Antimutagenic Effects of Selenium-Enriched Polysaccharides from Pyracantha fortuneana through Suppression of Cytochrome P450 1A Subfamily in the Mouse Liver
Abstract
:1. Introduction
2. Results
2.1. Anti-Mutagenic Effects of Se-PFPs in Mice
2.2. Antioxidative Activity of Se-PFPs in Liver of Mice
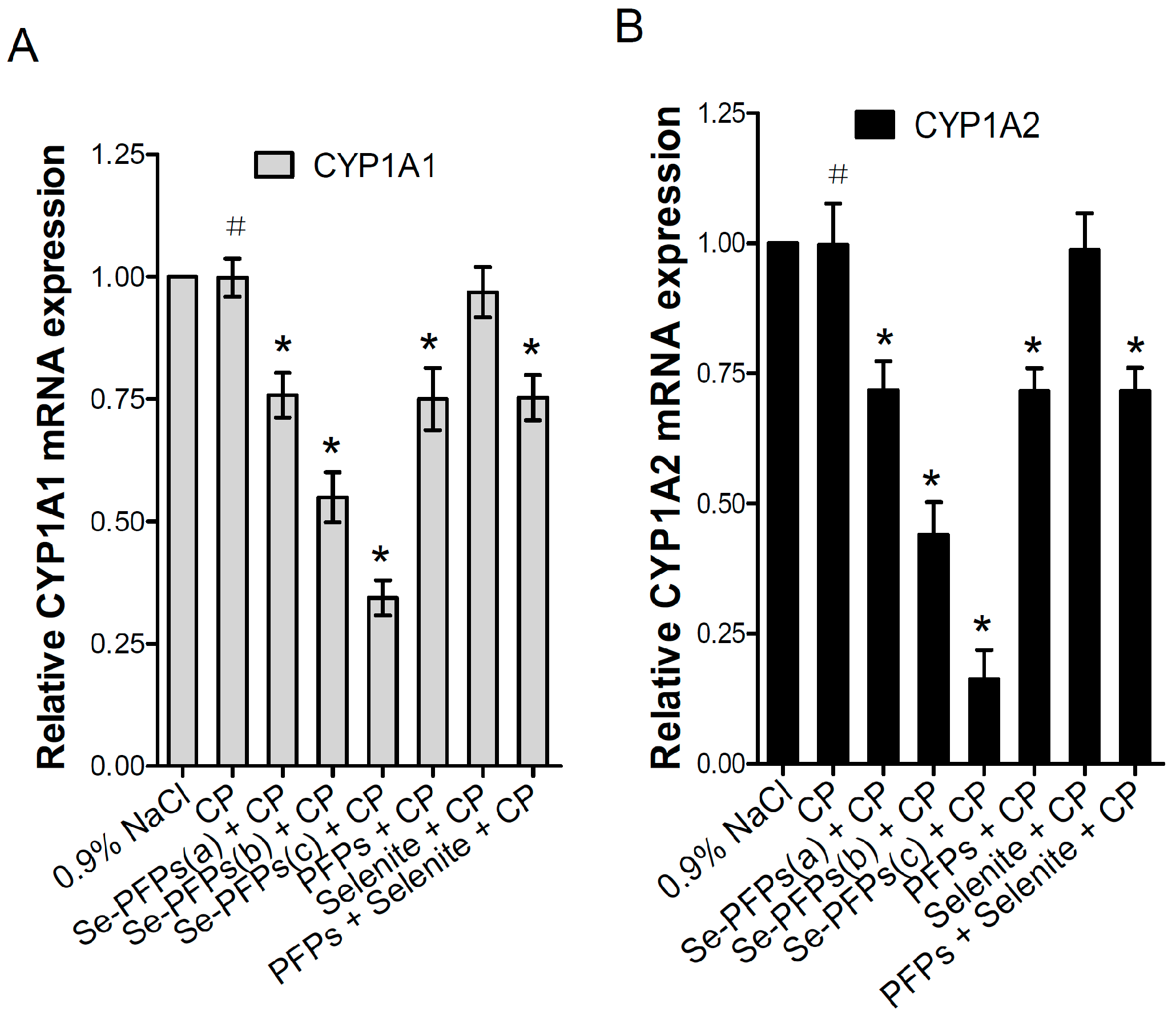
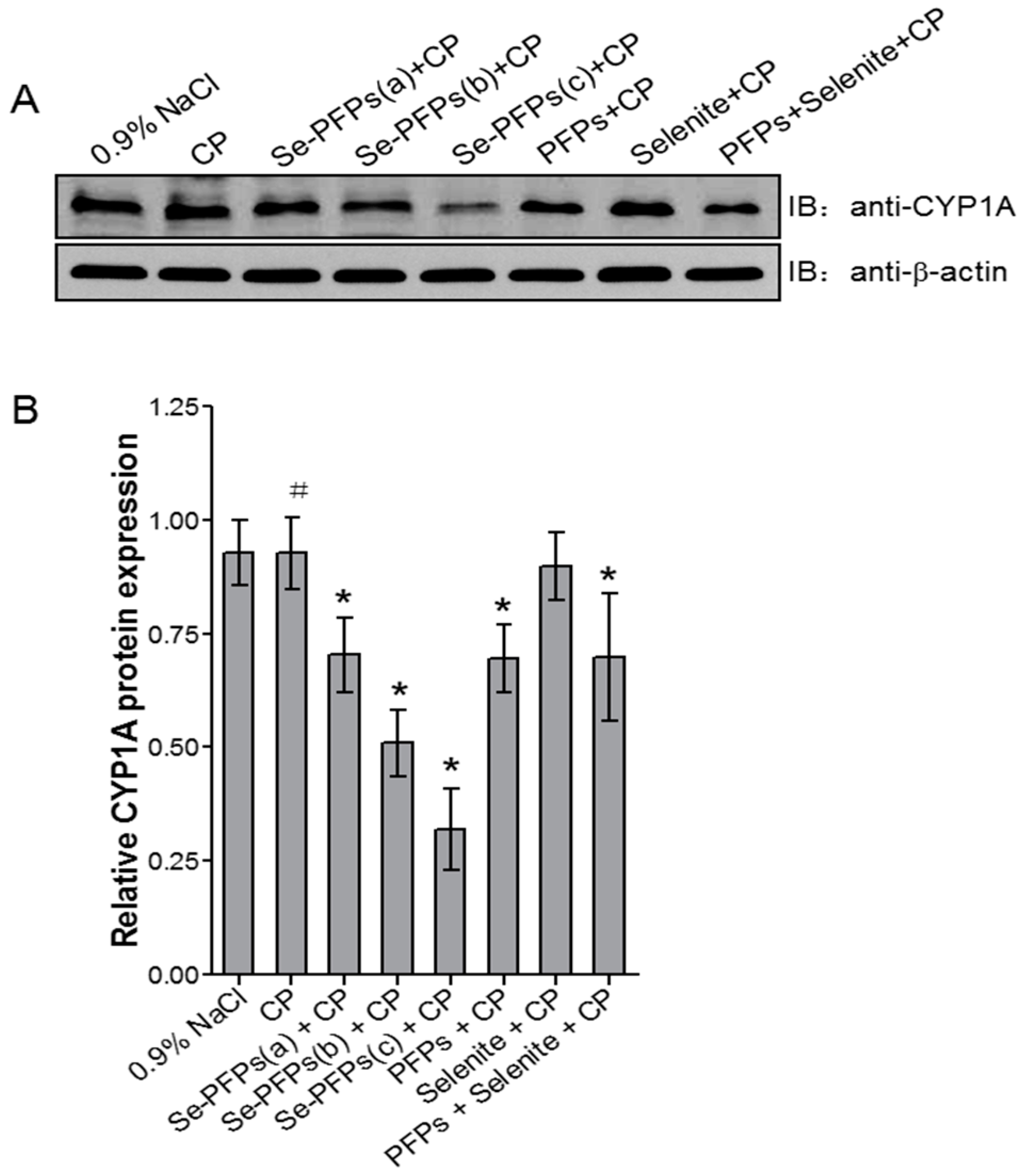
2.3. Influence of Se-PFPs on Heaptic Cytochrome P450 1A (CYP1A)
3. Discussions
4. Experimental Section
4.1. Materials and Chemicals
4.2. Preparation of Polysaccharides from Se-Enriched P. fortuneana (Se-PFPs)
4.3. Animals
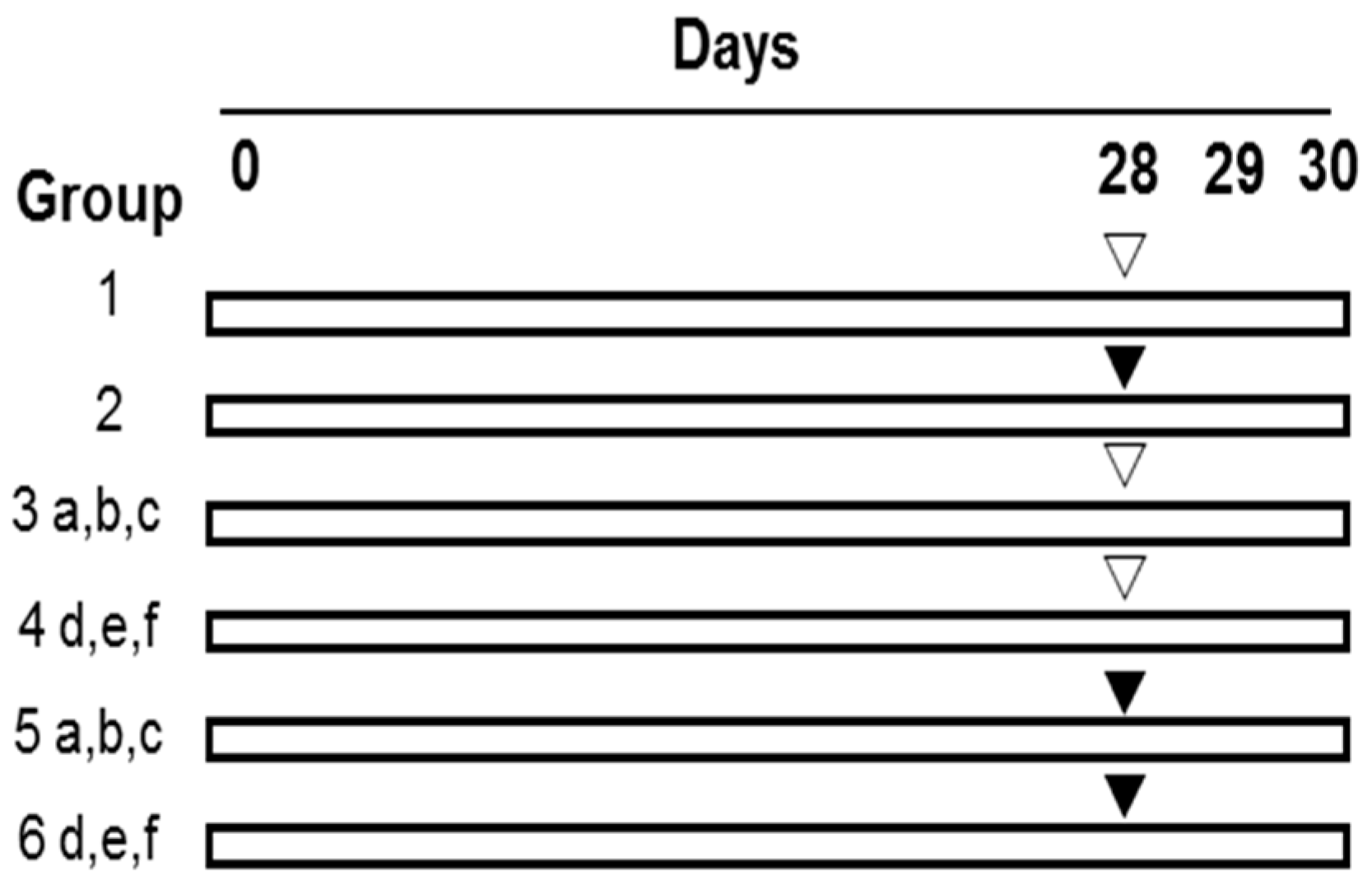
4.4. Experimental Design
4.5. Bone Marrow Micronucleus (MN) Assay
4.6. Peripheral Blood MN Assay
4.7. Measurement of Superoxide Dismutase (SOD) and Glutathione Peroxidase (GPx)
4.8. Measurement of Activity of Cytochrome P450 1A (CYP1A)
4.9. RT-PCR Analysis
4.10. Western Blot
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sarasin, A. An overview of the mechanisms of mutagenesis and carcinogenesis. Mutat. Res. 2003, 544, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Mathews, C.K. Deoxyribonucleotide metabolism, mutagenesis and cancer. Nat. Rev. Cancer 2015, 15, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Stratton, M.R.; Campbell, P.J.; Futreal, P.A. The cancer genome. Nature 2009, 458, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.A.; Bindal, N.; Bamford, S.; Cole, C.; Kok, C.Y.; Beare, D.; Jia, M.; Shepherd, R.; Leung, K.; Menzies, A.; et al. COSMIC: Mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011, 39, D945–D950. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.J.; Ratner, E.; Leng, S.; Zhai, R.; Nallur, S.; Babar, I.; Muller, R.U.; Straka, E.; Su, L.; Burki, E.A.; et al. A SNP in a let-7 microRNA complementary site in the KRAS 3′ untranslated region increases non-small cell lung cancer risk. Cancer Res. 2008, 68, 8535–8540. [Google Scholar] [CrossRef] [PubMed]
- Mayr, C.; Bartel, D.P. Widespread shortening of 3′UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell 2009, 138, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xu, L.; Wei, Q.; Song, X.; Sturgis, E.M.; Li, G. Significance of MDM2 and P14 ARF polymorphisms in susceptibility to differentiated thyroid carcinoma. Surgery 2013, 153, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.J.; Ashworth, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Karanika, S.; Karantanos, T.; Li, L.; Corn, P.G.; Thompson, T.C. DNA damage response and prostate cancer: Defects, regulation and therapeutic implications. Oncogene 2015, 34, 2815–2822. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C. Oxidative stress in Alzheimer’s disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.J. Pathogenesis of chronic hyperglycemia: From reductive stress to oxidative stress. J. Diabetes Res. 2014, 2014, 137919. [Google Scholar] [CrossRef] [PubMed]
- Perl, A. Oxidative stress in the pathology and treatment of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2013, 9, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Bernatsky, S.; Clarke, A.E.; Suissa, S. Hematologic malignant neoplasms after drug exposure in rheumatoid arthritis. Arch. Intern. Med. 2008, 168, 378–381. [Google Scholar] [CrossRef] [PubMed]
- Barton, T.S.; Robaire, B.; Hales, B.F. Epigenetic programming in the preimplantation rat embryo is disrupted by chronic paternal cyclophosphamide exposure. Proc. Natl. Acad. Sci. USA 2005, 102, 7865–7870. [Google Scholar] [CrossRef] [PubMed]
- Peiffer, R.L.; McCullen, R.; Alles, A.J.; Sulik, K.K. Relationship of cell death to cyclo-phosphamide-induced ocular malformations. Teratog. Carcinog. Mutagen. 1991, 11, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Huong, D.L.; Amoura, Z.; Duhaut, P.; Sbai, A.; Costedoat, N.; Wechsler, B.; Piette, J.C. Risk of ovarian failure and fertility after intravenous cyclophosphamide. A study in 84 patients. J. Rheumatol. 2002, 29, 2571–2576. [Google Scholar] [PubMed]
- Vaux, K.K.; Kahole, N.C.; Jones, K.L. Cyclophosphamide, methotrexate, and cytarabine embropathy: Is apoptosis the common pathway? Birth Defects Res. A Clin. Mol. Teratol. 2003, 67, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Ujházy, E.; Balonová, T.; Durisová, M.; Gajdosík, A.; Jansák, J.; Molnárová, A. Teratogenicity of cyclophosphamide in New Zealand white rabbits. Neoplasma 1993, 40, 45–49. [Google Scholar] [PubMed]
- Cohen, J.L.; Jao, J.Y. Enzymatic basis of cyclophosphamide activation by hepatic microsomes of the rat. J. Pharmacol. Exp. Ther. 1972, 174, 206–210. [Google Scholar]
- Deng, R.F.; Wang, S.G.; Li, G.R. Studies on the nutritional components of the fruit of the wild plant-firethorn. Acta Nutr. Sin. 1990, 1, 79–84. [Google Scholar]
- Dai, Y.; Zhou, G.X.; Kurihara, H.; Ye, W.C.; Yao, X.S. Fortuneanosides G-L, dibenzofuran glycosides from the fruit of Pyracantha fortuneana. Chem. Pharm. Bull. 2008, 56, 439–442. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wang, C.; Bu, Y.; Xiang, T.; Huang, X.; Wang, Z.; Yi, F.; Ren, G.; Liu, G.; Song, F. Antioxidative and immunoprotective effects of Pyracantha fortuneana (Maxim.) Li polysaccharides in mice. Immunol. Lett. 2010, 133, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Li, Z.; Yi, M.; Wang, X.; Peng, F.; Xiao, F.; Chen, T.; Wang, C.; Mushtaq, G.; Kamal, M.A. Hepatoprotective effects of polysaccharides from selenium-enriched Pyracantha fortuneana on mice liver injury. Med. Chem. 2015, 11, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.F.; Lei, D.J.; Song, G.H.; Zhang, H.; Xu, H.; Yu, L.J. Characterisation of water-soluble proanthocyanidins of Pyracantha fortuneana fruit and their improvement in cell bioavailable antioxidant activity of quercetin. Food Chem. 2015, 169, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Ramya, S.; Shanmugasundaram, T.; Balagurunathan, R. Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J. Trace Elem. Med. Biol. 2015, 32, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Kahya, M.C.; Naziroglu, M.; Cig, B. Melatonin and selenium reduce plasma cytokine and brain oxidative stress levels in diabetic rats. Brain Inj. 2015, 12, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F. Selenium, an antioxidant nutrient. Nutr. Clin. Care 2002, 5, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Molan, A.L. Antioxidant and prebiotic activities of selenium-containing green tea. Nutrition 2013, 29, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N. Effects of selenium and low levels of lead on mammary tumor development and growth in MMTV-infected female mice. Biol. Trace Elem. Res. 2008, 125, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, A.C.; Barbosa-Ribeiro, A.; Alves, V.; Silva, T.; Sarmento-Ribeiro, A.B. Selenium compounds induced ROS-dependent apoptosis in myelodysplasia cells. Biol. Trace Elem. Res. 2013, 154, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Zhu, L.Y.; Yang, B.S.; Shi, L.J.; Liu, Y.; Jiang, A.M.; Zhao, L.L.; Song, G.; Liu, T.F. Antitumor and immunomodulating activity of a polysaccharide from Sophora flavescens Ait. Int. J. Biol. Macromol. 2012, 51, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Xin, T.; Zhang, F.; Jiang, Q.; Chen, C.; Huang, D.; Li, Y.; Shen, W.; Jin, Y. Extraction, purification and antitumor activity of a water-soluble polysaccharide from the roots of Polygala tenuifolia. Carbohydr. Polym. 2012, 90, 1127–1131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lu, C.; Wu, C.; Xu, M.; Kou, X.; Kong, D.; Jing, G. Polysaccharide of Boschniakia rossica induces apoptosis on laryngeal carcinoma Hep2 cells. Gene 2014, 536, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Bian, F.; Yue, L.; Jin, H.; Hong, Z.; Shu, G. Selenium-dependent antitumor immuno-modulating activity of polysaccharides from roots of A. membranaceus. Int. J. Biol. Macromol. 2014, 69, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Hu, Y.; Luo, Y.; Yang, X. Selenium-containing polysaccharides from Ziyang green tea ameliorate high-fructose diet induced insulin resistance and hepatic oxidative stress in mice. Food Funct. 2015, 6, 3342–3350. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wang, C.; Wang, J.; Kumar, V.; Anwar, F.; Xiao, F.; Mushtaq, G.; Liu, Y.; Kamal, M.A.; Yuan, D. Inhibition on the growth of human MDA-MB-231 breast cancer cells in vitro and tumor growth in a mouse xenograft model by Se-containing polysaccharides from Pyracantha fortuneana. Nutr. Res. 2016, 36, 1243–1254. [Google Scholar] [PubMed]
- Hashimoto, T.; Nonaka, Y.; Minato, K.; Kawakami, S.; Mizuno, M.; Fukuda, I.; Kanazawa, K.; Ashida, H. Suppressive effect of polysaccharides from the edible and medicinal mushrooms, Lentinus edodes and Agaricus blazei, on the expression of cytochrome P450s in mice. Biosci. Biotechnol. Biochem. 2002, 66, 1610–1614. [Google Scholar] [CrossRef] [PubMed]
- Vondracek, J.; Umannova, L.; Machala, M. Interactions of the aryl hydrocarbon receptor with inflammatory mediators: Beyond CYP1A regulation. Curr. Drug Metab. 2011, 12, 89–103. [Google Scholar] [CrossRef] [PubMed]
- El-Bayoumy, K. The protective role of selenium on genetic damage and on cancer. Mutat. Res. 2001, 475, 123–139. [Google Scholar] [CrossRef]
- Heddle, J.A.; Hite, M.; Kirkhart, B.; Mavourvin, K.; Macgregor, J.T.; Nowell, G.W.; Salamone, M.F. The induction of micronuclei as a measure of genotoxicity: A report of the U.S. environmental protection agency Gene-Tox program. Mutat. Res. 1983, 123, 61–118. [Google Scholar] [CrossRef]
- Sinha, R.; El-Bayoumy, K. Apoptosis is a critical cellular event in cancer chemoprevention and chemotherapy by selenium compounds. Curr. Cancer Drug Targets 2004, 4, 13–28. [Google Scholar] [CrossRef] [PubMed]
- Rikiishi, H. Apoptotic cellular events for selenium compounds involved in cancer prevention. J. Bioenerg. Biomembr. 2007, 39, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Drake, E.N. Cancer chemoprevention: Selenium as a prooxidant, not an antioxidant. Med. Hypotheses 2006, 67, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Brozmanova, J.; Manikova, D.; Vlckova, V.; Chovanec, M. Selenium: A double-edged sword for defense and offence in cancer. Arch. Toxicol. 2010, 84, 919–938. [Google Scholar] [CrossRef] [PubMed]
- An, J.J.; Shi, K.J.; Wei, W.; Hua, F.Y.; Ci, Y.L.; Jiang, Q.; Li, F.; Wu, P.; Hui, K.Y.; Yang, Y.; et al. The ROS/JNK/ATF2 pathway mediates selenite-induced leukemia NB4 cell cycle arrest and apoptosis in vitro and in vivo. Cell Death Dis. 2013, 4, e973. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Li, Z.; Peng, F.; Xiao, F.; Ren, D.; Xue, H.; Chen, T.; Mushtaq, G.; Kamal, M.A. Combination of selenium-enriched green tea polysaccharides and Huo-ji polysaccharides synergistically enhances antioxidant and immune activity in mice. J. Sci. Food Agric. 2015, 95, 3211–3217. [Google Scholar] [CrossRef] [PubMed]
- Mate, J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Indo, H.P.; Yen, H.C.; Nakanishi, I.; Matsumoto, K.; Tamura, M.; Nagano, Y.; Matsui, H.; Gusev, O.; Cornette, R.; Okuda, T.; et al. A mitochondrial superoxide theory for oxidative stress diseases and aging. J. Clin. Biochem. Nutr. 2015, 56, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Coon, M.J. Cytochrome P450: Nature’s most versatile biological catalyst. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Bernhardt, R. Cytochromes P450 as versatile biocatalysts. J. Biotechnol. 2006, 124, 128–145. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Guengerich, F.P. Cytochrome P450 activation of arylamines and heterocyclic amines. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 27–49. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Lu, A.Y.H. CYP1A induction and human risk assessment: An evolving tale of in vitro and in vivo studies. Drug Metab. Dispos. 2007, 35, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q. Induction of CYP1A1. The AhR/DRE paradigm: Transcription, receptor regulation, and expanding biological roles. Curr. Drug Metab. 2001, 2, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.K.; Weber, G.F.; Crespi, C.L.; Waxman, D.J. Differential activation of cyclo-phosphamide and ifosphamide by cytochromes P-450 2B and 3A in human liver microsomes. Cancer Res. 1993, 53, 5629–5637. [Google Scholar] [PubMed]
- Ren, S.; Yang, J.S.; Kalhorn, T.F.; Slattery, J.T. Oxidation of cyclophosphamide to 4-hydroxy-cyclophosphamide and deschloroethyl-cyclophosphamide in human liver microsomes. Cancer Res. 1997, 57, 4229–4235. [Google Scholar] [PubMed]
- Morgan, E.T. Regulation of cytochrome P450 by inflammatory mediators: Why and how? Drug Metab. Dispos. 2001, 29, 207–212. [Google Scholar]
- Barger, J.L.; Kayo, T.; Pugh, T.D.; Vann, J.A.; Power, R.; Dawson, K.; Weindruch, R.; Prolla, T.A. Gene expression profiling reveals differential effects of sodium selenite, selenomethionine, and yeast-derived selenium in the mouse. Genes Nutr. 2011, 7, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Burk, R.F.; Norsworthy, B.K.; Hill, K.E.; Motley, A.K.; Byrne, D.W. Effects of chemical form of selenium on plasma biomarkers in a high-dose human supplementation trial. Cancer Epidemiol. Biomark. Prev. 2006, 15, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Endo, M.; Shinohara, F.; Echigo, S.; Rikiishi, H. Differential apoptotic response of human cancer cells to organoselenium compounds. Cancer Chemother Pharmacol. 2010, 66, 475–484. [Google Scholar] [CrossRef] [PubMed]
- Peters, U.; Takata, Y. Selenium and the prevention of prostate and colorectal cancer. Mol. Nutr. Food Res. 2008, 52, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, K.; Willett, W.C.; Morris, S.J.; Stampfer, M.J.; Spiegelman, D.; Rimm, E.B.; Giovannucci, E. Study of prediagnostic selenium level in toenails and the risk of advanced prostate cancer. J. Natl. Cancer Inst. 1998, 90, 1219–1224. [Google Scholar] [CrossRef] [PubMed]
- Ghadirian, P.; Maisonneuve, P.; Perret, C.; Kennedy, G.; Boyle, P.; Krewski, D.; Lacroix, A. A case-control study of toenail selenium and cancer of the breast, colon, and prostate. Cancer Detect. Prev. 2000, 24, 305–313. [Google Scholar] [PubMed]
- Kusunoki, Y.; Ikarashi, N.; Hayakawa, Y.; Ishii, M.; Kon, R.; Ochiai, W.; Machida, Y.; Sugiyama, K. Hepatic early inflammation induces downregulation of hepatic cytochrome P450 expression and metabolic activity in the dextran sulfate sodium-induced murine colitis. Eur. J. Pharm. Sci. 2014, 54, 17–27. [Google Scholar] [CrossRef] [PubMed]
- MacGregor, J.T.; Heddle, J.A.; Hite, M.; Margolin, B.H.; Ramel, C.; Salamone, M.F.; Tice, R.R.; Wild, D. Guidelines for the conduct of micronucleus assays in mammalian bone marrow erythrocytes. Mutat. Res. 1987, 189, 103–112. [Google Scholar] [CrossRef]
- Manoharan, K.; Banerjee, M.R. beta-Carotene reduces sister chromatid exchanges induced by chemical carcinogens in mouse mammary cells in organ culture. Cell Biol. Int. Rep. 1985, 9, 783–789. [Google Scholar] [CrossRef]
- Waters, M.D.; Brady, A.L.; Stack, H.F.; Brockman, H.E. Antimutagenicity profiles for some model compounds. Mutat. Res. 1990, 238, 57–85. [Google Scholar] [CrossRef]
- Hayashi, M.; Morita, T.; Kodama, Y.; Sofuni, T.; Ishidate, M., Jr. The micronucleus assay with mouse peripheral blood reticulocytes using acridine orange-coated slides. Mutat. Res. 1990, 245, 245–249. [Google Scholar] [CrossRef]
- Helsby, N.A.; Zhu, S.; Pearson, A.E.; Tingle, M.D.; Ferguson, L.R. Antimutagenic effects of wheat bran diet through modification of xenobiotic metabolising enzymes. Mutat. Res. 2000, 454, 77–88. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
| Group | Body Weight Changes during the Experiment (g) | Increased Body Weight (g) | Liver Index (%) | |
|---|---|---|---|---|
| Initial Body Weight | Final Body Weight | |||
| 0.9% NaCl | 25.47 ± 1.86 | 35.28 ± 2.37 | 9.81 ± 0.94 | 4.72 ± 0.23 |
| CP | 26.30 ± 1.05 | 35.47 ± 2.02 | 9.17 ± 1.45 | 5.06 ± 0.39 |
| Se-PFPs (a) + NaCl | 26.51 ± 1.50 | 36.27 ± 1.27 | 10.07 ± 1.04 | 5.98 ± 0.22 |
| Se-PFPs (b) + NaCl | 25.50 ± 1.31 | 34.93 ± 3.12 | 9.32 ± 1.84 | 5.28 ± 0.54 |
| Se-PFPs (c) + NaCl | 26.41 ± 1.51 | 36.43 ± 2.17 | 10.15 ± 1.21 | 5.08 ± 0.31 |
| PFPs + NaCl | 25.47 ± 1.92 | 35.10 ± 2.21 | 9.63 ± 1.62 | 5.62 ± 0.51 |
| Se + NaCl | 25.46 ± 2.06 | 35.37 ± 1.64 | 9.91 ± 1.24 | 5.44 ± 0.47 |
| PFPs + Se + NaCl | 26.57 ± 1.35 | 36.98 ± 2.17 | 10.42 ± 1.13 | 5.52 ± 0.65 |
| Se-PFPs (a) + CP | 25.93 ± 1.29 | 35.67 ± 2.14 | 9.76 ± 1.64 | 5.37 ± 0.45 |
| Se-PFPs (b) +CP | 25.60 ± 2.13 | 34.92 ± 2.67 | 9.34 ± 1.88 | 5.42 ± 0.61 |
| Se-PFPs (c) + CP | 25.69 ± 2.40 | 35.54 ± 1.87 | 9.88 ± 2.02 | 4.91 ± 0.69 |
| PFPs + CP | 26.60 ± 1.47 | 36.71 ± 2.24 | 10.12 ± 1.67 | 5.25 ± 0.56 |
| Se + CP | 26.52 ± 1.42 | 36.39 ± 2.53 | 9.87 ± 1.45 | 5.67 ± 0.46 |
| PFPs + Se + CP | 25.89 ± 2.14 | 35.32 ± 1.77 | 9.44 ± 2.55 | 5.38 ± 0.50 |
| Group | No. of Animal | Polysaccharide Dose (g/kg·BW) | Se Dose (μg/kg·BW) | No. of Analyzed Cells | MNPEC | Reduction (%) | |
|---|---|---|---|---|---|---|---|
| No. | % | ||||||
| 0.9% NaCl | 10 | 0 | 0 | 10,000 | 31 ± 3 | 0.31 ± 0.03 | |
| CP | 10 | 0 | 0 | 10,000 | 624 ± 17 | 6.24 ± 0.17 a | |
| Se-PFPs (a) + NaCl | 10 | 1.35 | 5 | 10,000 | 25 ± 5 | 0.25 ± 0.05 | |
| Se-PFPs (b) + NaCl | 10 | 2.7 | 10 | 10,000 | 26 ± 4 | 0.26 ± 0.04 | |
| Se-PFPs (c) + NaCl | 10 | 5.4 | 20 | 10,000 | 29 ± 4 | 0.29 ± 0.04 | |
| PFPs + NaCl | 10 | 2.7 | 0.108 | 10,000 | 28 ± 5 | 0.28 ± 0.05 | |
| Se + NaCl | 10 | 0 | 10 | 10,000 | 27 ± 4 | 0.27 ± 0.04 | |
| PFPs + Se + NaCl | 10 | 2.7 | 10 | 10,000 | 25 ± 4 | 0.25 ± 0.04 | |
| Se-PFPs (a) + CP | 10 | 1.35 | 5 | 10,000 | 281 ± 9 | 2.81 ± 0.09 b | 57.8 |
| Se-PFPs (b) +CP | 10 | 2.7 | 10 | 10,000 | 186 ± 7 | 1.86 ± 0.07 b | 73.9 |
| Se-PFPs (c) + CP | 10 | 5.4 | 20 | 10,000 | 112 ± 10 | 1.12 ± 0.10 b,d | 86.3 |
| PFPs + CP | 10 | 2.7 | 0.108 | 10,000 | 377 ± 10 | 3.77 ± 0.10 b | 41.7 |
| Se + CP | 10 | 0 | 10 | 10,000 | 362 ± 9 | 3.62 ± 0.09 b,c | 44.2 |
| PFPs + Se + CP | 10 | 2.7 | 10 | 10,000 | 253 ± 9 | 2.53 ± 0.09 b,c | 62.6 |
| Group | No. of Animal | Polysaccharides Dose (g/kg·BW) | Se Dose (μg/kg·BW) | No. of Analyzed Cells | MNRET | Reduction (%) | |
|---|---|---|---|---|---|---|---|
| No. | % | ||||||
| 0.9% NaCl | 10 | 0 | 0 | 10,000 | 33 ± 6 | 0.33 ± 0.06 | |
| CP | 10 | 0 | 0 | 10,000 | 649 ± 19 | 6.49 ± 0.19 a | |
| Se-PFPs (a) + NaCl | 10 | 1.35 | 5 | 10,000 | 26 ± 6 | 0.26 ± 0.06 | |
| Se-PFPs (b) + NaCl | 10 | 2.7 | 10 | 10,000 | 27 ± 5 | 0.27 ± 0.05 | |
| Se-PFPs (c) + NaCl | 10 | 5.4 | 20 | 10,000 | 31 ± 6 | 0.31 ± 0.06 | |
| PFPs + NaCl | 10 | 2.7 | 0.108 | 10,000 | 28 ± 5 | 0.28 ± 0.05 | |
| Se + NaCl | 10 | 0 | 10 | 10,000 | 27 ± 4 | 0.27 ± 0.04 | |
| PFPs + Se + NaCl | 10 | 2.7 | 10 | 10,000 | 26 ± 5 | 0.26 ± 0.05 | |
| Se-PFPs (a) + CP | 10 | 1.35 | 5 | 10,000 | 299 ± 10 | 2.99 ± 0.10 b | 56.8 |
| Se-PFPs (b) +CP | 10 | 2.7 | 10 | 10,000 | 198 ± 8 | 1.98 ± 0.08 b | 73.2 |
| Se-PFPs (c) + CP | 10 | 5.4 | 20 | 10,000 | 107 ± 11 | 1.07 ± 0.11 b,d | 87.9 |
| PFPs + CP | 10 | 2.7 | 0.108 | 10,000 | 398 ± 12 | 3.98 ± 0.12 b | 40.7 |
| Se + CP | 10 | 0 | 10 | 10,000 | 381 ± 11 | 3.81 ± 0.11 b,c | 43.5 |
| PFPs + Se + CP | 10 | 2.7 | 10 | 10,000 | 273 ± 10 | 2.73 ± 0.10 b | 61.0 |
| Group | SOD Activity (U/mg) | GPx Activity (U/mg) |
|---|---|---|
| 0.9% NaCl | 199.3 ± 17.5 | 241 ± 21.6 |
| CP | 83.2 ± 7.9 a | 99.7 ± 12.1 a |
| Se-PFPs (a) + CP | 131.4 ± 16.0 b | 157.7 ± 19.2 b |
| Se-PFPs (b) + CP | 168.1 ± 5.0 b | 198.9 ± 15.2 b |
| Se-PFPs (c) + CP | 185.6 ± 9.2 b,d | 222.8 ± 10.9 b,d |
| PFPs + CP | 132.4 ± 9.3 b | 158.9 ± 12.4 b |
| Se + CP | 127.7 ± 12.8 b,c | 149.2 ± 10.5 b,c |
| PFPs + Se + CP | 152.6 ± 12.9 b | 183.1 ± 15.5 b |
| Group | EROD Activity (pmol/min/mg Protein) | MROD Activity (pmol/min/mg Protein) |
|---|---|---|
| 0.9% NaCl | 38.17 ± 1.95 | 61.27 ± 9.29 |
| CP | 38.10 ± 3.18 a | 61.1 ± 7.93 a |
| Se-PFPs (a) + CP | 28.93 ± 2.36 b | 44.00 ± 5.03 b |
| Se-PFPs (b) + CP | 20.97 ± 2.06 b,d | 26.93 ± 2.68 b,d |
| Se-PFPs (c) + CP | 13.13 ± 3.56 b,d | 10.00 ± 1.77 b,d |
| PFPs + CP | 28.57 ± 1.51 b | 43.88 ± 3.45 b |
| Se + CP | 36.97 ± 2.12 c | 60.47 ± 5.6 c |
| PFPs + Se + CP | 28.73 ± 2.41 b,e | 43.87 ± 3.72 b,e |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, F.; Guo, X.; Li, Z.; Li, C.; Wang, C.; Lv, W.; Wang, J.; Xiao, F.; Kamal, M.A.; Yuan, C. Antimutagenic Effects of Selenium-Enriched Polysaccharides from Pyracantha fortuneana through Suppression of Cytochrome P450 1A Subfamily in the Mouse Liver. Molecules 2016, 21, 1731. https://doi.org/10.3390/molecules21121731
Peng F, Guo X, Li Z, Li C, Wang C, Lv W, Wang J, Xiao F, Kamal MA, Yuan C. Antimutagenic Effects of Selenium-Enriched Polysaccharides from Pyracantha fortuneana through Suppression of Cytochrome P450 1A Subfamily in the Mouse Liver. Molecules. 2016; 21(12):1731. https://doi.org/10.3390/molecules21121731
Chicago/Turabian StylePeng, Fan, Xin Guo, Zhihong Li, Changzheng Li, Changdong Wang, Weiran Lv, Junjie Wang, Fangxiang Xiao, Mohammad Amjad Kamal, and Chengfu Yuan. 2016. "Antimutagenic Effects of Selenium-Enriched Polysaccharides from Pyracantha fortuneana through Suppression of Cytochrome P450 1A Subfamily in the Mouse Liver" Molecules 21, no. 12: 1731. https://doi.org/10.3390/molecules21121731
APA StylePeng, F., Guo, X., Li, Z., Li, C., Wang, C., Lv, W., Wang, J., Xiao, F., Kamal, M. A., & Yuan, C. (2016). Antimutagenic Effects of Selenium-Enriched Polysaccharides from Pyracantha fortuneana through Suppression of Cytochrome P450 1A Subfamily in the Mouse Liver. Molecules, 21(12), 1731. https://doi.org/10.3390/molecules21121731






