved to be non-toxic even at the highest tested concentration, namely 1000 µM, with a recorded cell viability value not significantly different from the control (not significant by the ANOVA test).

Table 2.
In vitro toxicity of imidazopyranotacrines, and tacrine in HepG2 cells.
3. Materials and Methods
3.1. General Information
Melting points were determined on a Kofler apparatus, and are uncorrected. IR spectra were recorded on a Shimadzu FT-IR 8201 PC spectrophotometer (Shimadzu, Kyoto, Japan), and only significant absorption bands are reported. 1H-NMR and 13C-NMR spectra were recorded on a Bruker Avance DPX250 (Bruker Avance DPX250, Bruker BioSpin, Fällanden, Switzerland) or VARIAN Mercury-300 (Palo Alto, CA, USA) spectrometers at 250 or 300 MHz and at 62 or 75 MHz, respectively in CDCl3, DMSO-d6 or CD3CN at 250 or 300 MHz and at 62 or 75 MHz, respectively, using solvent peaks as internal reference. Elemental analyses were performed on a Carlo-Erba CHNS apparatus, at CNQO (Madrid, Spain). TLC were performed on precoated Merck (Kenilworth, NJ, USA) silica gel aluminum sheets 60 F254 and detection by UV light at 254 nm or by charring with either ninhydrin, anisaldehyde or phosphomolybdic-H2SO4 reagents. Anhydrous solvents were used in all experiments. Flash column chromatography was performed on silica gel 60 (230 mesh). Commercial grade reagents were used as supplied from Acros (Geel, Belgium) or Aldrich (Saint Louis, MO, USA).
3.2. General Procedure for the Preparation of 1-Methylimidazole-4H-pyran Derivatives
To a solution of the 2-(1-methyl-1H-imidazol-2-yl)methylene)malononitrile (15) in ethanol (1 mmol/10 mL), the corresponding 1,3-dicarbonyle (1.1 mmol), and some drops of piperidine were added. The mixture was stirred at room temperature (rt) and the reaction was monitored by TLC. The precipitated solid was isolated by filtration, washed with cold ethanol, and purified by silica gel flash chromatography using AcOEt/Hexane (1:1) as eluent to give pure compounds.
3.3. General Procedure for the Preparation of 1-Methylimidazole-4H-pyran Derivatives from 1- or 2-Naphthol
A mixture of 1 mmol of 2-((1-methyl-1H-(benz)imidazol-2-yl) methylene)malononitrile and 1- or 2-naphthol (1 mmol) in ethanol (10 mL) containing 2–3 drops of piperidine was heated under reflux for overnight. The reaction mixture was cooled and the precipitated product filtered, washed with cold ethanol and purified by silica gel flash chromatography using AcOEt or acetone as eluent to give pure compounds.
3.4. General Method for the Friedländer Reaction
Aluminum chloride (1.5 equiv) was suspended in dry 1,2-dichloroethane at rt. Then, the 4H-pyran (1 equiv) and the selected cycloalcanone (1.5 equiv) were added, and the reaction mixture was heated at reflux for 24 h. When the reaction was over (thin layer chromatography analysis), an aqueous solution of sodium hydroxide (10%) was added dropwise to the mixture until the aqueous solution became basic. After stirring for 30 min, the precipitate was filtered off and washed with water. The resultant solid was purified by silica gel flash chromatography using AcOEt as eluent to give pure compounds.
2-(1-Methyl-1H-imidazol-2yl)methylene)malononitrile (15). To a solution of 1-methyl-1H-imidazole-2-carbaldehyde (14) in ethanol (1.1 g, 10 mmol), 1 equiv (1.58 g, 10 mmol) of malononitrile and 2–3 drops of piperidine were added, and the mixture was stirred at rt overnight. The precipitated product was filtered off and washed with cold ethanol to afford 1.47 g (9.3 mmol) of 2-(1-methyl-1H-imidazol-2-yl) methylene)malononitrile (15) (93% yield): mp 200–202 °C; IR (KBr) max 2210 (CN) cm−1; 1H-NMR (250 MHz, DMSO-d6) δ 8.15 (s, 1H), 7.58 (s, 1H), 7.40 (s, 1H), 3.79 (s, 3H); 13C-NMR (62.9 MHz, CD3CN) δ 142.7, 141.1, 134.0, 129.2, 115.8, 114.1, 79.3, 34.1. Anal. Calcd. for C8H6N4: C, 60.75; H, 3.82; N, 35.42. Found: C, 60.50; H, 4.03; N, 35.61.
Ethyl 6-amino-5-cyano-2-methyl-4-(1-methyl-1H-imidazol-2-yl)-4H-pyran-3-carboxylate (16). According to the general procedure, 2-(1-methyl-1H-imidazol-2-yl)methylene)malononitrile (15) (160 mg, 1.01 mmol), ethyl acetoacetate (150 mg, 1.15 mmol), and piperidine (2-3 drops), gave 270 mg (0.93 mmol) of the title compound 16 (93% yield): mp 230 °C; IR (KBr) max 3390 (NH2), 2318 (CN), 1689 (CO) cm−1; 1H-NMR (300 MHz, DMSO-d6) δ 6.96–6.94 (m, 3H), 6.69 (s, 1H), 4.59 (s, 1H), 3.99–3.87 (m, 2H), 3.65 (s, 3H), 2.30 (s, 3H), 0.97 (t, J = 7.1Hz, 3H); 13C-NMR (74.5 MHz, DMSO-d6) δ 165.2, 158.7, 157.6, 149.6, 126.6, 120.7, 119.8, 105.0, 60.0, 54.5, 32.1, 30.1, 18.2, 13.8. Anal. Calcd. for C14H16N4O3: C, 58.32; H, 5.59; N, 19.43. Found: C, 58.09; H, 5.49; N, 19.32.
5 -Acetyl-2-amino-6-methyl-4-(1-methyl-1H-imidazol-2-yl)-4H-pyran-3-carbonitrile (17). In the same manner, 2-(1-methyl-1H-imidazol-2-yl)methylene)malononitrile (15) (160 mg, 1.01 mmol), 2,4-pentadione (115 mg, 1.15 mmol), and piperidine (2–3 drops), gave 230 mg (0.89 mmol) of the title compound 17 (90% yield): mp 220 °C; IR (KBr) max 3394 (NH2), 2218 (CN), 1616 (CO) cm−1; 1H-NMR (300 MHz, DMSO-d6) δ 7.00–6.96 (s, 3H), 6.71 (s, 1H), 4.76 (s, 1H), 3.66 (s, 3H), 2.32 (s, 3H), 2.01 (s, 3H) ; 13C-NMR (74.5 MHz, DMSO-d6) δ 197.5, 159.0, 155.9, 148.8, 126.7, 121.6, 119.9, 113.2, 54.2, 32.3, 30.9, 29.6, 18.8. Anal. Calcd. for C13H14N4O2: C, 60.45; H, 5.46; N, 21.69. Found: C, 60.23; H, 5.42; N, 21.55.
2-Amino-7,7-dimethyl-4-(1-methyl-1H-imidazol-2-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (18). 2-(1-Methyl-1H-imidazol-2-yl)methylene)malononitrile (15) (160 mg, 1.01 mmol), dimedone (155 mg, 1.1 mmol) and piperidine (2–3 drops), gave 205 mg (0.69 mmol) of the title compound 18 (69% yield): mp 192–193 °C; IR (KBr) max 3367 (NH2), 2183 (CN), 1689 (CO) cm−1; 1H-NMR (300 MHz, DMSO-d6) δ 7.01 (s, 2H), 6.92 (s, 1H), 6.66 (s, 1H), 4.44 (s, 1H), 3.70 (s, 3H), 2.56–2.03 (m, 4H), 1.03 (s, 3H), 0.99 (s, 3H); 13C-NMR (74.5 MHz, DMSO-d6) δ 196.0, 162.6, 158.9, 149.3, 126.5, 120.6, 119.9, 111.3, 55.5, 49.8, 32.3, 32.1, 25.5, 26.9, 26.5. Anal. Calcd. for C16H18N4O2: C, 64.41; H, 6.08; N, 18.78. Found: C, 64.50; H, 6.02; N, 18.94.
2-Amino-4-(1-methyl-1H-imidazol-2-yl)-4H-benzo[h]chromene-3-carbonitrile (20). According to the general procedure, 2-(1-methyl-1H-imidazol-2-yl)methylene)malononitrile (15) (160 mg, 1.01 mmol), 1-naphtol (146 mg, 1.01 mmol) and piperidine (2–3 drops), gave 228 mg (0.75 mmol) of the title compound 20 (75% yield): mp > 260 °C; IR (KBr) max 3433 (NH2), 2376 (CN) cm−1; 1H-NMR (250 MHz, CD3CN) δ 7.95–7.90 (m, 2H), 7.78–7.75 (m, 1H), 7.53–7.43 (m, 2H), 7.30 (d, J = 8.91 Hz, 1H), 7.15 (br,s, 2H), 7.98 (s, 1H), 6.67 (s, 1H), 5.56 (s, 1H), 3.65 (s, 3H); 13C-NMR (62.9 MHz, CD3CN) δ 160.4, 148.9, 146.8, 130.7, 130.6, 129.8, 128.5, 127.3, 126.6, 125.1, 123.2, 121.9, 120.5, 116.8, 113.1, 53.7, 32.4, 31.5. Anal. Calcd. for C18H14N4O: C, 71.51; H, 4.67; N, 18.53. Found: C, 71.38; H, 4.51; N, 18.46.
2-Amino-1-(1-methyl-1H-imidazol-2-yl)-1H-benzo[f]chromene-3-carbonitrile (19). Following the same procedure, 2-(1-methyl-1H-imidazol-2-yl)methylene)malononitrile (15) (160 mg, 1.01 mmol), 2-naphtol (146 mg, 1.01 mmol) and piperidine (2–3 drops), gave 218 mg (0.72 mmol) of the title compound 19 (72% yield): mp > 260 °C; IR (KBr) max 3440 .(NH2), 2183 (CN) cm−1; 1H-NMR (250 MHz, DMSO-d6) δ 7.96–7.92 (m, 2H), 7.78–7.75 (m, 1H), 7.51–7.43 (m, 2H), 7.30 (d, J = 8.9 Hz, 2H), 6.99 (d, J = 0.8 Hz, 1H), 6.67 (d, J = 0.9 Hz, 1H), 5.67 (s, 1H), 3.66 (s, 3H); 13C-NMR (62.9 MHz, DMSO-d6) δ 160.4, 148.9, 146.8, 130.7, 130.4, 129.7, 128.5, 127.3, 126.6, 125.0, 123.2, 121.9, 120.5, 116.8, 113.1, 53.7, 32.4, 31.4. Anal. Calcd. for C18H14N4O: C, 71.51; H, 4.67; N, 18.53. Found: C, 71.47; H, 4.50; N, 18.72.
2-Amino-4-(1-methyl-1H-imidazol-2-yl)-4H-pyrano[3,2-h]quinoline-3-carbonitrile (21). Using the procedure described above, 2-(1-methyl-1H-imidazol-2-yl) methylene)malononitrile (15) (160 mg, 1.01 mmol), 8-hydroxyquinoline (147 mg, 1.01 mmol) and piperidine (2–3 drops), gave 142 mg (0.47 mmol) of the title compound 21 (47% yield): mp 244–246 °C; IR (KBr) max 3398 (NH2), 2191 (CN) cm−1; 1H-NMR (250 MHz, CDCl3) δ 8.93–8.91 (m, 1H), 8.22 (dd, J = 8.3 Hz, J = 1.6 Hz, 1H), 7.59–7.51 (m, 2H), 7.12 (d, J = 8.5 Hz, 1H), 6.90–6.84 (m, 4H), 5.83 (s, 1H), 3.56 (s, 3H); 13C-NMR (62.9 MHz, CDCl3) δ 159.4, 148.9, 146.7, 142.3, 136.5, 134.9, 127.1, 125.7, 124.9, 122.6, 121.3, 120.9, 115.8, 117.1, 52.0, 33.9, 31.7. Anal. Calcd. for C17H13N5O: C, 67.32; H, 4.32 ; N, 23.09. Found: C, 67.55; H, 4.11; N, 23.25.
Ethyl 5-amino-2-methyl-4-(1-methyl-1H-imidazol-2-yl)-4,6,7,8-tetrahydrocyclopenta[b]pyrano[3,2-e]pyridine-3-carboxylate (1). According to the procedure described above, ethyl 6-amino-5-cyano-2-methyl-4-(1-methyl-1H-imidazol-2-yl)-4H-pyran-3-carboxylate (16) (290 mg, 1.0 mmol), AlCl3 (200 mg, 1.53 mmol), and cyclopentanone (130 mg, 1.54 mmol), gave 191 mg (0.54 mmol) of the title compound 1 (54% yield): mp 160–161 °C; IR (KBr) max 3344 (NH2); 1704 (CO) cm−1; 1H-NMR (250 MHz, CDCl3) δ 6.85 (s, 1H), 6.66 (s, 1H,), 5.43 (s, 1H′), 5.11 (br s, 2H), 4.14–4.02 (m, 2H), 3.42 (s, 3H), 2.87–2.77 (m, 2H), 2.65–2.59 (m, 2H), 2.47 (s, 3H), 2.05–2.02 (m, 2H), 1.14–2.05 (m, 3H); 13C-NMR (62.9 MHz, CDCl3) δ 166.4, 162.4, 161.3, 155.6, 150.1, 147.0, 126.2, 122.6, 118.1, 101.6, 94.5, 60.4, 34.4, 34.3, 32.9, 27.0, 22.1, 19.3, 14.1. Anal. Calcd. for C19H22N4O3: C, 64.39; H, 6.26; N, 15.81. Found: C, 64.16; H, 6.37; N, 15.77.
Ethyl 5-amino-2-methyl-4-(1-methyl-1H-imidazol-2-yl)-6,7,8,9-tetrahydro-4H-pyrano[2,3-b]quinoline-3-carboxylate (2). Ethyl 6-amino-5-cyano-2-methyl-4-(1-methyl-1H-imidazol-2-yl)-4H-pyran-3-carboxylate (16) (300 mg, 1.04 mmol), cyclohexanone (150 mg, 1.53 equiv), and AlCl3 (200 mg, 1.53 mmol), gave 272 mg (0.74 mmol) of the title compound 2 (72% yield): mp 222–224 °C; IR (KBr) max 3343 (NH2); 1701 (CO) cm−1; 1H-NMR (250 MHz, CDCl3) δ 6.86 (s, 1H), 6.67 (s, 1H), 5.42 (s, 1H), 5.15 (br s, 2H), 4.22–3.99 (m, 2H), 3.43 (s, 3H), 2.70–2.68 (m, 2H), 2.49 (s, 3H), 2.35–2.17 (m, 2H), 1.73–1.15 (m, 4H), 1.13–1.10 (m, 3H); 13C-NMR (62.9 MHz, CDCl3) δ 166.5, 161.4, 154.3, 153.4, 152.1, 147.0, 126.2, 122.6, 113.6, 101.7, 94.3, 60.5, 34.5, 33.0, 32.5, 22.8, 22.5, 22.3, 19.4, 14.2. Anal. Calcd. for C20H24N4O3: C, 65.20; H, 6.57; N, 15.21. Found: C, 65.41; H, 6.33; N, 15.02.
Ethyl 5-amino-2-methyl-4-(1-methyl-1H-imidazol-2-yl)-4,6,7,8,9,10-hexahydrocyclohepta[b]pyrano[3,2-e]pyridine-3-carboxylate (3). Following the general procedure, ethyl 6-amino-5-cyano-2-methyl-4-(1-methyl-1H-imidazol-2-yl)-4H-pyran-3-carboxylate (16) (300 mg, 1.04 mmol), AlCl3 (200 mg, 1.53 mmol), cycloheptanone (170 mg, 1.51 mmol), gave 295 mg (0.77 mmol) of the title compound 3 (74% yield): mp 196–198 °C; IR (KBr) max 3343 (NH2); 1701 (CO) cm−1; 1H-NMR (250 MHz, CDCl3) δ 6.82 (s, 1H), 6.65 (s, 1H), 5.35 (s, 1H), 5.19 (br s, 2H), 4.16–4.01 (m, 2H), 3.40 (s, 3H), 2.81–2.77 (m, 2H,), 2.45–2.40 (m, 5H), 1.79–1.42 (m, 6H,), 1.12–1.14 (m, 3H); 13C-NMR (62.9 MHz, CDCl3) δ 166.3, 161.2, 160.8, 152.9, 151.1, 146.8, 126.1, 122.6, 118.8, 101.7, 95.1, 60.3, 38.5, 34.5, 32.9, 32.0, 29.6, 26.6, 26.0, 25.5, 19.2, 14.1. Anal. Calcd. for C21H26N4O3: C, 65.95; H, 6.85; N, 14.65. Found: C, 66.01; H, 6.66; N, 14.84.
1-(5-Amino-2-methyl-4-(1-methyl-1H-imidazol-2-yl)-6,7,8,9-tetrahydro-4H-pyrano[2,3-b]quinolin-3-yl)ethan-1-one (4). In accordance with the general procedure, 5-acetyl-2-amino-6-methyl-4-(1-methyl-1H-imidazol-2-yl)-4H-pyran-3-carbonitrile (17) (260 mg, 1.0 mmol), cyclohexanone (150 mg, 1.53 mmol), and AlCl3 (200 mg, 1.53 mmol), gave 175 mg (0.52 mmol) of the title compound 4 (52% yield): mp 243–245 °C; IR (KBr) max 3359 (NH2); 1689 (CO) cm−1; 1H-NMR (250 MHz, CDCl3): δ 6.84 (s, 1H), 6.68 (s, 1H), 5.39 (s, 1H), 5.07 (br s, 2H), 3.37 (s, 3H), 2.65–2.49 (m, 2H), 2.36 (s, 3H), 2.23–2.15 (m, 5H), 1.75–1.67 (m, 4H); 13C-NMR (62.9 MHz, CDCl3) δ 199.1, 159.2, 154.5, 153.2, 151.9, 146.2, 126.3, 123.3, 113.5, 109.4, 93.9, 35.2, 33.0, 32.4, 29.7, 22.7, 22.4, 22.2, 19.6. Anal. Calcd. for C19H22N4O2: C, 67.44; H, 6.55; N, 16.56. Found: C, 67.31; H, 6.63; N, 16.44.
11-Amino-7,7-dimethyl-10-(1-methyl-1H-imidazol-2-yl)-2,3,6,7,8,10-hexahydrochromeno[2,3-b]cyclopenta[e]pyridin-9(1H)-one (5). According to the procedure, 2-amino-7,7-dimethyl-4-(1-methyl-1H-imidazol-2-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (18) (300 mg, 1.0 mmol), anhydrous AlCl3 (200 mg, 1.5 mmol) in dry ClCH2CH2Cl (10 mL), and cyclopentanone (130 mg, 1.54 mmol) gave 185 mg (0.51 mmol) of the title compound 5 (51% yield): mp > 260 °C; IR (KBr) max 3442 (NH2), 1643 (CO) cm−1; 1H-NMR (250 MHz, CDCl3) δ 6.80 (s, 1H), 6.67 (s, 1H), 5.29 (s, 1H), 4.98 (br s, 2H), 3.41 (s, 3H), 2.60–2.57 (m, 2H), 2.43–2.22 (m, 4H), 2.15–2.06 (m, 2H), 1.96–1.04 (m, 2H), 0.99 (s, 6H); 13C-NMR (62.9 MHz, CDCl3) δ 196.0, 165.7, 162.0, 155.4, 150.2, 146.7, 126.6, 122.0, 118.4, 109.0, 95.4, 50.5, 41.2, 34.0, 32.7, 31.8, 30.3, 28.3, 28.2, 26.9, 22.0. Anal. Calcd. for C21H24N4O2: C, 69.21; H, 6.64; N, 15.37. Found: C, 69.01; H, 6.44; N, 15.55.
11-Amino-3,3-dimethyl-12-(1-methyl-1H-imidazol-2-yl)-2,3,4,7,8,9,10,12-octahydro-1H-chromeno[2,3-b]quinolin-1-one (6). Using the same procedure, 2-amino-7,7-dimethyl-4-(1-methyl-1H-imidazol-2-yl)-5-oxo-5,6,7,8-tetrahydro-4H-chromene-3-carbonitrile (18) (300 mg, 1.0 mmol), AlCl3 (200 mg, 1.53 mmol), and cyclohexanone (150 mg, 1.53 mmol), gave 132 mg (0.35 mmol) of the title compound 6 (35% yield): mp > 260 °C; IR (KBr) max 3341 (NH2); 1702 (CO) cm−1; 1H-NMR (250 MHz, CDCl3) δ 6.94 (s, 1H), 6.69 (s, 1H), 5.36 (s, 1H), 4.99 (br s, 2H), 3.49 (s, 3H), 2.75–2.61 (m, 4H), 2.40–2.20 (m, 4H), 1.83–1.31 (m, 4H), 1.14 (s, 6H); 13C-NMR (62.9 MHz, CDCl3) δ 196.3, 166.0, 161.2, 154.4, 152.4, 146.9, 127.1, 122,3, 114,1 109.4, 95.4, 50.8, 41.6, 33.0, 32.5, 32.2, 30.8, 28.6, 22.9, 22.6, 22.4; Anal. Calcd. for C22H26N4O2: C, 69.82; H, 6.92; N, 14.80. Found: C, 69.66; H, 6.78; N, 14.67.
13-(1-Methyl-1H-imidazol-2-yl)-9,10,11,13-tetrahydrobenzo[5,6]chromeno[2,3-b]cyclopenta[e]pyridin-12-amine (7). In accordance with the general procedure, 2-amino-1-(1-methyl-1H-imidazol-2-yl)-1H-benzo[f]chromene-3-carbonitrile (19) (300 mg, 1.0. mmol), AlCl3 (200 mg, 1.53 mmol), cyclopentanone (130 mg, 1.54 mmol), gave 202 mg (0.55 mmol) of the title compound 7 (55% yield): mp > 260 °C; IR (KBr) max 3352 (NH2) cm−1; 1H-NMR (250 MHz, CDCl3) δ 8.20 (d, J = 8.4 Hz, 1H), 7.86–7.82 (m, 2H),7.50–7.39 (m, 3H), 6.96 (s, 1H), 6.59 (s, 1H), 6.29 (s, 1H), 5.32 (br s, 2H), 3.08 (s, 3H), 2.99–2.91 (m, 2H), 2.77–2.64 (m, 2H), 2.21–2.09 (m, 2H); 13C-NMR (62.9 MHz, CDCl3) δ 162.8, 156.6, 150.6, 149.5, 147.8, 131.7, 130.8, 130.3, 128.5, 127.5, 126.3, 124.7, 123.5, 123.1, 118.0, 117.6, 110.5, 94.7, 34.8, 34.4, 32.9, 27.3, 22.1. Anal. Calcd. for C23H20N4O: C, 74.98; H, 5.47; N, 15.21. Found: C, 75.01; H, 5.51; N, 15.02.
14-(1-Methyl-1H-imidazol-2-yl)-9,11,12,14-tetrahydro-10H-benzo[5,6]chromeno[2,3-b]quinolin-13-amine (8). Following the general procedure, 2-amino-1-(1-methyl-1H-imidazol-2-yl)-1H-benzo[f]chromene-3-carbonitrile (19) (300 mg, 1.0 mmol), AlCl3 (200 mg, 1.53 mmol), and cyclohexanone (150 mg, 1.53 mmol), gave 225 mg (0.59 mmol) of the title compound 8 (59% yield): mp > 260 °C; IR (KBr) max 3351 (NH2) cm−1; 1H-NMR (250 MHz, CDCl3) δ 8.20 (d, J = 8.3 Hz, 1H), 7.84–7.80 (m, 2H), 7.52–7.37 (m, 3H), 6.94 (s, 1H), 6.56 (s, 1H), 6.25 (s, 1H), 5.37 (br s, 2H), 3.08 (s, 3H), 2.88–2.72 (m, 2H), 2.44–2.26 (m, 2H), 1.85–1.77 (m, 4H); 13C-NMR (62.9 MHz, CDCl3) δ 154.5, 154.4, 152.6, 149.5, 147.6, 131.7, 130.7, 130.3, 128.5, 127.4, 126.1, 124.6, 123.4, 122.9, 118.0, 112.9, 110.5, 94.4, 34.7, 32.8, 32.6, 22.9, 22.7, 22.5. Anal. Calcd. for C24H22N4O: C, 75.37; H, 5.80; N, 14.65. Found: C, 75.30; H, 5.82; N, 14.38.
15-(1-Methyl-1H-imidazol-2-yl)-9,10,11,12,13,15-hexahydrobenzo[5,6]chromeno[2,3-b]cyclohepta[e]pyridin-14-amine (9). Following the general procedure, 2-amino-1-(1-methyl-1H-imidazol-2-yl)-1H-benzo[f]chromene-3-carbonitrile (19) 300 mg (1.0. mmol), AlCl3 (200 mg, 1.53 mmol), and cycloheptanone (170 mg, 1.51 mmol), gave 238 mg (0.6 mmol) of the title compound 9 (60% yield): mp > 260 °C; IR (KBr) max 3355 (NH2 cm−1); 1H-NMR (250 MHz, CDCl3) δ 8.21 (d, J = 8.2 Hz, 1H), 7.84–7.80 (m, 2H), 7.52–7.37 (m, 3H), 6.95 (s, 1H), 6.57 (s, 1H), 6.32 (s, 1H), 5.43 (br s, 2H), 3.09 (s, 3H), 2.96–2.92 (m, 2H), 2.57–2.53 (m, 2H), 1.87–1.53 (m, 6H); 13C-NMR (62.9 MHz, CDCl3) δ 161.1, 154.1, 151.7, 149.5, 147.6, 131.7, 130.7, 130.3, 128.5, 127.4, 126.2, 124.6, 123.4, 123.0, 118.3, 117.9, 110.6, 95.4, 38.9, 35.0, 32.8, 32.3, 26.9, 26.2, 25.7. Anal. Calcd. For C25H24N4O: C, 75.73; H, 6.10; N, 14.13. Found: C, 75.66; H, 6.23; N, 14.01.
7-(1-Methyl-1H-imidazol-2-yl)-9,10,11,12-tetrahydro-7H-benzo[7,8]chromeno[2,3-b]quinolin-8-amine (10). Following the general procedure, 2-amino-4-(1-methyl-1H-imidazol-2-yl)-4H-benzo[h]chromene-3-carbonitrile (20) (300 mg, 1.0 mmol), cyclohexanone (150 mg, 1.53 mmol), and AlCl3 (200 mg, 1.53 mmol), gave 198 mg (0.52 mmol) of the title compound 10 (52% yield): mp > 260 °C; IR (KBr) max 3355 (NH2) cm−1; 1H-NMR (250 MHz, CDCl3) δ 8.27 (d, J = 8.3 Hz, 1H), 7.85–7.81 (m, 2H),7.50–7.40 (m, 3H), 6.95 (s, 1H), 6.58 (s, 1H), 6.26 (s, 1H), 5.32 (br s, 2H), 3.09 (s, 3H), 2.81–2.80 (m, 2H), 2.45–2.25 (m, 2H), 1.86–1.78 (m, 4H); 13C-NMR (62.9 MHz, CDCl3) δ 154.6, 154.5, 152.6, 149.5, 147.7, 131.7, 130.7, 130.3, 128.5, 127.5, 126.1, 124.7, 123.5, 122.9, 118.0, 113.0, 110.6, 94.5, 34.8, 32.8, 32.7, 22.9, 22.7, 22.5. Anal. Calcd. for C24H22N4O: C, 75.37; H, 5.80; N, 14.65. Found: C, 75.12; H, 5.75; N, 14.58.
7-(1-Methyl-1H-imidazol-2-yl)-7,9,10,11,12,13-hexahydrobenzo[7,8]chromeno[2,3-b]cyclohepta[e]pyridin-8-amine (11). Following the general procedure, 2-amino-4-(1-methyl-1H-imidazol-2-yl)-4H-benzo[h]chromene-3-carbonitrile (20) (300 mg, 1.0 mmol), cycloheptanone (170 mg, 1.51 mmol), and AlCl3 (200 mg, 1.53 mmol), gave 245 mg (0.62 mmol) of the title compound 11 (62% yield): mp > 260 °C; IR (KBr) max 3350 (NH2) cm−1; 1H-NMR (250 MHz, CDCl3) δ 8.21 (d, J = 8.3 Hz, 1H), 7.84–7.80 (m, 2H), 7.51–7.42 (m, 3H), 6.99 (s, 1H), 6.58 (s, 1H), 6.25 (s, 1H), 5.43 (br s, 2H), 3.00 (s, 3H), 2.96–2.92 (m, 2H), 2.57–2.52 (m, 2H), 1.88–1.27 (m, 6H); 13C-NMR (62.9 MHz, CDCl3) δ 161.1, 154.1, 151.7, 149.5, 147.6, 131.6, 130.7, 130.3, 128.5, 127.4, 126.1, 124.7, 123.4, 123.0, 118.3, 117.9, 110.6, 95.3, 38.9, 34.9, 32.8, 32.3, 26.9, 26.5, 25.7. Anal. Calcd. for C25H24N4O: C, 75.73; H, 6.10; N, 14.13. Found: C, 75.88; H, 6.02; N, 14.25.
7-(1-Methyl-1H-imidazol-2-yl)-7,9,10,11-tetrahydrocyclopenta[5′,6′]pyrido[3′,2′:5,6]pyrano[3,2-h]quinolin-8-amine (12). In accordance with the general procedure, 2-amino-4-(1-methyl-1H-imidazol-2-yl)-4H-pyrano[3,2-h]quinoline-3-carbonitrile (21) (1.0 mmol, 300 mg), AlCl3 (200 mg, 1.5 mmol) and cyclopentanone (130 mg, 1.54 mmol), gave 238 mg (0.65 mmol) of title compound 12 (65% yield): mp > 260 °C; IR (KBr) max 3349 (NH2) cm−1; 1H-NMR (250 MHz, CDCl3) δ 9.00–8.98 (m, 1H), 8.11–8.07 (m, 1H), 7.47–7.44 (m, 2H), 7.34–7.23 (m, 1H), 6.94 (s, 1H), 6.71 (s, 1H), 5.89 (s, 1H), 4.92 (br s, 2H), 3.10 (s, 3H), 2.95–2.87 (m, 2H), 2.70–2.59 (m, 2H), 2.15–2.04 (m, 2H); 13C-NMR (62.9 MHz, CDCl3) δ 163.1, 156.7, 150.4, 150.2, 147.8, 146.1, 138.5, 135.6, 128.6, 126.4, 126.3, 123.3, 122.5, 122.1, 117.5, 116.3, 93.5, 36.9, 34.3, 33.0, 27.1, 22.2. Anal. Calcd. for C22H19N5O: C, 71.53; H, 5.18; N, 18.96. Found: C, 71.48; H, 5.33; N, 19.01.
7-(1-Methyl-1H-imidazol-2-yl)-7,9,10,11,12,13-hexahydrocyclohepta[5′,6′]pyrido[3′,2′:5,6]pyrano[3,2-h]quinolin-8-amine (13). In accordance with the general procedure, 2-amino-4-(1-methyl-1H-imidazol-2-yl)-4H-pyrano[3,2-h]quinoline-3-carbonitrile (21) (300 mg, 1.0 mmol), AlCl3 (200 mg, 1.5 mmol), and cycloheptanone (170 mg, 1.51 mmol), gave 158 mg (0.40 mmol) of title compound 13 (40% yield): mp 206–207 °C; IR (KBr) max 3429 (NH2) cm−1; 1H-NMR (250 MHz, CDCl3) δ 9.00–8.98 (m, 1H), 8.06–8.03 (m, 1H), 7.43–7.36 (m, 2H), 7.28–7.21 (m, 1H), 6.89 (s, 1H), 6.65 (s, 1H), 5.77 (s, 1H), 4.99 (br s, 2H), 3.04 (s, 3H), 2.90–2.76 (m, 2H), 2.63–2.47 (m, 2H), 1.77–1.46 (m, 6H); 13C-NMR (62.9 MHz, CDCl3) δ 161.5, 153.9, 151.1, 150.3, 147.6, 145.9, 138.3, 135.5, 128.4, 126.4, 126.3, 123.2, 122.3, 122.0, 118.1, 116.4, 94.2, 38.4, 37.1, 32.8, 32.1, 26.8, 26.1, 25.5. Anal. Calcd. for C24H23N5O: C, 72.52; H, 5.83; N, 17.62. Found: C, 72.67; H, 5.85; N, 17.41.
3.5. In Vitro Toxicity in HepG2 Cells
3.5.1. Cell Culture and Treatment
The human hepatoma HepG2 line cells were purchased from American Type Culture Collection. The cells were cultured in Eagle’s Minimum Essential Medium (Ozyme, Montigny-le-Bretonneux, France) supplemented with 10% fetal bovine serum, 1X non-essential amino acids, 100 units/mL penicillin and 10 mg/mL streptomycin (Dutscher, Brumath, France). Cultures were kept under a CO2/air (5%/95%) humidified atmosphere at 37 °C. Prior to the experiment, cells were seeded in 96-well culture plates at a density of 0.1 × 106 cells per well. After 24 h of incubation, the culture medium was refreshed and 100 µL of the test compounds or DMSO (0.1%) were added. Compounds were tested at 7 concentrations (1–1000 µM) in triplicate.
3.5.2. Measurement of Cell Viability
Cell viability, measured as the mitochondrial activity of living cells, was determined by quantitative colorimetric assay with 3-[4,5 dimethylthiazol-2-yl]-2,5-diphenyl-tetrazolium bromide (MTT) [33]. Briefly, after 24 h of treatment, cells were incubated with 50 µL MTT (0.5 mg/mL, Sigma Aldrich) at 37 °C for 2 h. Plates were centrifuged, MTT was removed and 100 µL DMSO was distributed per well. The absorbance was read at 570 nm by microplate spectrophotometry. Cell viability was expressed as percentage over controls (DMSO, 0.1%).
3.6. Measurement of the Inhibitory Potency against EeAChE
The inhibitory activity on AChE from Electrophorus electricus (type VI-S, Sigma, Milan, Italy), was evaluated spectrophotometrically by the method of Ellman et al. [27]. AChE stock solution was prepared by dissolving EeAChE lyophilized powder (Sigma) in 0.1 M phosphate buffer (pH = 8.0) containing 0.1% Triton X-100. Stock solutions of the tested compounds (1 mM) were prepared in MeOH/H2O 50/50 (v/v) and diluted in MeOH/H2O 50/50 (v/v). Five increasing concentrations of each inhibitor were used, able to give an inhibition of the enzymatic activity in the range of 20–80%. The assay solution consisted of a 0.1 M phosphate buffer pH 8.0, with the addition of 340 μM 5,5′-dithio-bis(2-nitrobenzoic acid), 0.02 unit/mL of EeAChE and 550 μM of substrate (acetylthiocholine iodide, ATCh). Assay solutions with and without inhibitor were preincubated at 37 °C for 20 min followed by the addition of substrate. Blank solutions containing all components except EeAChE were prepared in parallel to account for the non-enzymatic hydrolysis of the substrate. Initial rate assays were performed at 37 °C with a Jasco V-530 double beam spectrophotometer. The rate of increase in the absorbance at 412 nm was followed for 210 s. The velocity in the presence and absence of inhibitor were compared and % inhibition was calculated. The results were plotted by placing the percentage of inhibition in function of the decimal log of the final inhibitor concentration. Linear regression and IC50 values were calculated using Microcal Origin 3.5 software (Microcal Software, Kiev, Ukraine).
3.7. Measurement of the Inhibitory Potency against eqBuChE
The % inhibitory activity on BuChE from horse serum (lyophilized powder, Sigma-Aldrich), was evaluated spectrophotometrically by the method of Ellman et al. [27]. BuChE stock solutions were first dissolved in 0.1 M phosphate buffer (pH 8.0) and then aliquoted in small vials. Compounds were dissolved in DMSO at 10 mM. The reaction was carried out in a final volume of 3 mL of a 0.1 M phosphate-buffered solution at pH 8.0, containing 5,5′-dithiobis-2-nitrobenzoic acid (DTNB, 2625 µL, 0.35 mM, final concentration), eqBuChE (60 µL, 0.05 U/mL final concentration), tested compound (3 µL, 10 µM, final concentration) and 1% w/v Bovine Serum Albumin phosphate-buffered (pH 8.0) solution (BSA, 40 µL). The inhibition with respect to control without compound was determined by pre-incubating this mixture at rt with each compound for 10 min. After this period, butyrylthiocholine iodide (150 µL, 0.5 mM final concentration) was added and incubated for 15 min at rt. Then the absorbances were measured at 412 nm in a spectrophotometer plate reader (iEMS Reader MF, Labsystems). % inhibition given by each compound was calculated and data are expressed as mean ± SEM of at least three different experiments.
3.8. Kinetic Analysis of the Inhibition of EeAChE by Compound 8
To obtain estimates of the inhibition mechanism, reciprocal plots of 1/v vs. 1/[S] were constructed at different concentrations of the substrate acetylthiocholine (0.111–0.557 mM) by using Ellman′s method [27] and EeAChE (type VI-S, Sigma). Four concentrations of inhibitor were selected for this study: 0.850, 2.124, 3.399, 6.798 μM. The plots were assessed by a weighted least-squares analysis that assumed the variance of the velocity (v) to be a constant percentage of v for the entire data set. Data analysis was performed with GraphPad Prism 4.03 software (GraphPad Software Inc., San Diego, CA, USA). The determination of the non-competitive inhibition constant Ki value was carried out using Dixon plot, i.e., plotting the apparent 1/v vs. inhibitor concentration [I] [34] and confirmed by Cornish-Bowden plot ([substrate]/v vs. [I]) [28].
3.9. Oxygen Radical Absorbance Capacity Assay
The radical scavenging activity of imidazopyranotacrines 1–13 was determined by the ORAC-FL method [29,30] using a Varioskan Flash plate reader with built-in injectors (Thermo Scientific). Trolox, 2,2′-azobis(amidinopropane) dihydrochloride (AAPH) and fluorescein (FL) were purchased from Sigma-Aldrich. The reaction was performed at 37 °C in 75 mM phosphate buffer (pH = 7.4), and the final volume reaction mixture was 200 µL. The final concentrations were 1–8 µM for Trolox and 0.1–1 µM for the tested compounds. Briefly, in a black 96-well microplate (Nunc), antioxidant (20 µL) and fluorescein (120 µL) were incubated for 15 min at 37 °C. Then, 2,2′-azobis(amidinopropane) dihydrochloride (60 µL) was added using the built-in injector. The fluorescence at λem 535 nm (λexc = 485 nm) was measured each minute during 60 min. All assays were made in triplicate and at least three different assays were performed for each sample. Antioxidant curves were first normalized to the curve of the blank and then, the area under the fluorescence decay curve (AUC) was calculated as:
where f0 is the initial fluorescence reading at 0 min and fi is the fluorescence value at time i.
AUC = 1 + sum(fi/f0)
The net AUC corresponding to a sample was calculated as follows:
Net AUC = AUCantioxidant − AUCblank
Regression equations were calculated by plotting the net AUC against the antioxidant concentration. The ORAC value was obtained by dividing the slope of the latter curve by the slope of the Trolox curve obtained in the same assay. ORAC values was expressed as µmol of Trolox equivalent/µmol of imidazopyranotacrines. Data are expressed as mean ± SD.
4. Conclusions
Although the etiology of AD is a matter of debate, a number of biological targets [35] such as inhibition of Aβ oligomerization and deposition in senile plaques, halting neurofibrillary tangle formation, and inhibition of γ- and β-secretase [36] have been identified and exploited to design new therapies. However, the recent failure in clinical phase III of semagacestat [37], γ-secretase inhibitor, as well as the clinical phases discontinuation of tideglusib [38], a GSK-3 inhibitor targeting tau protein, clearly show that the soundest hypotheses for AD have not yet provided any drug for AD patients cure. Thus, this may be the moment to repurpose old strategies such as the search for non-hepatotoxic tacrines.
In this context, in this article we have reported our most recent endeavors on this topic [19], describing a number of imidazopyranotacrines, a new type of tacrine analogues [39,40,41,42,43], as modest and selective EeAChE inhibitors showing good antioxidant power. Particularly attractive in this family of compounds is the imidazopyranotacrine 4, which is a modest AChEI, but a potent antioxidant agent, endowed with a very interesting safety profile on HepG2 cells, i.e., showing lack of hepatotoxicity even at very high concentration (1000 µM). Other tacrine analogues such as 8 and 10 are significantly more potent AChEIs than 4 and show good antioxidant power, but also, unfortunately, higher hepatotoxicity, although compare favorably with tacrine especially at high concentrations (≥ 300 μM). Kinetic analysis showed that compound 8 is a non-competitive AChEI, a mechanism that we have confirmed by computational chemistry by performing the docking analysis of both enantiomers of compound 8 on AChE. In addition, modeling results showed that the best binding positions for compound 8 on BuChE were clustered rather far from the active site, at the top of the gorge, explaining thus why this compound is a poor eqBuChE inhibitor (Supplementary Materials).
We have also calculated the Admission, Distribution, Metabolism, Excretion (ADME) properties of some selected imidazopyranotacrines. Thus, about 45 physically significant descriptors and pharmacologically relevant properties of both enantiomers of compounds 2, 4, 8 and 10 were predicted and some of the important properties were analyzed. All compounds showed significant values for the properties analyzed and showed drug-like characteristics based on Lipinski′s rule of five (see Table S1, Supplementary Materials). Drugs currently used for neurological disorders treatment are generally CNS acting drugs, which show values of MW, Hydrogen Bond (HB) donor, acceptors and rotatable bonds, etc., in general, in a smaller range than non-CNS therapeutics. Thus, factors that are relevant to the success of CNS drugs were analyzed. CNS drugs show values of MW < 450, HB donor < 3, HB acceptors < 7, QPlogPo/w < 5, PSA < 90, number of rotatable bonds < 8 and hydrogen bonds < 8. Thus, the enantiomers of compounds 2, 4, 8 and 10 satisfied all the characteristic of CNS acting drugs. The solubility of organic molecules in water has a significant impact on many ADME-related properties. These compounds present solubility values within the limits. The partition coefficient (QPlogPo/w), critical for estimation of absorption within the body, ranged between 2.82 and 4.75. The percentage human oral absorption for the compounds is high, 100%. The most used parameter for Blood Brain (BB) barrier penetration is log BB. The log BB of many prescribed CNS drugs is >−0.5 and compounds with log BB <−1.0 penetrate poorly into the brain, yet some commercial CNS drugs have log BB <−1.0. For compounds 2, 4, 8 and 10, these values lie within the indicated limits of −3 < QPlogBB < 1.2.
In conclusion, some imidazopyranotacrine derivatives showing selective modest AChE inhibition, whose pharmacokinetic parameters are within the acceptable range defined for human use, thereby indicating their potential as drug-like molecules and possible brain penetration, have been discovered for the potential treatment of AD.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/4/400/s1.
Acknowledgments
A.B. thanks MESRES (Ministère de l′Enseignement Supérieur et de la Recherche Scientifique, Algeria) for partial financial support. J.M.-C. thanks María do Carmo Carreiras (School of Pharmacy, University of Lisbon, Lisbon, Portugal) for critically reading and correcting the manuscript.
Author Contributions
Houssem Boulebd and Abdelmalek Bouraiou carried out the synthesis and spectra analysis and interpretation. Mourad Chioua took care of the elemental analyses. Helene Martin and Alexandre Bonet performed the hepatotoxicity analyses. Lhassane Ismaili carried out the cholinesterase and ORAC analysis. Manuela Bartolini and Vincenza Andrisano performed the cholinesterase inhibition and kinetic studies. José Marco-Contelles wrote the manuscript. Ignacio Moraleda and Isabel Iriepa performed molecular modeling study. Ali Belfaitah conceived the project and conducted collaborative project with José Marco-Contelles and Lhassane Ismaili (Monitoring, coordination and management), analysis of results and spectra analysis and interpretation (chemistry). All authors contributed to and have approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Soler-López, M.; Badiola, N.; Zanzoni, A.; Aloy, P. Towards Alzheimer′s root cause: ECSIT as an integrating hub between oxidative stress, inflammation and mitochondrial dysfunction. Hypothetical role of the adapter protein ECSIT in familial and sporadic Alzheimer′s disease pathogenesis. Bioessays 2012, 34, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Reitz, C.; Brayne, C.; Mayeux, R. Epidemiology of Alzheimer disease. Nat. Rev. Neurol. 2011, 7, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Schneider, L.S.; Mangialasche, F.; Andreasen, N.; Feldman, H.; Giacobini, E.; Jones, R.; Mantua, V.; Mecocci, P.; Pani, L.; Winblad, B.; et al. Clinical trials and late-stage drug development for Alzheimer′s disease: An appraisal from 1984 to 2014. J. Intern. Med. 2014, 275, 251–283. [Google Scholar] [CrossRef] [PubMed]
- Ittner, L.M.; Götz, J. Amyloid-β and tau–a toxic pas de deux in Alzheimer′s disease. Nat. Rev. Neurosci. 2011, 12, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Ávila, J. Tau phosphorylation and aggregation in Alzheimer′s disease pathology. FEBS Lett. 2006, 580, 2922–2927. [Google Scholar] [CrossRef] [PubMed]
- Rosini, M.; Simoni, E.; Milelli, A.; Minarini, A.; Melchiorre, C. Oxidative stress in Alzheimer′s disease: Are we connecting the dots? J. Med. Chem. 2014, 57, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.L.; Xu, J.Q.; Kochanek, K.D. Deaths: Final Data for 2010; National Center for Health Statistics: Hyattsville, MD, USA, 2013. [Google Scholar]
- Chacón, M.A.; Reyes, A.E.; Inestrosa, N.C. Acetylcholinesterase induces neuronal cell loss, astrocyte hypertrophy and behavioral deficits in mammalian hippocampus. J. Neurochem. 2003, 87, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Terry, A.V.; Buccafusco, J.J.; Wilson, C. Cognitive dysfunction in neuropsychiatric disorders: selected serotonin receptor subtypes as therapeutic targets. Behav. Brain Res. 2008, 195, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Gura, T. Hope in Alzheimer′s fight emerges from unexpected places. Nat. Med. 2008, 14, 894. [Google Scholar] [CrossRef] [PubMed]
- Racchi, M.; Mazzucchelli, M.; Porrello, E.; Lanni, C.; Govoni, S. Acetylcholinesterase inhibitors: Novel activities of old molecules. Pharmacol. Res. Off. J. Ital. Pharmacol. Soc. 2004, 50, 441–451. [Google Scholar]
- Castro, A.; Martínez, A. Targeting Beta-Amyloid Pathogenesis Through Acetylcholinesterase Inhibitors. Curr. Pharm. Des. 2006, 12, 4377–4387. [Google Scholar] [CrossRef] [PubMed]
- Rosini, M. Polypharmacology: The rise of multitarget drugs over combination therapies. Future Med. Chem. 2014, 6, 485–487. [Google Scholar] [CrossRef] [PubMed]
- León, R.; García, A.G.; Marco-Contelles, J. Recent advances in the multitarget-directed ligands approach for the treatment of Alzheimer′s disease. Med. Res. Rev. 2013, 33, 139–189. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Cacabelos, R.; Oset-Gasque, M.J.; Samadi, A.; Marco-Contelles, J. Novel tacrine-related drugs as potential candidates for the treatment of Alzheimer′s disease. Bioorg. Med. Chem. Lett. 2013, 23, 1916–1922. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.; Di Nicola, M.; Velluto, L.; D′Angelo, C.; Costantini, E.; Lahiri, D.K.; Kamal, M.A.; Yu, Q.S.; Greig, N.H. Selective acetyl and butyrylcholinesterase inhibitors reduce amyloid-beta ex vivo activation of peripheral chemo-cytokines from Alzheimer′s disease subjects: Exploring the cholinergic anti-inflammatory pathway. Curr. Alzheimer Res. 2014, 11, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Watkins, P.B.; Zimmerman, H.J.; Knapp, M.J.; Gracon, S.I.; Lewis, K.W. Hepatotoxic effects of tacrine administration in patients with Alzheimer′s disease. JAMA 1994, 271, 992–998. [Google Scholar] [CrossRef] [PubMed]
- Soukup, O.; Jun, D.; Zdarova-Karasova, J.; Patocka, J.; Musilek, K.; Korabecny, J.; Krusek, J.; Kaniakova, M.; Sepsova, V.; Mandikova, J.; et al. A resurrection of 7-MEOTA: A comparison with tacrine. Curr. Alzheimer Res. 2013, 10, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.; Lamba, D.; Zhang, L.; Lou, Y.; Xu, C.; Kang, D.; Chen, L.; Xu, Y.; Zhang, L.; De Simone, A.; et al. Novel tacrine-benzofuran hybrids as potent multitarget-directed ligands for the treatment of Alzheimer′s disease: Design, synthesis, biological evaluation, and X‑ray crystallography. J. Med. Chem. 2016, 59, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Grau, A.; Marco, J.L. Friedländer reaction on 2-amino-3-cyano-4H-pyrans: Synthesis of derivatives of 4H-pyran[2,3-b]quinoline, new tacrine analogues. Bioorg. Med. Chem. Lett. 1997, 7, 3165–3170. [Google Scholar] [CrossRef]
- Marco, J.L.; de los Ríos, C.; Carreiras, M.C.; Baños, J.E.; Badía, A.; Vivas, N.M. Synthesis and acetylcholinesterase/butyrylcholinesterase inhibition activity of new tacrine-like analogues. Bioorg. Med. Chem. 2001, 9, 727–732. [Google Scholar] [CrossRef]
- León, R.; Marco-Contelles, J.; García, A.G.; Villarroya, M. Synthesis, acetylcholinesterase inhibition and neuroprotective activity of new tacrine analogues. Bioorg. Med. Chem. 2005, 13, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Marco-Contelles, J.; León, R.; López, M.G.; García, A.G.; Villarroya, M. Synthesis and biological evaluation of new 4H-pyrano[2,3-b]quinoline derivatives that block acetylcholinesterase and cell calcium signals, and cause neuroprotection against calcium overload and free radicals. Eur. J. Med. Chem. 2006, 41, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Kaptein, B.; Kellogg, R.M.; van Bolhuis, F. Synthesis, molecular structure, and complexation of 1,4-dihydropyridines containing ligands for intramolecular complexation of metal electrophiles. Recl. Trav. Chim. Pays-Bas 1990, 109, 388–395. [Google Scholar] [CrossRef]
- Okubo, T.; Yoshikawa, R.; Chaki, S.; Okuyama, S.; Nakazato, A. Design, synthesis and structure-affinity relationships of aryloxyanilide derivatives as novel peripheral benzodiazepine receptor ligands. Bioorg. Med. Chem. 2004, 12, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Marco-Contelles, J.; Pérez-Mayoral, E.; Samadi, A.; Carreiras, M.C.; Soriano, E. Recent Advances in the Friedländer Reaction. Chem. Rev. 2009, 109, 2652–2671. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L.; Courtney, K.D.; Andres, V., Jr.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Fang, L.; Kraus, B.; Lehmann, J.; Heilmann, J.; Zhang, Y.; Decker, M. Design and synthesis of tacrine–ferulic acid hybrids as multi-potent anti-Alzheimer drug candidates. Bioorg. Med. Chem. Lett. 2008, 18, 2905–2909. [Google Scholar] [CrossRef] [PubMed]
- Cornish-Bowden, A. A simple graphical method for determining the inhibition constants of mixed, uncompetitive and non-competitive inhibitors (Short Communication). Biochem. J. 1974, 137, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- O′Brien, P.J.; Irwin, W.; Diaz, D.; Howard-Cofield, E.; Krejsa, C.M.; Slaughter, M.R.; Gao, B.; Kaludercic, N.; Angeline, A.; Bernardi, P.; et al. High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch. Toxicol. 2006, 80, 580–604. [Google Scholar] [CrossRef] [PubMed]
- Schoonen, W.G.E.J.; Westerink, W.M.A.; de Roos, J.A.D.M.; Débiton, E. Cytotoxic effects of 100 reference compounds on Hep G2 and HeLa cells and of 60 compounds on ECC-1 and CHO cells. I mechanistic assays on ROS, glutathione depletion and calcein uptake. Toxicol. In Vitro 2005, 19, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Denizot, F.; Lang, R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J. Immunol. Methods 1986, 89, 271–277. [Google Scholar] [CrossRef]
- Dixon, M.; Webb, E.C. Enzymes, 2nd ed.; Longmans, Green and Co.: London, UK, 1964; pp. 54–166. [Google Scholar]
- Martínez, A. Emerging Drugs and Targets for Alzheimer′s Disease: Neuronal Plasticity, Neuronal Protection and Other Miscellaneous Strategies; Royal Society of Chemistry: London, UK, 2010; Volume 2, pp. 1–24. [Google Scholar]
- Grill, J.D.; Cummings, J.L. Current therapeutic targets for the treatment of Alzheimer′s disease. Expert Rev. Neurother. 2010, 10, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Haass, C. Semagacestat′s fall: Where next for AD therapies? Nat. Med. 2013, 19, 1214–1215. [Google Scholar]
- Lovestone, S.; Boada, M.; Dubois, B.; Hull, M.; Rinne, J.O.; Huppertz, H.-J.; Calero, M.; Andrés, M.V.; Gómez-Carrillo, B.; León, T.; et al. A phase II trial of tideglusib in Alzheimer′s disease. J. Alzheimer′s Dis. 2015, 45, 75–88. [Google Scholar]
- Benchekroun, M.; Bartolini, M.; Egea, J.; Romero, A.; Soriano, E.; Pudlo, M.; Luzet, V.; Andrisano, V.; Jimeno, M.L.; López, M.G.; et al. Novel tacrine-grafted Ugi adducts as multipotent anti-Alzheimer drugs: a synthetic renewal in tacrine-ferulic acid hybrids. Chem. Med. Chem. 2015, 10, 523–539. [Google Scholar] [CrossRef] [PubMed]
- Nepovimova, E.; Uliassi, E.; Korabecny, J.; Pena-Altamira, L.E.; Samez, S.; Pesaresi, A.; Garcia, G.E.; Bartolini, M.; Andrisano, V.; Bergamini, C.; et al. Multitarget drug design strategy: quinone-tacrine hybrids designed to block amyloid-beta aggregation and to exert anticholinesterase and antioxidant effects. J. Med. Chem. 2014, 57, 8576–8589. [Google Scholar] [CrossRef] [PubMed]
- Esquivias-Pérez, M.; Maalej, E.; Romero, A.; Chabchoub, F.; Samadi, A.; Marco-Contelles, J.; Oset-Gasque, M.J. Nontoxic and neuroprotective β-naphthotacrines for Alzheimer′s disease. Chem. Res. Toxicol. 2013, 26, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Sun, J.; Fang, L.; Liu, M.; Peng, S.; Liao, H.; Lehmann, J.; Zhang, Y. Tacrine-ferulic acid-nitric oxide (NO) donor trihybrids as potent, multifunctional acetyl- and butyrylcholinesterase inhibitors. J. Med. Chem. 2012, 55, 4309–4321. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bachiller, M.I.; Pérez, C.; González-Muñoz, G.C.; Conde, S.; López, M.G.; Villarroya, M.; García, A.G.; Rodríguez-Franco, M.I. Novel tacrine-8-hydroxyquinoline hybrids as multifunctional agents for the treatment of Alzheimer′s disease, with neuroprotective, cholinergic, antioxidant, and copper-complexing properties. J. Med. Chem. 2010, 53, 4927–4937. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of all compounds (1)–(21) are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).