Abstract
In this work, a computational study of a series of N-substitued-4-piperidones curcumin analogues is presented. The molecular structure of the neutral molecules and their radical anions, as well as their reactivity, are investigated. N-substituents include methyl and benzyl groups, while substituents on the aromatic rings cover electron-donor and electron-acceptor groups. Substitutions at the nitrogen atom do not significantly affect the geometry and frontier molecular orbitals (FMO) energies of these molecules. On the other hand, substituents on the aromatic rings modify the distribution of FMO. In addition, they influence the capability of these molecules to attach an additional electron, which was studied through adiabatic (AEA) and vertical electron affinities (VEA), as well as vertical detachment energy (VDE). To study electrophilic properties of these structures, local reactivity indices, such as Fukui (f+) and Parr (P+) functions, were calculated, and show the influence of the aromatic rings substituents on the reactivity of α,β-unsaturated ketones towards nucleophilic attack. This study has potential implications for the design of curcumin analogues based on a 4-piperidone core with desired reactivity.
1. Introduction
Curcumin is a natural polyphenol, isolated from the herb Curcuma longa L. It has been used in Indian ayurvedic, Chinese and Arabian medicine from ancient times [1,2]. Nowadays, curcumin is used in the food industry as a coloring and flavoring agent. A currently active field of research deals with the multiple biological activities displayed by curcumin and their derivatives [1,3,4,5,6]. Nevertheless, for these applications, curcumin presents some disadvantages, namely poor water solubility, poor bioavailability, and fast metabolic degradation [7,8,9].
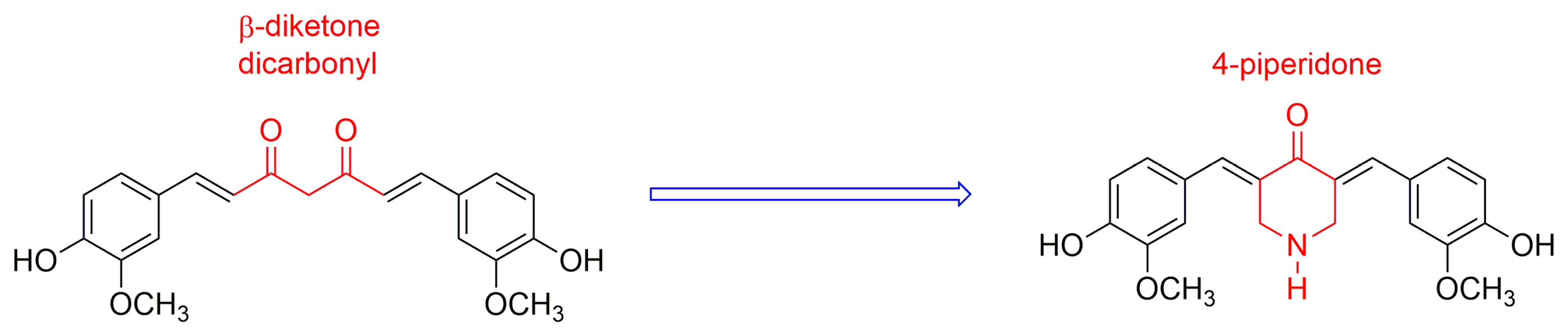
In this context, the search for curcumin derivatives and analogues that overcome the above-mentioned disadvantages has become an active area of research [1,2]. The synthesis of new curcumin derivatives has been guided by several approaches to increase the bioavailability of these compounds without losing their pharmacological action [1,2]. The β-diketone moiety, present in curcumin, is a specific substrate for liver enzymes, which contributes to their fast metabolism [10]. Additionally, this feature has been also associated to the generation of reactive oxygen species (ROS), which are involved in several mechanisms of cellular apoptosis [11,12]. Among other possibilities, the replacement of β-diketone moiety by a carbonyl group conjugated by the two sides (Figure 1) has emerged as a new promising alternative [13,14].

Figure 1.
4-piperidone structure derived from curcumin.
Among these compounds, those whose central linker is a 4-piperidone scaffold have shown a high potential as anti-inflammatory and anticancer agents [13,15]. These kinds of compounds have a preferential affinity for thiols, in contrast to amino or hydroxyl groups [16]. Apparently, the central heterocyclic ring fits a primary binding site, being this interaction influenced by the nature of the substituents at the nitrogen atom [17]. The presence of 4-piperidone alkylated at the nitrogen atom, along with phenyl rings bearing a great variety of substituents, constitutes the key features of a series of monocarbonyl analogues of curcumin, whose anti-inflammatory and anti-cancer activities have been recently reported [14,15]. Moreover, dimers linked by the nitrogen atoms, have also shown potent cytotoxicity against cancer cells [18].
As far as we know, despite the relevant articles that deal with the pharmacological properties of curcumin derivatives having the 4-piperidone moiety mentioned above, no theoretical reports about the electronic structure and reactivity of this kind of compounds has been published. Based on the above, in this work we carry out a density functional theory (DFT) study of two series of 4-piperidone curcumin (4-PC) analogues, with methyl or benzyl group at the nitrogen atom (Table 1). To gain insight about the chemical reactivity and the determinants of the biological activity of these compounds, both neutral and radical anions are studied. In particular, the effect of aromatic ring substituents on the geometrical and molecular orbital properties, as well as their capability to attach an extra electron, are investigated.

Table 1.
Molecules studied in this work.
2. Results and Discussion
2.1. Geometry and Electronic Structure Analysis of Neutral Species
The series of 4-PC derivatives were chosen with the aim of studying the influence of substituents in the aromatic rings and at the nitrogen atom on reactivity. The neutral closed shell molecules were studied at the B3LYP/6-31G(d) level. A conformational analysis at the PM6 level was carried out on the rotation around N11-C12 and C12-CAr1 bonds of compound 7 (Figure S1, Supplementary Materials), and the minimum energy geometry found was used as a model input for the benzyl series. Plots of all optimized structures are presented in the supporting information section. The main minimum energy geometrical parameters are presented in Table S1 (Supplementary Materials).
Compounds 1–6 did not show significant structural differences arising from variations in the substituents on the aromatic rings, except for a slight shortening of the C2-C3 bond and an elongation of the C3-C4 bond, for 4, 5, and 6 when compared with 1, 2, and 3. The replacement of a benzyl by a methyl group as a substituent at the nitrogen atom did not modify geometrical parameters of compounds 7–12, except for the elongation of the N11-C12 bond. These results show that substitution at the nitrogen atom by a methyl or benzyl groups do not significantly affect the molecular geometry.
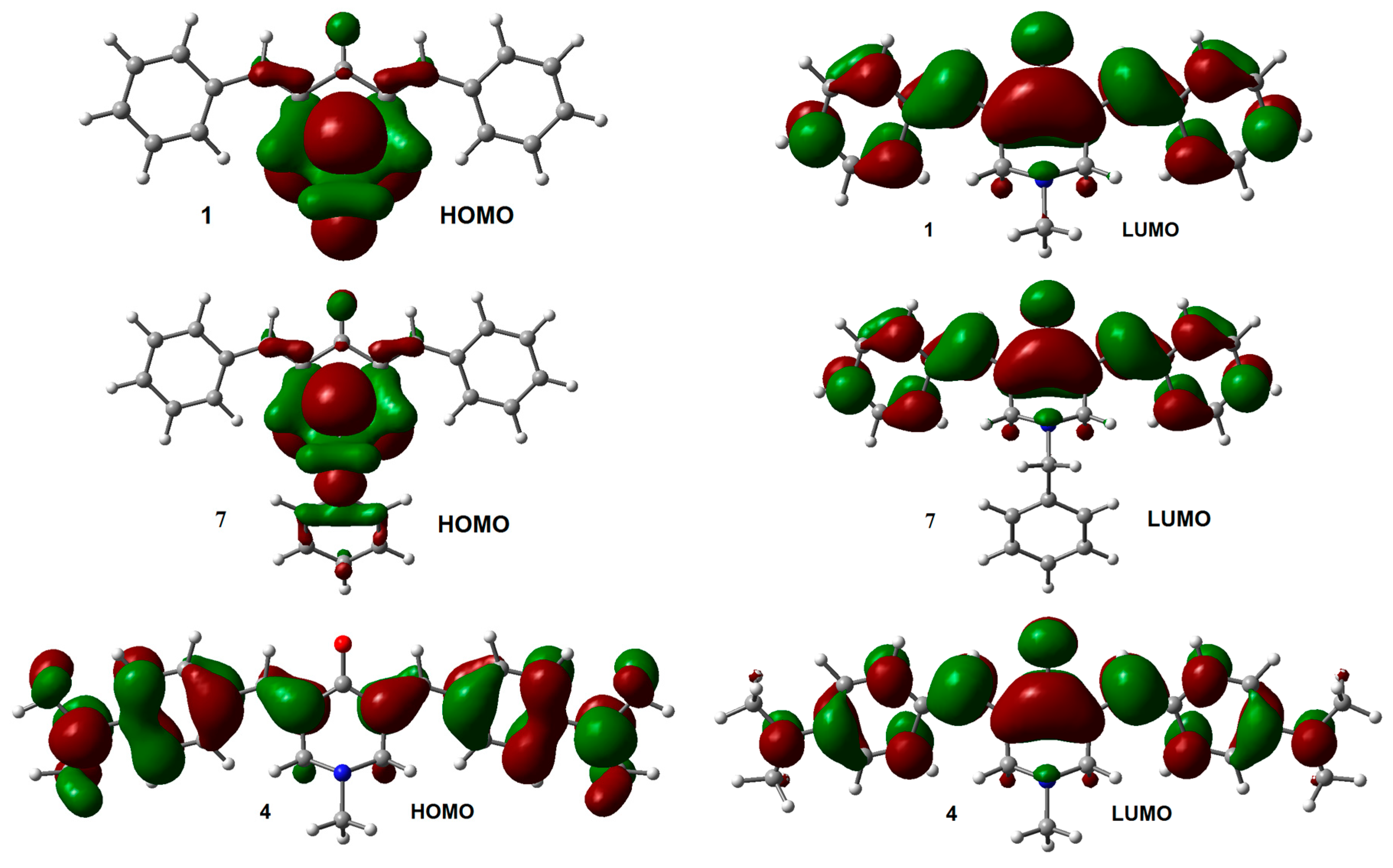
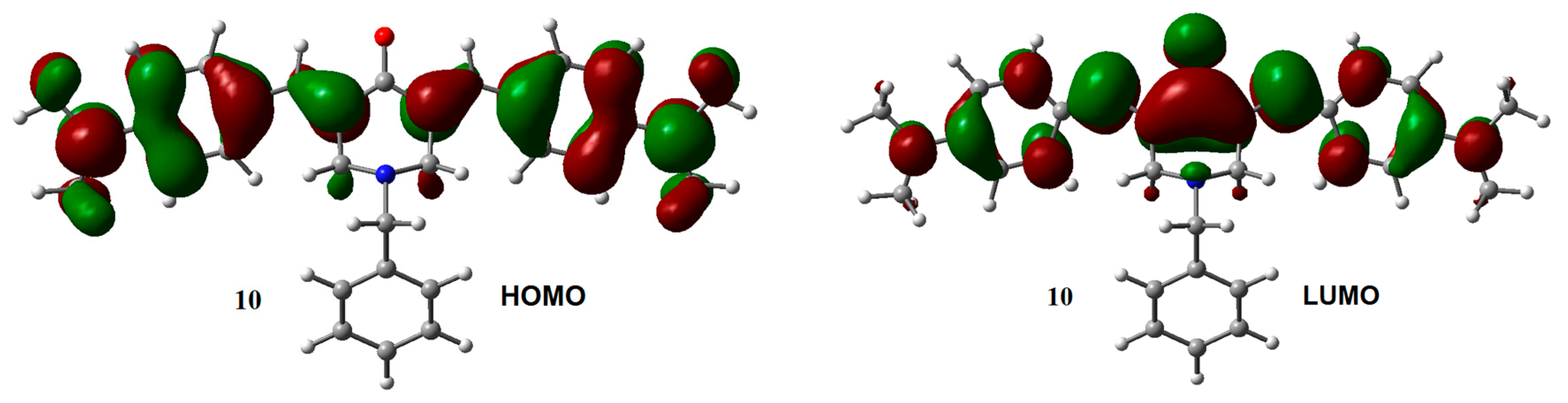
The influence of the substituent on the electronic structure of these molecules was studied by the analysis of their frontier molecular orbitals (FMO), observing differences in FMO distribution patterns for different type of substituents. A similar behavior was observed for the HOMO and LUMO distribution among the members of this series with N-methyl (1–6) and N-benzyl (7–12) substituents. For both unsubstituted aromatic rings molecules (1 and 7), the HOMO was fully localized on the 4-piperidone ring and the exo double bonds, while the LUMO was distributed through most part of the molecules, except over the substituents at the nitrogen atom. Contrary, the halogen substituted aromatic rings (compounds 2, 3, 8, and 9), contributed to the HOMO (Figure S2, Supplementary Materials). Instead, the dimethyl-amine derivatives displayed the HOMO localized on the exo double bonds as well as over the aromatic rings (compounds 4 and 10, Figure 2). Electro withdrawing substituents –CN and –CF3 (compounds 5, 6, 11, and 12) distributed their HOMO in a similar way that unsubstituted 1 and 7 do. There are no significant effects from the substituents on the LUMO distribution, for all molecules.
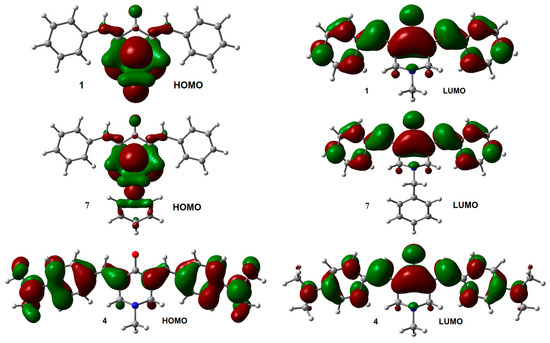
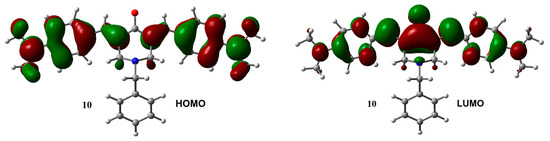
Figure 2.
HOMO and LUMO orbitals for neutral species of 1, 4, 7, and 10. Isosurface value = 0.02 e/Å3.
The energies of HOMO and LUMO for the N-methyl derivatives 1–6 were lower than those for the N-benzyl derivatives 7–12 (Table 2), except for the 4-dimethylamino derivatives (compounds 4 and 10) where they were the same for both HOMO orbitals (−4.87 eV), and almost the same for the LUMO orbitals (−1.51 eV and −1.50 eV for 4 and 10 respectively). The HOMO-LUMO gap (GAPH-L) was higher for 1–6 compared with their analogs 7–12 (Table 2), except for 4 whose gap was lower than the one obtained for 10. The effect of halogens on the energy level of FMO was very similar; decreasing both, HOMO and LUMO energies, like the unsubstituted 1 and 7 derivatives. On the other hand, the effect of dimethylamine group increased the energy levels of the FMO, being more remarkably for HOMO than for LUMO, which leads to a smaller GAPH-L respect to the other analogs.

Table 2.
HOMO, LUMO, and GAP HOMO-LUMO (GAPH-L) energy, electronic chemical potential (μ), hardness (η) and electrophilicity (ώ) a.
Global reactivity indices, such as electronic chemical potential (μ), hardness (η), and electrophilicity (ώ) were also obtained for all molecules according with their definitions (Table 2) [19,20]. η indicates the resistance of the molecule to change their electron density distribution and it was very similar for all compounds, except for 4 and 10, where the presence of dimethyl-amine substituents decreased η significantly. On the other hand, μ indicates the change in free energy when electrons are added or removed from the molecule, and ώ is a measurement of the trend of a molecule to act as an electrophile, and is obtained from μ and η. Values of ώ increased for 2, 3, 5, and 6 compared with 1, while it was reduced when compared with 4. A similar behavior was observed for the benzyl analogues 7 to 12, being both series very similar respect to their values of ώ. These results show that the tendency to increase ώ is CN > CF3 > Cl = Br > N(CH3)2, in agreement with basic concepts of organic chemistry.
2.2. Radical Anions and Electron Affinities
All molecular orbitals and properties of anion radical species were calculated at the B3LYP/6-31G(d) level. AEAs, VEAs, and VDEs were also calculated at the B3LYP/6-311+G(2df,p)//B3LYP/6-31G(d) level.
Among the main mechanisms invoked to explain the biological activities of this kind of molecules are those involving free radicals [21,22,23]. It has been previously shown that the reduction potentials of this kind of molecules, which present a α,β-unsaturated ketone moiety, can affect directly their biological activity [24,25]. In order to study the anion radical species derived from these curcuminoids, an orbital analysis, as well as electron affinity calculations, were carried out, in a similar way to that performed on α,β-unsaturated analogues [26].
In Table S2 (Supplementary Materials) the main geometrical parameters, at the B3LYP/6-31G(d) level, for the anion radicals from all molecules are presented. The most significant variations, with regard to the neutral species, were the elongation of C7=C8 and C9=O9 double bonds.
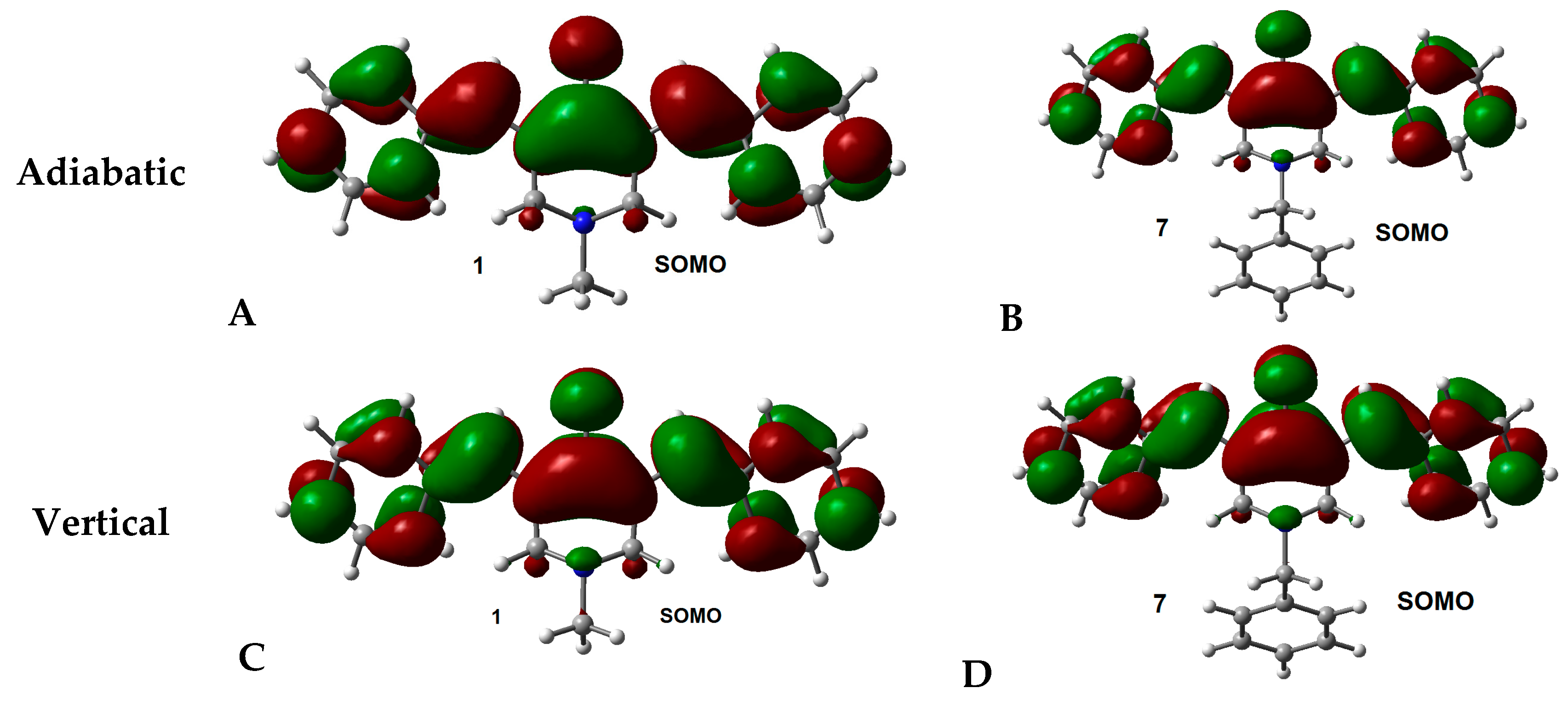
To study the distribution of the excess electron density in the anion radical of all molecules, the single occupied molecular orbital (SOMO) of the adiabatic and vertical cases were obtained. In dipole-bound anions, the SOMO is distributed in a limited part of the molecule, while in valence-bound anions the SOMO is distributed over the whole molecule. In Figure 3, the SOMO of adiabatic and vertical anions for molecules 1 (representative example for series 1–6) and 7 (representative example for series 7–12), are displayed (others are presented in Figure S3, Supplementary Materials). The representations of SOMOs show that all molecules display a delocalized distribution of the orbital and, therefore, suggest a valence-bound anion in both cases, vertical and adiabatic. LUMOs for vertical and adiabatic anions present the same distribution for 1 to 6 (Figure S3, Supplementary Materials), but are different for 7–12 (Figure S4, Supplementary Materials).
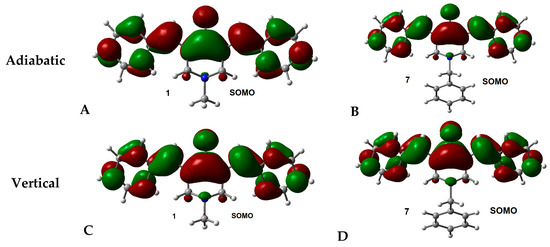
Figure 3.
SOMO of adiabatic and vertical anions for molecules 1 and 7. Isosurface value = 0.02 e/Å3.
Calculated AEAs, VEAs, and VDEs at B3LYP/6-31G(d) and B3LYP/6-311+G(2df,p)// B3LYP/6-31G(d) are presented in Table 3. In order to ensure the adequacy of our approach, AEAs for compounds 1 and 7 were also obtained from geometries optimized at the B3LYP/6-311+G(2df,p) level, and the results (1.398 eV and 1.407 eV respectively) were very close to those obtained at the B3LYP/6-311+G(2df,p)//B3LYP/6-31G(d) level. This shows that high level optimization is not necessary to obtain reliable results. Positive VEA and AEA indicates the favorable tendency of the molecule to attach an electron, the first represents the generation of radical anion without the geometrical rearrangement, while the second indicates that the radical anion is in their final optimized geometry. A positive VDE indicates that the energy of the neutral molecule is higher than their anion, and it is stable with respect to the vertical electron auto-detachment. VEA and VDE can be interpreted as lower and upper bounds of AEA, as long as the nuclear configuration of the anion and the neutral molecule are similar. AEAs for all molecules were positive, which showed that all have a favorable tendency to form stable anion radicals. Positive VEAs for all molecules also indicate that they have a favorable tendency to attach an extra electron. Positive VDEs for all studied molecules indicate that the electron auto-detachment from the anion radical is unlikely to occur.

Table 3.
Calculated Adiabatic Electron affinities (AEAs), vertical electron affinities (VEAs), and vertical detachment energies (VDEs) for molecules 1–12 in vacuum with the B3LYP/6-31G(d) a, and B3LYP/6-311+G(2df,p)// B3LYP/6-31G(d) b, basis sets c.
To study the effect of substituents at the aromatic rings regarding the attachment and detachment of an electron, we correlated the AEAs, VEAs, and VDEs with the Hammett sigma constant [27,28,29]. In both series, 1–6 and 7–12, we found for AEA a correlation coefficient (R2) of 0.89 (1–6) and 0.90 (7–12) for values calculated with the 6-31G(d) basis set and 0.94 (1–6) and 0.91 (7–12) for values calculated with the B3LYP/6-311+G(2df,p) basis set (Figures S5 and S6, Supplementary Materials), which indicates a better fit for values calculated with the larger basis. Similarly for VEAs, we found a correlation coefficient (R2) of 0.90 (1–6 and 7–12) for values calculated with the 6-31G(d) basis set and 0.91 (1–6 and 7–12) for values calculated with the B3LYP/6-311+G(2df,p) basis set. These results indicate that substituents on the aromatic ring have a significant role on the capability of these molecules to attach an additional electron. VDEs values obtained with the 6-31G(d) and 6-311+G(2df,p) basis sets present similar correlations. Series 1–6 exhibits a R2 of 0.81, while series 7–12 present a R2 of 0.89 with both basis sets. The correlation of AEAs, VEAs, and VDEs with the Hammet parameters show that using an extended basis set, such as B3LYP/6-311+G(2df,p), to calculate electronic energy and properties, do not significantly improve the results.
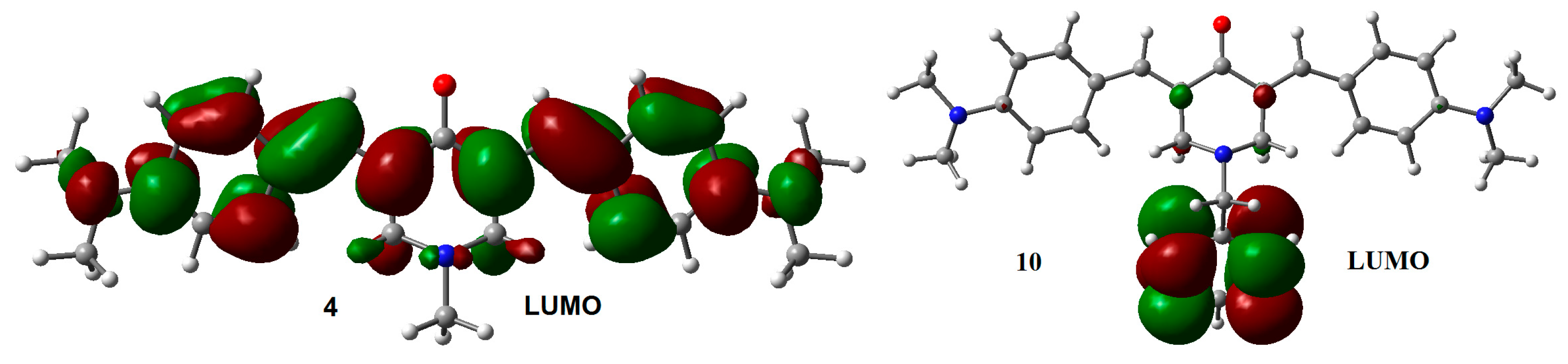
Compounds 4 and 10, with the strongest electro-donor p-N(CH3)2 group, on the aromatic rings, presented the lowest AEAs, followed by the unsubstituted compounds 1 and 7, and by the p-Cl compounds 2 and 8 and the p-Br derivatives 3 and 9, which can be considered weak electron-donor substituents at long distances. Compounds with strong electron-acceptor substituents as –CF3 (6 and 12) and –CN (5 and 11) on para position of the aromatic rings had higher AEAs. The relationship between substituents on the aromatic rings with VEAs and VDEs was the same that for AEAs. These results also agree with basic organic chemistry principles.
A particularly interesting issue is the difference observed in the VDE values between compounds 4 and 10. The slightly larger difference in the VDE between 4 and 10, compared with the difference between the other coupled analogs, suggests that the excess electron density arising from the p-N(CH3)2 substituents on the lateral aromatic rings, should increases the probability to populate excited states, allowing electron transfer between the central aromatic ring and the rest of the structure. The latter can explain the difference observed in the VDE between 10 and 4. LUMO distribution in 4 and 10 (Figure 4) support this explanation.
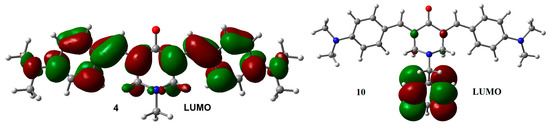
Figure 4.
LUMO for adiabatic radical anions 4 and 10. Isosurface value = 0.02 e/Å3.
2.3. Local Reactivity
To study the effect of substituents on the reactivity towards nucleophiles, Fukui function (f+), and Parr function (P+) were calculated [30,31]. We focused on the atoms that can experience a nucleophilic attack, atoms C7 and C9 at the α,β-unsaturated ketone C7=C8-C9=O9 fragment. It has been previously demonstrated that the addition of biological nucleophiles to this structures plays a key role in the anticancer activity of these curcumin analogues [32], with selective reactivity towards the thiol group, a soft nucleophile [16]. Nucleophile-electrophile reactions occur following the hard and soft acid and base (HSAB) theory, which indicates that nucleophiles react preferentially with electrophiles of similar hardness or softness [33]. The oxygen carbonyl atom O9 withdraw electron density from C7=C8, which creates a deficiency of electron density at C7. The later gives as a result a relatively hard carbonyl carbon atom (C9) for a 1,2-addition, and a relatively soft β-carbon atom (C7) for a 1,4-addition.
Fukui function (f+) and Parr function (P+) for nucleophilic attack toward C7 and C9 are presented in Table 4. According to f+, C7 is slightly more reactive than C9 toward nucleophiles for all molecules. P+ indicate reactivity in the same direction, but a more significant difference in the electrophilic reactivity for C7 with regard to C9 is predicted.

Table 4.
Fukui function (f+) and Parr function (P+) for nucleophilic attack at C7 and C9.
According to both reactivity indices, analogues with N(CH3)2 substituents in the aromatic rings (4 and 10) present the greatest difference in reactivity between C7 and C9, while analogues with CN substituents in the aromatic rings (5 and 11) present the lowest difference.
3. Materials and Methods
The calculations were carried out using the Gaussian09 program package [34], running in a Supermicro cluster of blades. In order to find the minimum energy conformation, compound 7 geometry was optimized at the DFT B3LYP/6-31G(d) level and, later, a semi-empirical PM6 level conformational analysis was carried out on the rotations around N11-C12 and C12-CAr bonds. After that, compound 7 was re-optimized at DFT B3LYP/6-31G(d) starting from the minimum energy conformation found at PM6 level. This minimum energy conformation found for 7 was used as the initial model for the optimization of 8–12. No symmetry constraints were imposed to the optimization of the other molecules, which were performed at DFT B3LYP/6-31G(d) level. No imaginary vibrational frequencies were found at the optimized geometries, indicating that they are true minima of the potential energy surface.
Electron affinities and vertical detachment energies were calculated as follow:
where E = Eelec + zero-point vibrational energies (ZPVE).
Adiabatic electron affinity (AEA) = E(optimized neutral) − E(optimized anion)
Vertical electron affinity (VEA) = E(optimized neutral) − E(anion at optimized neutral geometry)
Vertical detachment energy (VDE) = E(neutral at optimized anion geometry) − E(optimized anion)
Gas phase energies were also obtained at the B3LYP/6-311+G(2df,p) level, through single point calculations based on B3LYP/6-31G(d) optimized geometries. ZPVE obtained at B3LYP/6-31G(d) were used as correction for B3LYP/6-311+G(2df,p) energies. This methodology has shown good results in similar calculations [35]. In order to ensure the adequacy of our approach, AEAs for compounds 1 and 7 were also obtained from geometries optimized at the B3LYP/6-311+G(2df,p) level.
Global reactivity indices, such as electronic chemical potential (μ), hardness (η), and electrophilicity (ώ), were obtained for all molecules according with their definitions [20,21]. Local indices, Fukui function (f+) and Parr function (P+), for electrophilic attack were calculated as previously described [31,31].
4. Conclusions
In this work, we have studied the molecular structure of twelve N-substitued-4-piperidone curcumin analogues, focusing on the effect of substituents on geometries, frontier molecular orbitals, electron affinities, and global, as well as local, reactivity indices. Methyl and benzyl substituents on the nitrogen atom do not have significant effects on the geometries and frontier molecular orbitals of both neutral and radical anion species, showing that the proper choice of groups located at the nitrogen atom can be a nice procedure to modify relevant properties linked to biological activities, such as lipophilicity, solubility, etc., without affecting the reactivity of the molecule. On the other hand, substituents on the aromatic rings influence the distribution of HOMO orbitals of the neutral molecules, but do not affect the SOMO of radical anions. Additionally, the LUMO do not seems to be affected by the substituents, not in neutral form, neither as a radical anionic species. Except for compound 10 where the LUMO orbital displays a significant population at the methylene group.
Adiabatic and vertical electron affinities (AEA and VEA), as well as vertical detachment energy (VDE), were calculated at the B3LYP/6-31G(d) and B3LYP/6-311+G(2df,p) levels. The effect of substituents on the aromatic rings was studied by correlations with the Hammett sigma constant. As expected, for AEAs the correlation coefficients present a better fit with the 6-311+G(2df,p) than with the 6-31G(d) basis set. For VEAs and VDEs no differences were found. The analysis of the SOMO orbital distribution for both adiabatic and vertical radical anions, as well as the difference between AEA and VEA, indicates that for all studied molecules, the formed anion radicals are valence-bound types.
The Fukui (f+) and Parr functions (P+) for electrophilic attack were calculated and show the influence of the substituent on the aromatic rings on the reactivity of α,β-unsaturated ketone systems toward nucleophiles. C7 showed to be more reactive toward nucleophilic attack than C9, which indicates that 1,4-addition is more favorable than 1,2-addition. Furthermore, the difference in local reactivity on these electrophilic sites was influenced by the nature of the substituent present in the aromatic rings. The results found in this work can be useful for the design of curcumin analogues based on a 4-piperidone core with desired reactivity.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/21/12/1658/s1.
Acknowledgments
We are grateful to Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) Grant 1140753 (Ramiro Araya-Maturana) and FONDECYT/POSTDOCTORADO 3140286 (Maximiliano Martínez-Cifuentes). Powered@NLHPC: This research was partially supported by the supercomputing infrastructure of the NLHPC (ECM-02).
Author Contributions
Maximiliano Martínez-Cifuentes and Ramiro Araya-Maturana designed research; Maximiliano Martínez-Cifuentes performed the computational calculations; Maximiliano Martínez-Cifuentes, Ramiro Araya-Maturana and Boris Weiss-López analyzed the data; Maximiliano Martínez-Cifuentes and Ramiro Araya-Maturana wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martinez-Cifuentes, M.; Weiss-Lopez, B.E.; Santos, L.S.; Araya-Maturana, R. Heterocyclic curcumin derivatives of pharmacological interest: Recent progress. Curr. Top. Med. Chem. 2015, 15, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Bairwa, K.; Grover, J.; Kania, M.; Jachak, S.M. Recent developments in chemistry and biology of curcumin analogues. Rsc Adv. 2014, 4, 13946–13978. [Google Scholar] [CrossRef]
- Zhou, H.; Beevers, C.S.; Huang, S. The targets of curcumin. Curr. Drug. Targets 2011, 12, 332–347. [Google Scholar] [CrossRef] [PubMed]
- Epstein, J.; Sanderson, I.R.; MacDonald, T.T. Curcumin as a therapeutic agent: The evidence from in vitro, animal and human studies. Br. J. Nutr. 2010, 103, 1545–1557. [Google Scholar] [CrossRef] [PubMed]
- Teiten, M.H.; Eifes, S.; Dicato, M.; Diederich, M. Curcumin-the paradigm of a multi-target natural compound with applications in cancer prevention and treatment. Toxins 2010, 2, 128–162. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Phermacol. Scl. 2009, 30, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.; El-meligy, A.; Lungu, G.; Fetaih, H.; Dessouki, A.; Stoica, G.; Barhoumi, R. Curcumin induces apoptosis in a murine mammary gland adenocarcinoma cell line through the mitochondrial pathway. Eur. J. Pharmacol. 2011, 668, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of curcumin: Problems and promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Moron, E.; Calderon-Montano, J.M.; Salvador, J.; Robles, A.; Lopez-Lazaro, M. The dark side of curcumin. Int. J. Cancer 2010, 126, 1771–1775. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.A.; Shao, L.L.; Wang, Y.; Zhao, C.G.; Chu, Y.H.; Xiao, J.; Zhao, Y.; Li, X.K.; Yang, S.L. Exploration and synthesis of curcumin analogues with improved structural stability both in vitro and in vivo as cytotoxic agents. Bioorgan. Med. Chem. 2009, 17, 2623–2631. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Thomas, S.G.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Sung, B.; Tharakan, S.T.; Misra, K.; Priyadarsini, I.K.; Rajasekharan, K.N.; et al. Biological activities of curcumin and its analogues (congeners) made by man and mother nature. Biochem. Pharmacol. 2008, 76, 1590–1611. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Surh, Y.-J.; Shishodia, S. The Molecular Targets and Therapeutic uSes of Curcumin in Health and Disease, Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2007. [Google Scholar]
- Bazzaro, M.; Anchoori, R.K.; Mudiam, M.K.R.; Issaenko, O.; Kumar, S.; Karanam, B.; Lin, Z.H.; Vogel, R.I.; Gavioli, R.; Destro, F.; et al. Alpha,beta-unsaturated carbonyl system of chalcone-based derivatives is responsible for broad inhibition of proteasomal activity and preferential killing of human papilloma virus (hpv) positive cervical cancer cells. J. Med. Chem. 2011, 54, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, Y.; Cai, Y.; Wang, J.; Weng, B.; Tang, Q.; Chen, X.; Pan, Z.; Liang, G.; Yang, S. Discovery and evaluation of piperid-4-one-containing mono-carbonyl analogs of curcumin as anti-inflammatory agents. Bioorg. Med. Chem. 2013, 21, 3058–3065. [Google Scholar] [CrossRef] [PubMed]
- Nunes, L.M.; Hossain, M.; Varela-Ramirez, A.; Das, U.; Ayala-Marin, Y.M.; Dimmock, J.R.; Aguilera, R.J. A novel class of piperidones exhibit potent, selective and pro-apoptotic anti-leukemia properties. Oncol. Lett. 2016, 11, 3842–3848. [Google Scholar] [CrossRef] [PubMed]
- Pati, H.N.; Das, U.; Sharma, R.K.; Dimmock, J.R. Cytotoxic thiol alkylators. Mini-Rev. Med. Chem. 2007, 7, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Das, U.; Sharma, R.K.; Dimmock, J.R. 1,5-diaryl-3-oxo-1,4-pentadienes: A case for antineoplastics with multiple targets. Curr. Med. Chem. 2009, 16, 2001–2020. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Das, U.; Varela-Ramirez, A.; Lema, C.; Aguilera, R.J.; Balzarini, J.; de Clercq, E.; Dimmock, S.G.; Gorecki, D.K.J.; Dimmock, J.R. Bis 3,5-bis(benzylidene)-4-oxo-1-piperidinyl amides: A novel class of potent cytotoxins. Chemmedchem 2011, 6, 1892–1899. [Google Scholar] [CrossRef] [PubMed]
- Domingo, L.R.; Rios-Gutierrez, M.; Perez, P. Applications of the conceptual density functional theory indices to organic chemistry reactivity. Molecules 2016, 21, 22. [Google Scholar] [CrossRef] [PubMed]
- Chattaraj, P.K.; Sarkar, U.; Roy, D.R. Electrophilicity index. Chem. Rev. 2006, 106, 2065–2091. [Google Scholar] [CrossRef] [PubMed]
- Ao, G.Z.; Chu, X.J.; Ji, Y.Y.; Wang, J.W. Antioxidant properties and pc12 cell protective effects of a novel curcumin analogue (2e,6e)-2,6-bis(3,5-dimethoxybenzylidene)cyclohexanone (mch). Int. J. Mol. Sci. 2014, 15, 3970–3988. [Google Scholar] [CrossRef] [PubMed]
- Leon-Gonzalez, A.J.; Auger, C.; Schini-Kerth, V.B. Pro-oxidant activity of polyphenols and its implication on cancer chemoprevention and chemotherapy. Biochem. Pharmacol. 2015, 98, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Nimiya, Y.; Wang, W.C.; Du, Z.Y.; Sukamtoh, E.; Zhu, J.L.; Decker, E.; Zhang, G.D. Redox modulation of curcumin stability: Redox active antioxidants increase chemical stability of curcumin. Mol. Nutr. Food Res. 2016, 60, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.K.; Ferstl, E.M.; Davis, M.C.; Herold, M.; Kurtkaya, S.; Camalier, R.F.; Hollingshead, M.G.; Kaur, G.; Sausville, E.A.; Rickles, F.R.; et al. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg. Med. Chem. 2004, 12, 3871–3883. [Google Scholar] [CrossRef] [PubMed]
- Modzelewska, A.; Pettit, C.; Achanta, G.; Davidson, N.E.; Huang, P.; Khan, S.R. Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorg. Med. Chem. 2006, 14, 3491–3495. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Cifuentes, M.; Salazar, R.; Escobar, C.A.; Weiss-Lopez, B.E.; Santos, L.S.; Araya-Maturana, R. Correlating experimental electrochemistry and theoretical calculations in 2′-hydroxy chalcones: The role of the intramolecular hydrogen bond. RSC Adv. 2015, 5, 50929–50937. [Google Scholar] [CrossRef]
- Pandith, A.H.; Islam, N. Electron transport and nonlinear optical properties of substituted aryldimesityl boranes: A dft study. PLoS ONE 2014, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.M.; Pluth, M.D. Linear free energy relationships reveal structural changes in hydrogen-bonded host-guest interactions. J. Org. Chem. 2014, 79, 11797–11801. [Google Scholar] [CrossRef] [PubMed]
- Irfan, A.; Al-Sehemi, A.G.; Al-Assiri, M.S. Push-pull effect on the electronic, optical and charge transport properties of the benzo 2,3-b thiophene derivatives as efficient multifunctional materials. Comp. Theor. Chem. 2014, 1031, 76–82. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density functional approach to the frontier-electron theory of chemical reactivity. J. Am. Chem. Soc. 1984, 106, 4049–4050. [Google Scholar] [CrossRef]
- Domingo, L.R.; Perez, P.; Saez, J.A. Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic parr functions. RSC Adv. 2013, 3, 1486–1494. [Google Scholar] [CrossRef]
- Moore, T.W.; Zhu, S.; Randolph, R.; Shoji, M.; Snyder, J.P. Liver s9 fraction-derived metabolites of curcumin analogue ubs109. ACS Med. Chem. Lett. 2014, 5, 288–292. [Google Scholar] [CrossRef] [PubMed]
- LoPachin, R.M.; Gavin, T.; DeCaprio, A.; Barber, D.S. Application of the hard and soft, acids and bases (hsab) theory to toxicant-target interactions. Chem. Res. Toxicol. 2012, 25, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Revision a.01, Gaussian 09, Gaussian Inc.: Wallingford, CT, USA, 2009.
- Liu, H.N.; Ding, Y.Q.; Walker, L.A.; Doerksen, R.J. Computational study on the effect of exocyclic substituents on the ionization potential of primaquine: Insights into the design of primaquine-based antimalarial drugs with less methemoglobin generation. Chem. Res. Toxicol. 2015, 28, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Not available.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).