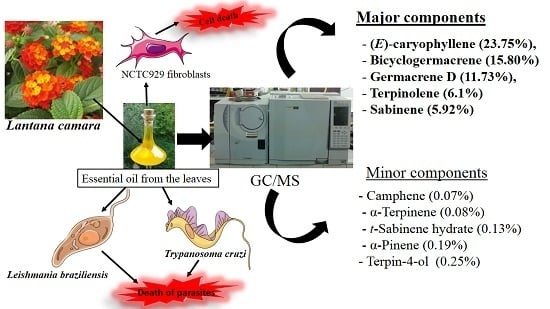
Chemical Characterization and Trypanocidal, Leishmanicidal and Cytotoxicity Potential of Lantana camara L. (Verbenaceae) Essential Oil
Abstract
:1. Introduction
2. Results
2.1. GC/MS Analysis of L. camara Leaf Essential Oil
| Compounds | RI a | RI b | Oil Composition (%) |
|---|---|---|---|
| α-Pinene | 939 | 937 | 0.19 |
| Camphene | 953 | 951 | 0.07 |
| Sabinene | 976 | 675 | 5.92 |
| β-Pinene | 980 | 983 | 0.45 |
| Myrcene | 991 | 990 | 0.31 |
| α-Terpinene | 1018 | 1015 | 0.08 |
| p-Cymene | 1026 | 1026 | 2.73 |
| (Z)-β-Ocimene | 1040 | 1037 | 0.68 |
| (E)-β-Ocimene | 1050 | 1054 | 0.93 |
| γ-Terpinene | 1062 | 1061 | 1.84 |
| Terpinolene | 1088 | 1079 | 6.01 |
| Terpin-4-ol | 1177 | 1174 | 0.25 |
| α-Terpineol | 1189 | 1193 | 1.02 |
| t-Sabinene hydrate | 1254 | 1257 | 0.13 |
| α-Copaene | 1376 | 1376 | 0.93 |
| β-Elemene | 1391 | 1389 | 1.50 |
| β-Caryophyllene | 1404 | 1401 | 3.46 |
| (E)-Caryophyllene | 1418 | 1423 | 23.75 |
| Aromandendrene-allo | 1461 | 1460 | 2.17 |
| α-Humulene | 1454 | 1451 | 4.04 |
| Germacrene D | 1480 | 1480 | 11.73 |
| Valencene | 1491 | 1489 | 8.32 |
| Bicyclogermacrene | 1494 | 1497 | 15.80 |
| Cubebol | 1514 | 1518 | 1.47 |
| δ-Cadinene | 1513 | 1509 | 0.26 |
| Spathulenol | 1576 | 1573 | 1.98 |
| Caryophyllene oxide | 1581 | 1585 | 2.67 |
| Total identified (%) | - | - | 98.69 |
2.2. Effect of L. camara Leaf Essential Oil against T. cruzi
| Nifurtimox (µg/mL) | %AE | Essential Oil (µg/mL) | %AE |
|---|---|---|---|
| - | - | 250 | 67.39 ± 0.26 |
| - | - | 125 | 22.04 ± 5.89 |
| 100 | 100 ± 0.46 | - | - |
| - | - | 62.5 | 0 ± 3.06 |
| 50 | 93 ± 0.66 | - | - |
| 10 | 84 ± 0.62 | - | - |
| 1 | 43 ± 0.93 | - | - |
| 0.5 | 13 ± 2.50 | - | - |
| 0.1 | 0 ± 1.54 | - | - |
| IC50 (µg/mL) | 3.02 ± 0.75 | 201.94 ± 1.2 |
2.3. Effect of Essential Oil from L. camara Leaves against Leishmania braziliensis
| Pentamidine (µg/mL) | %AP | Essential Oil (µg/mL) | %AE |
|---|---|---|---|
| - | - | 250 | 100 ± 0.76 |
| - | - | 125 | 100 ± 1.25 |
| - | - | 100 | 100 ± 2.23 |
| 100 | 93.9 ± 0.3 | - | - |
| - | - | 80 | 94.95 ± 1.46 |
| - | - | 70 | 36.4 ± 2.22 |
| - | - | 62.5 | 16.44 ± 0.90 |
| - | - | 50 | 15.9 ± 1.50 |
| 50 | 93.9 ± 0.1 | - | - |
| 25 | 89.2 ± 0.6 | - | - |
| 12.5 | 80.6 ± 0.2 | - | - |
| 6.25 | 54.2 ± 0.3 | - | - |
| 3.125 | 15.5 ± 1.1 | - | - |
| IC50 (µg/mL) | 5.69 ± 0.42 | 72.31 ± 0.89 |
2.4. Effect of L. camara Leaf Essential Oil on NCTC929 Fibroblasts
| Nifurtimox (µg/mL) | %C | Essential Oil (µg/mL) | %C |
|---|---|---|---|
| 600 | 100 ± 4.4 | - | - |
| - | - | 500 | 100 ± 1.49 |
| 400 | 100 ± 3.8 | - | - |
| - | - | 250 | 14.57 ± 0.72 |
| 200 | 100 ± 0.7 | - | - |
| - | - | 125 | 7.28 ± 1.18 |
| 100 | 64 ± 1.7 | - | - |
| - | - | 62.5 | 6.06 ± 7.72 |
| 50 | 7.0 ± 2.3 | - | - |
| - | - | 31.25 | 0.0 ± 4.09 |
| 25 | 1.4 ± 1.4 | - | - |
| IC50 (µg/mL) | 82.39 ± 2.16 | 301.42 ± 3.1 |
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material and Isolation of Essential Oil
4.3. Gas Chromatography Coupled with Mass Spectrometry (GC/MS) Analysis
4.4. Cell Lines Used
4.5. Trypanocidal Assay
4.6. Leishmanicidal Assay
4.7. Cytotoxicity Assay
4.8. Statistical Analysis
5. Conclusion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- WHO-World Health Organization. Leishmaniasis Magnitude of the Problem, 2010. Available online: http://www.who.int/leishmaniasis/burden/magnitude/burden_magnitude/en/print.html (assessed on 1 October 2015).
- Shaw, J.J. Taxonomy of the genus Leishmania: Present and future trends and their implications. Mem. Inst. Oswaldo Cruz 1994, 89, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Desjeux, P. Leishmaniasis: Public health aspects and control. Clin. Dermatol. 1996, 14, 417–423. [Google Scholar] [CrossRef]
- Ashford, R.W. The leishmaniases as model zoonoses. Ann. Trop. Med. Parasitol. 1997, 91, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Herwaldt, B.L. Leishmaniasis. Lancet 1999, 354, 1191–1199. [Google Scholar] [CrossRef]
- Croft, S.L.; Sundar, S.; Fairlamb, A.H. Drug resistance in Leishmaniasis. Clin. Microbiol. Rev. 2006, 19, 111–126. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.K.; de Toledo, J.S.; Falade, M.; Terrao, M.C.; Kamchonwongpaisan, S.; Kyle, D.E.; Uthaipibull, C. Current treatment and drug discovery against Leishmania spp. and Plasmodium spp.: A review. Curr. Drug Targets 2009, 10, 178–192. [Google Scholar] [CrossRef]
- WHO-World Health Organization. Neglected Tropical Diseases–Innovative and Intensified Disease Management; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Mishra, B.B.; Singh, R.K.; Srivastava, A.; Tripathi, V.J.; Tiwari, V.K. Fighting against leishmaniasis: Search of alkaloids as future true potential anti-leishmanial agents. Mini-Rev. Med. Chem. 2009, 9, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Croft, S.L.; Coombs, G.H. Leishmaniasis-current chemotherapy and recent advances in the search for novel drugs. Trends Parasitol. 2003, 19, 502–508. [Google Scholar] [CrossRef] [PubMed]
- Natera, S.; Machuca, C.; Padrón-Nieves, M.; Romero, A.; Diaz, E.; Ponte-Sucre, A. Leishmania spp.: Proficiency of drug-resistant parasites. Int. J. Antimicrob. Agents 2007, 29, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Clem, A.A. Current perspective on Leishmaniasis. J. Glob. Infect. Dis. 2010, 2, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A., Jr.; Rassi, A.; Marin-Neto, J.A.M. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Mougabure-Cueto, G.; Picollo, M.I. Insecticide resistance in vector Chagas disease: Evolution, mechanisms and management. Acta Trop. 2015, 149, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Coura, J.R.; de Castro, S.L. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz 2002, 97, 3–24. [Google Scholar] [CrossRef]
- Soeiro, M.N.; de Castro, S.L. Trypanosoma cruzi targets for new chemotherapeutic approaches. Expert Opin. Ther. Targets 2009, 13, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.G.; Almeida, J.R.G.S.; Macêdo, R.O.; Barbosa-Filho, J.M. A review of natural products with antileishmanial activity. Phytomedicine 2005, 12, 514–535. [Google Scholar] [CrossRef] [PubMed]
- Ndjonka, D.; Rapado, L.N.; Silber, A.M.; Liebau, E.; Wrenger, C. Natural products as a source for treatting negleted parasitic diseases. Int. J. Mol. Sci. 2013, 14, 3395–3439. [Google Scholar] [CrossRef] [PubMed]
- Derda, M.; Hadas, E. The use of phytotherapy in diseases caused by parasitic protozoa. Acta Parasitol. 2014, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Makkar, H.P.; Dawra, R.K. A review of the noxious plant Lantana camara. Toxicon 1988, 26, 975–987. [Google Scholar] [CrossRef]
- Ghisalberti, E.L. Lantana camara Linn (Review). Fitoterapia 2000, 71, 467–485. [Google Scholar] [CrossRef]
- Day, M.D.; Wiley, C.J.; Playford, J.; Zalucki, M.P. Lantana: Current Management Status and Future Prospects; ACIAR Monograph 102; Australian Centre for International Agricultural Research: Canberra, Australia, 2003.
- Kalita, S.; Kumar, G.; Karthik, L.; Rao, K.V.B. A review on medicinal properties of Lantana camara. Res. J. Pharm. Technol. 2012, 5, 771–775. [Google Scholar]
- Bhagwat, S.A.; Breman, E.; Thekaekara, T.; Thornton, T.F.; Willis, K.J. A battle lost? Report on two centuries of invasion and management of Lantana camara L. in Australia, India and South Africa. PLoS ONE 2012, 7, e32407. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Makkar, H.P.; Dawra, R.K.; Negi, S.S. A review of the toxicity of Lantana camara (Linn) in animals. Clin. Toxicol. 1981, 18, 1077–1094. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.K.; Shukla, B.N. Antifungal activity of some plant extracts against Fusarium oxysporum sp. causing wilt of linseed. J. Mycol. Plant Pathol. 2002, 32, 266–267. [Google Scholar]
- Braga, F.G.; Bouzada, M.L.M.; Fabri, R.L.; Matos, M.O.; Moreira, F.O.; Scio, E.; Coimbra, E.S. Antileishmanial and antifungal activity of plants used in traditional medicine in Brazil. J. Ethnopharmacol. 2007, 111, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Deena, M.J.; Thoppil, J.E. Antimicrobial activity of the essential oil of Lantana camara. Fitoterapia 2000, 71, 453–455. [Google Scholar] [CrossRef]
- Begum, S.; Wahab, A.; Siddiqui, B.S. Pentacyclic tri-terpenoids from the aerial parts of Lantana camara. Chem. Pharm. Bull. 2003, 51, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.P.; Chauhan, N.S.; Padh, H.; Rajani, M. Search for antibacterial and antifungal agents from selected Indian medicinal plants. J. Ethnopharmacol. 2006, 107, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Seth, R.; Mohan, M.; Singh, P.; Haider, S.Z.; Gupta, S.; Bajpai, I.; Singh, D.; Dobhal, R. Chemical composition and antibacterial properties of the essential oil and extracts of Lantana camara Linn. from Uttarakhand (India). Asian Pac. J. Trop. Biomed. 2012, 2, S1407–S1411. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 1995; p. 456. [Google Scholar]
- Ibrahim, M.A.; Mohammed, A.; Isah, M.B.; Aliyu, A.B. Anti-trypanosomal activity of African medicinal plants: A review update. J. Ethnopharmacol. 2014, 154, 26–54. [Google Scholar] [CrossRef] [PubMed]
- Machado, R.R.P.; Júnior, W.V.; Lesche, B.; Coimbra, E.S.; de Souza, N.B.; Abramo, C.; Soares, G.L.G.; Kaplan, M.A.C. Essential oil from leaves of Lantana camara: A potential source of medicine against leishmaniasis. Braz. J. Pharmacogn. 2012, 22, 1011–1017. [Google Scholar] [CrossRef]
- Morais-Teixeira, E.; Carvalho, A.S.; Costa, J.C.S.; Duarte, S.L.; Mendonça, J.S.; Boechat, N.; Rabello, A. In vitro and in vivo activity of meglumine antimoniate produced at Farmanguinhos-Fiocruz, Brazil, against Leishmania (Leishmania) amazonensis, L (L.) chagasi and L (Viannia) braziliensis. Mem. Inst. Oswaldo Cruz. 2008, 103, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.B.; Sghaier, R.M.; Guesmi, F.; Kaabi, B.; Mejri, M.; Attia, H.; Laouini, D.; Smaal, I. Evaluation of antileishmanial, cytotoxic and antioxidant activities of essential oils extracted from plants issued from leishmaniasis-endemic region of Sned (Tunisia). Nat. Prod. Res. 2011, 25, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Sharma, S.; Pattabhi Pattabhi, V.; Mahato, S.B.; Sharma, P.D. A review of the hepatotoxic plant Lantana camara. Crit. Rev. Toxicol. 2007, 37, 313–352. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Díaz, R.A.; Ibánez-Escribano, A.; Burillo, J.; de la Heras, L.; del Prado, G.; Agulló-Ortuno, M.T.; Julio, L.F.; Gonzáles-Coloma, A. Thrypanocidal, trichomonacidal and cytotoxic components of cultivated Artemisia absinthium Linnaeus (Asteraceae) essential oil. Mem. Inst. Oswaldo Cruz. 2015, 110, 693–699. [Google Scholar] [CrossRef] [PubMed]
- Charneau, S.; de Mesquita, M.L.; Bastos, I.M.; Santana, J.M.; de Paula, J.E.; Grellier, P.; Espindola, L.S. In vitro investigation of Brazilian Cerrado plant extract activity against Plasmodium falciparum, Trypanosoma cruzi and T. brucei gambiense. Nat. Prod. Res. 2015, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.V.; Dutra, L.M.; Salvador, M.J.; Ribeiro, L.H.; Gadelha, F.R.; de Carvalho, J.E. Chemical composition of the essential oils of Annona pickelii and Annona salzmannii (Annonaceae), and their antitumor and trypanocidal activities. Nat. Prod. Res. 2013, 27, 997–1001. [Google Scholar] [CrossRef] [PubMed]
- Guenther, E. Individual Essential Oils of the Plant Families Rutaceae and Labiatae; Robert, E., Ed.; Krieger Publishing Company, Inc.: New York, NY, USA, 1975; Volume 3, pp. 316–319. [Google Scholar]
- Le Senne, A.; Muelas-Serrano, S.; Fernandez-Portillo, C.; Escario, J.A.; Gómez-Barrio, A. Biological characterization of a beta-galactosidase expressing clone of Trypanosoma cruzi CL strain. Mem. Inst. Oswaldo Cruz 2002, 97, 1101–1105. [Google Scholar] [CrossRef] [PubMed]
- Roldós, V.; Nakayama, H.; Rolón, M.; Montero-Torres, A.; Trucco, F.; Torres, S.; Vega, C.; Marrero-Ponce, Y.; Heguaburu, V.; Yaluff, G.; et al. Activity of a hydroxybibenzyl bryophyte constituent against Leishmania spp. and Trypanosoma cruzi: In silico, in vitro and in vivo activity studies. Eur. J. Med. Chem. 2008, 43, 1797–1807. [Google Scholar] [CrossRef] [PubMed]
- Vega, C.; Rólon, M.; Martínez-Fernández, A.R.; Escario, J.A.; Gómez-Barrio, A. A new pharmacological screening assay with Trypanosoma cruzi epimastigotes expressing β-galactosidase. Parasitol. Res. 2005, 95, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barros, L.M.; Duarte, A.E.; Morais-Braga, M.F.B.; Waczuk, E.P.; Vega, C.; Leite, N.F.; De Menezes, I.R.A.; Coutinho, H.D.M.; Rocha, J.B.T.; Kamdem, J.P. Chemical Characterization and Trypanocidal, Leishmanicidal and Cytotoxicity Potential of Lantana camara L. (Verbenaceae) Essential Oil. Molecules 2016, 21, 209. https://doi.org/10.3390/molecules21020209
Barros LM, Duarte AE, Morais-Braga MFB, Waczuk EP, Vega C, Leite NF, De Menezes IRA, Coutinho HDM, Rocha JBT, Kamdem JP. Chemical Characterization and Trypanocidal, Leishmanicidal and Cytotoxicity Potential of Lantana camara L. (Verbenaceae) Essential Oil. Molecules. 2016; 21(2):209. https://doi.org/10.3390/molecules21020209
Chicago/Turabian StyleBarros, Luiz Marivando, Antonia Eliene Duarte, Maria Flaviana Bezerra Morais-Braga, Emily Pansera Waczuk, Celeste Vega, Nadghia Figueiredo Leite, Irwin Rose Alencar De Menezes, Henrique Douglas Melo Coutinho, João Batista Teixeira Rocha, and Jean Paul Kamdem. 2016. "Chemical Characterization and Trypanocidal, Leishmanicidal and Cytotoxicity Potential of Lantana camara L. (Verbenaceae) Essential Oil" Molecules 21, no. 2: 209. https://doi.org/10.3390/molecules21020209
APA StyleBarros, L. M., Duarte, A. E., Morais-Braga, M. F. B., Waczuk, E. P., Vega, C., Leite, N. F., De Menezes, I. R. A., Coutinho, H. D. M., Rocha, J. B. T., & Kamdem, J. P. (2016). Chemical Characterization and Trypanocidal, Leishmanicidal and Cytotoxicity Potential of Lantana camara L. (Verbenaceae) Essential Oil. Molecules, 21(2), 209. https://doi.org/10.3390/molecules21020209