Alpha- and Beta-Cyclodextrin Inclusion Complexes with 5-Fluorouracil: Characterization and Cytotoxic Activity Evaluation
Abstract
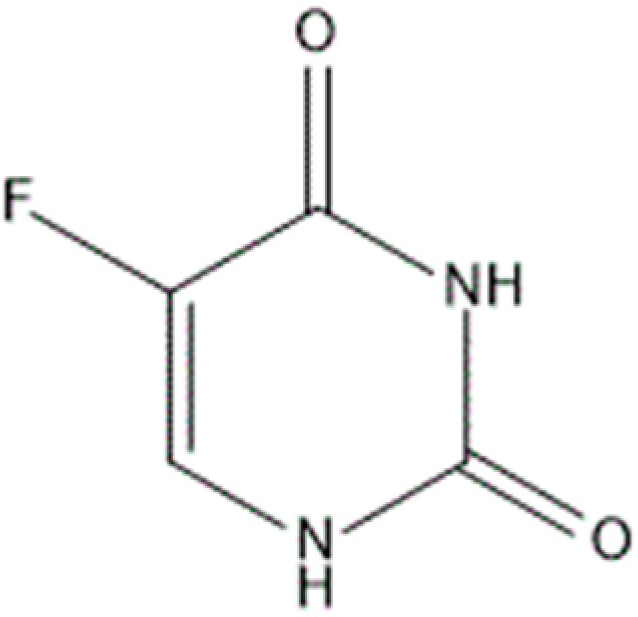
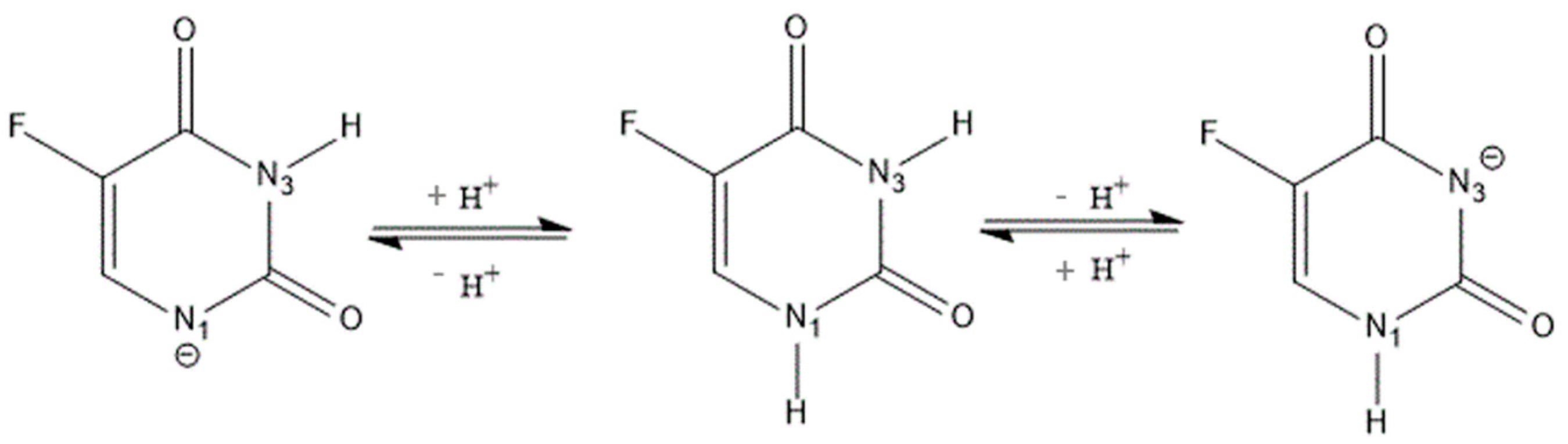
:1. Introduction
2. Results
2.1. Characterization of Inclusion Complexes in Solution
2.2. Characterization of Inclusion Complexes in Solid State
2.3. Molecular Docking Studies
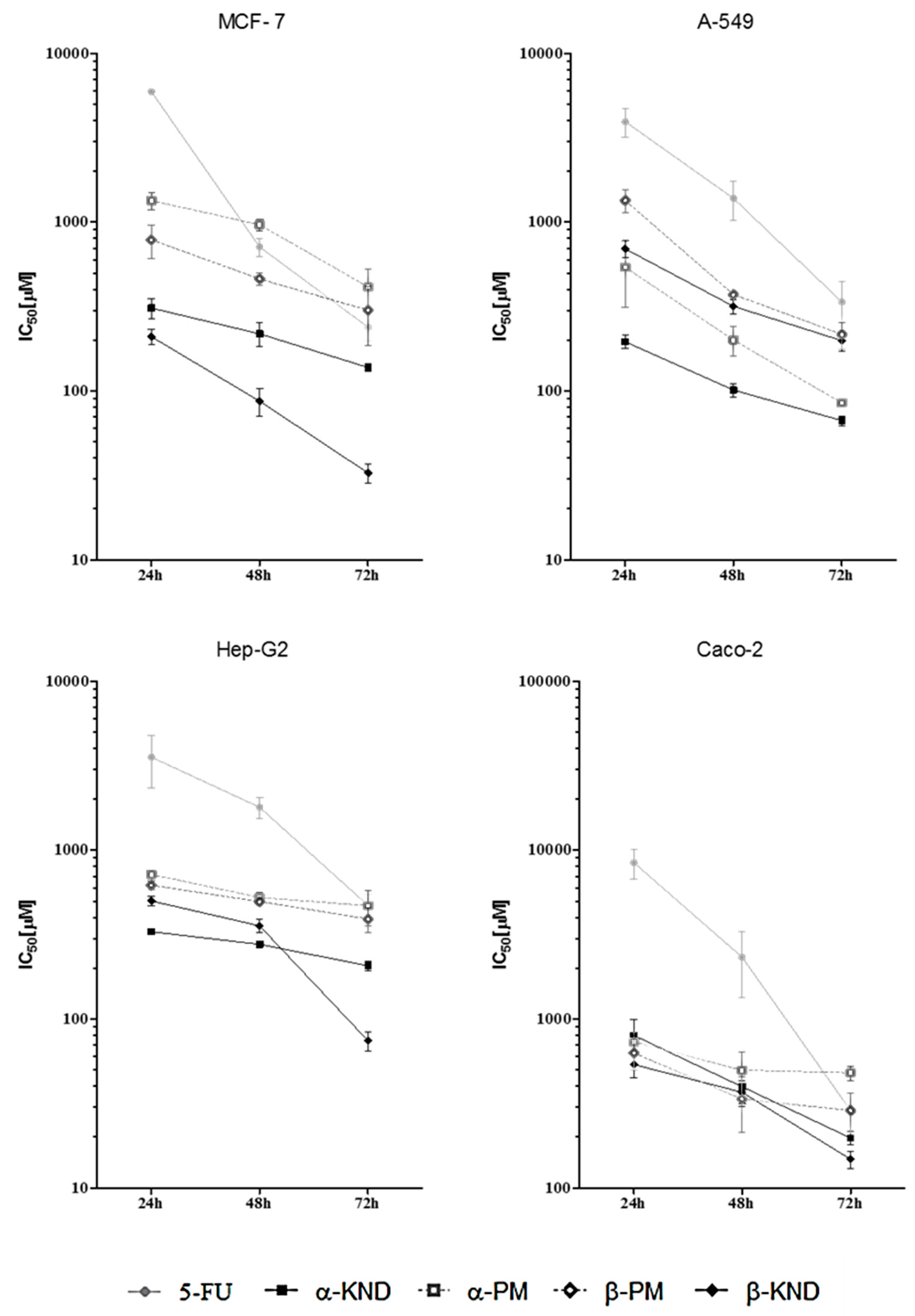
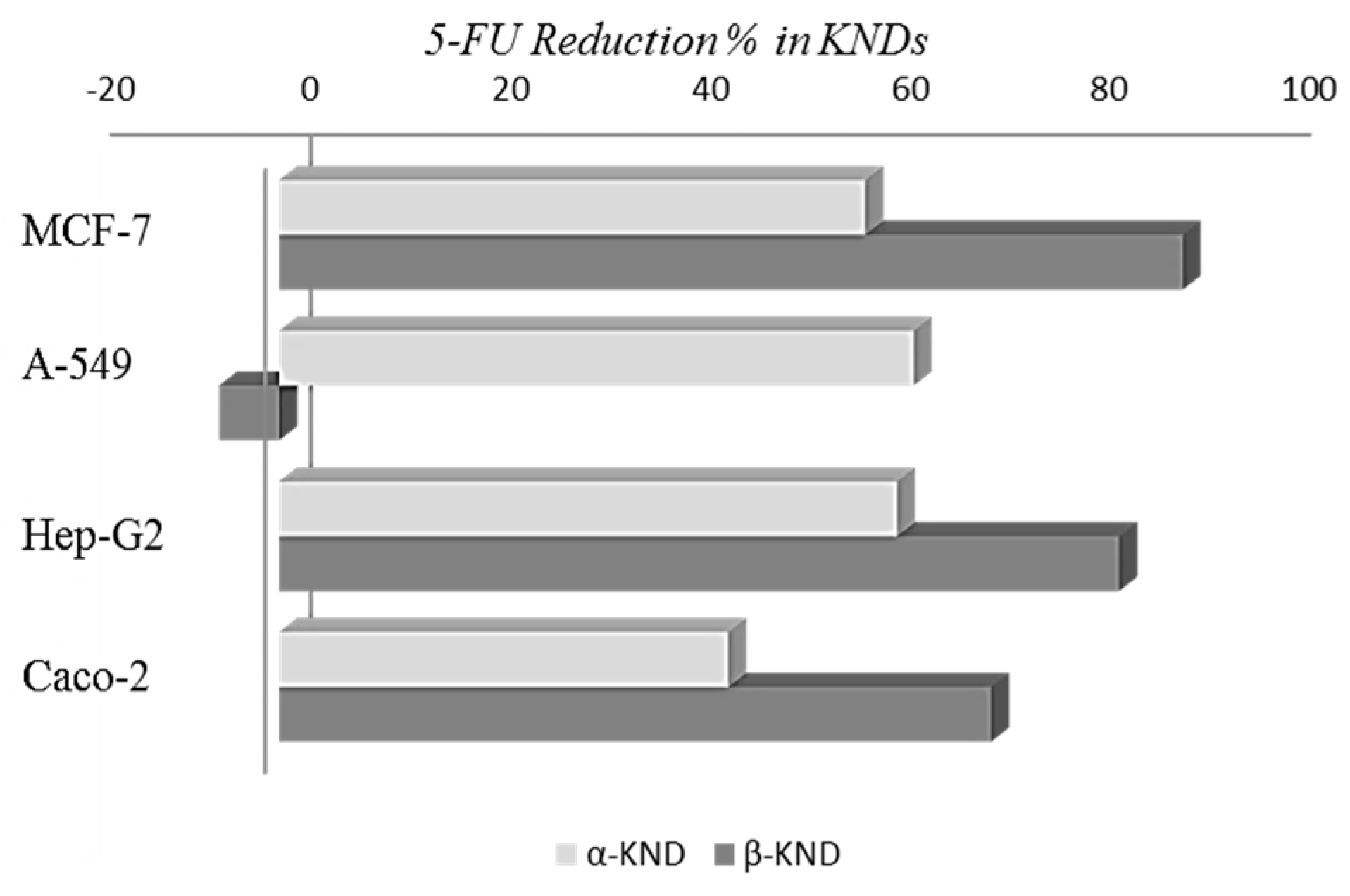
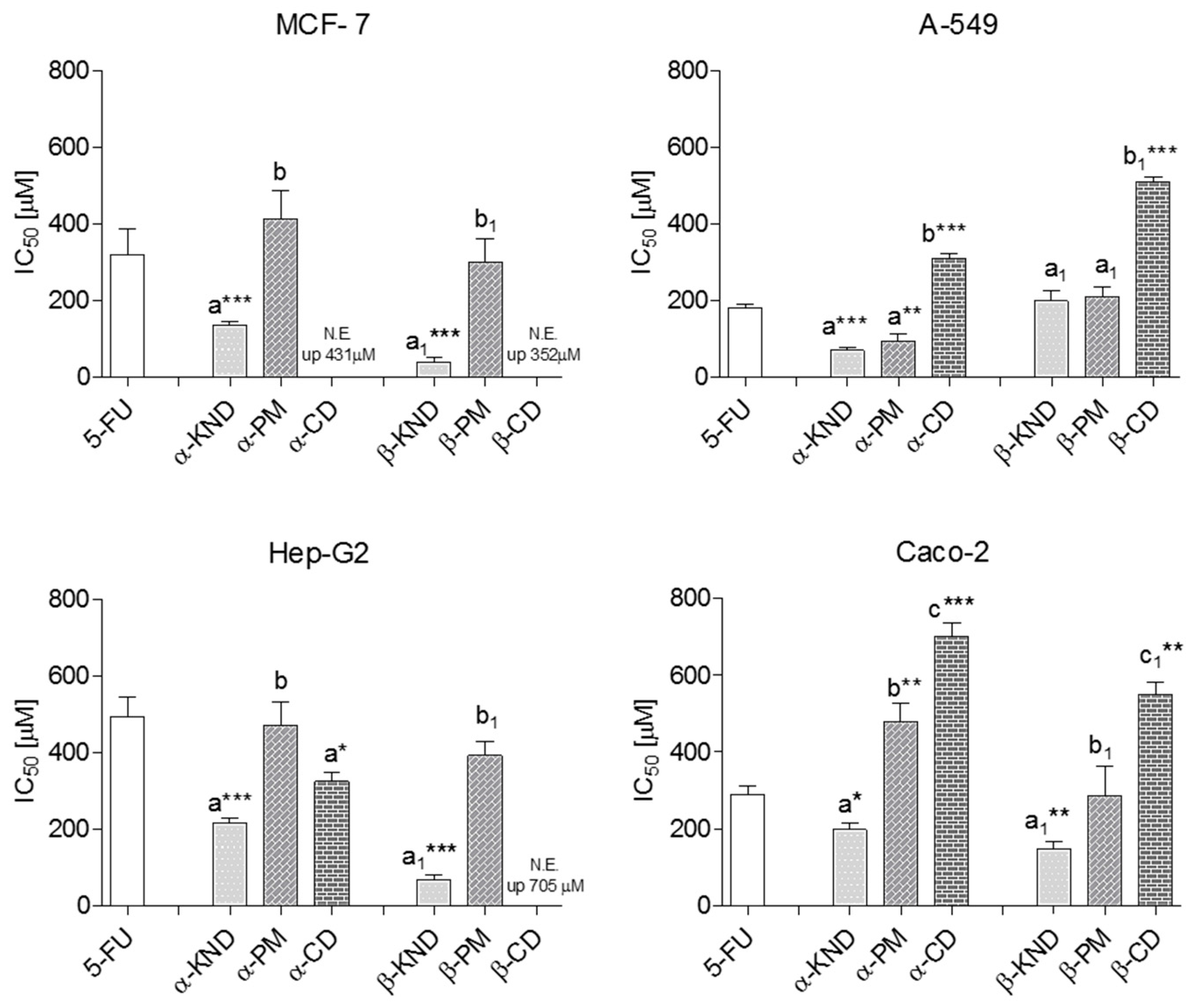
2.4. Cytotoxicity
3. Materials and Methods
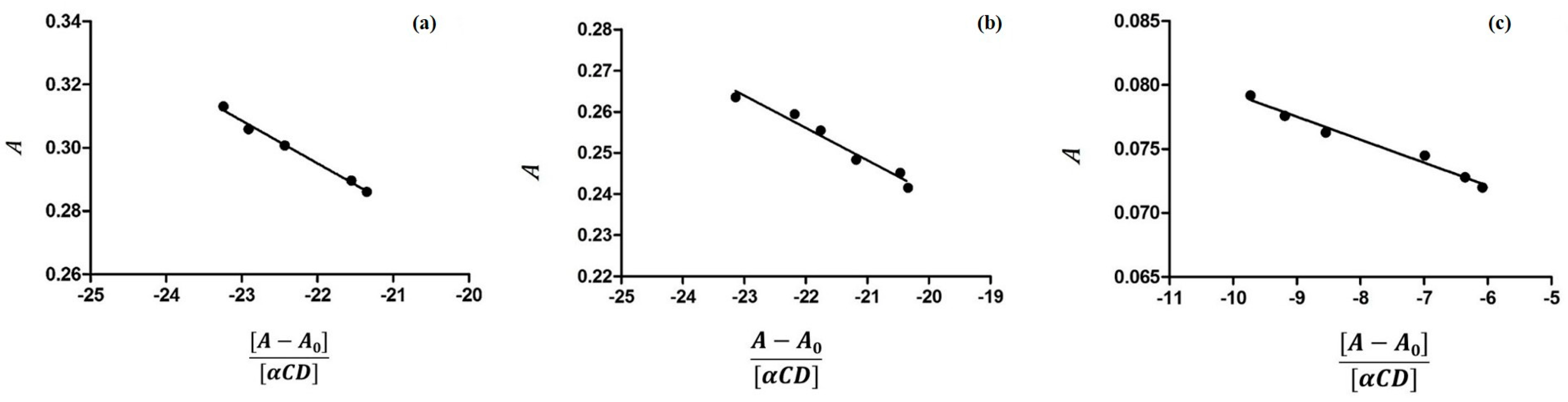
3.1. Determination of Binding Constants by UV-VIS Spectroscopy
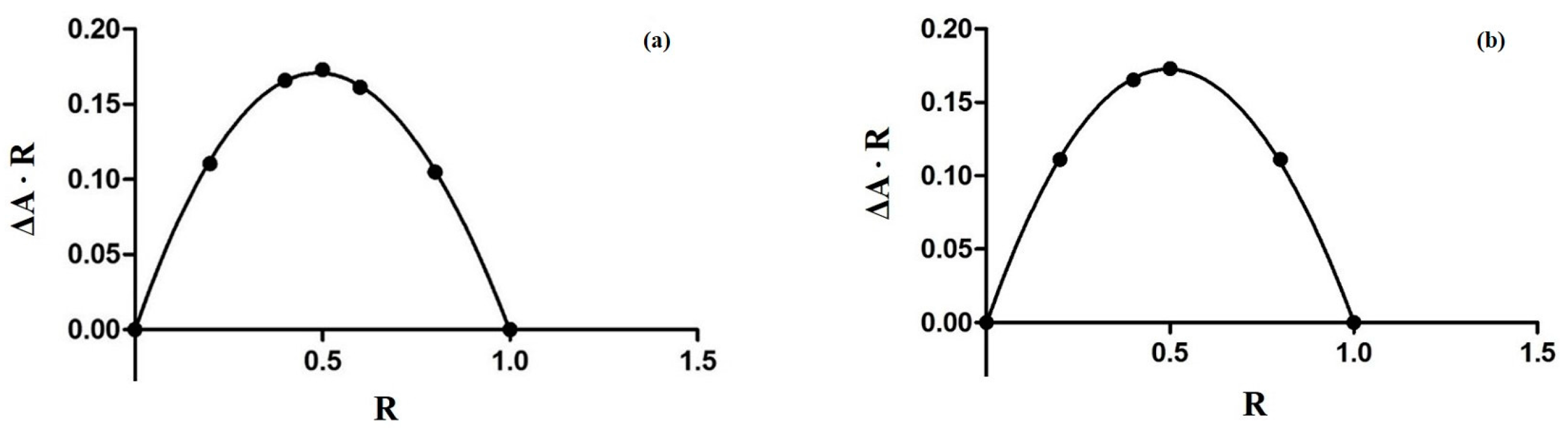
3.2. The Job Plot Method for the Determination of Stoichiometry
3.3. Preparation of Solid Binary System
3.4. X-ray Powder Diffraction (XRD)
3.5. Fourier Transform Infrared (FT-IR) Spectroscopy
3.6. Molecular Docking Studies
3.7. MTT Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Straub, J.O. Combined environmental risk assessment for 5-fluorouracil and capecitabine in Europe. Integr. Environ. Assess. Manag. 2010, 6, 540–566. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.H.; Sowers, L.C.; Cagin, T.; Goddard, W.A. First Principles Calculation of pKa Values for 5-Substituted Uracils. J. Phys. Chem. A 2001, 105, 274–280. [Google Scholar] [CrossRef]
- Heidelberger, C.; Chaudhuri, N.K.; Danneberg, P.; Mooren, D.; Griesbach, L.; Duschinsky, R.; Schnitzer, R.J.; Pleven, E.; Scheiner, J. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature 1957, 179, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Caballero, G.A.; Ausman, R.K.; Quebbeman, E.J. Long-term, ambulatory, continuous IV infusions of 5-FU for the treatment of advanced adenocarcinomas. Cancer Treat. Rep. 1985, 69, 13–15. [Google Scholar] [PubMed]
- Labianca, R.; Pessi, M.A.; Zamparelli, G. Treatment of Colorectal Cancer. Current Guidelines and Future Prospects for Drugs Therapy. Drugs 1997, 53, 593–607. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.L.; Zheng, W.S.; Chen, S.H.; Han, Y.X.; Jiang, J.D. Development of rectal delivered thermo-reversible gelling film encapsulating a 5-fluorouracil hydroxypropyl-β-cyclodextrin complex. Carbohydr. Polym. 2016, 137, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Daniel, B.; Longley, D.; Harkin, P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar]
- Noordhuis, P.; Holwerda, U.; Van der Wilt, C.L.; Van Groeningen, C.J.; Smid, K.; Meijer, S.; Pinedo, H.M.; Peters, G.J. 5-Fluorouracil incorporation into RNA and DNA in relation to thymidylate synthase inhibition of human colorectal cancers. Ann. Oncol. 2004, 7, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Fraile, R.J.; Baker, L.H.; Buroker, T.R.; Horwitz, J.; Vaitkevicius, V.K. Pharmacokinetics of 5-fluorouracil administered orally, by rapid intravenous and by slow infusion. Cancer Res. 1980, 40, 2223–2228. [Google Scholar] [PubMed]
- Van Kuilenburg, A.B. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur. J. Cancer 2004, 40, 939–950. [Google Scholar] [CrossRef] [PubMed]
- Kinno, R.; Kii, Y.; Uchiyama, M.; Owan, Y.; Yamazaki, T.; Fukui, T. 5-Fluorouracil–induced Leukoencephalopathy with Acute Stroke-like Presentation Fulfilling Criteria for Recombinant Tissue Plasminogen Activator Therapy. J. Stroke Cerebrovasc. Dis. 2014, 23, 387–389. [Google Scholar] [CrossRef] [PubMed]
- Chinembiri, T.N.; Gerber, M.; du Plessis, L.; du Preez, J.; du Plessis, J. Topical Delivery of 5-Fluorouracil from Pheroid™ Formulations and the in Vitro Efficacy against Human Melanoma. AAPS PharmSciTech 2015, 16, 1390–1399. [Google Scholar] [CrossRef] [PubMed]
- Troy, D.B. (Ed.) Remington: The Science and Practice of Pharmacy, 21st ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2006.
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M. Is the pathobiology of chemotherapy-induced alimentary tract mucositis influenced by the type of drug administered? Cancer Chemother. Pharm. 2009, 63, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Kalepu, S.; Nekkanti, V. Insoluble drug delivery strategies: Review of recent advances and business prospects. Acta Pharm. Sin. B 2015, 5, 442–453. [Google Scholar] [CrossRef] [PubMed]
- Szejtli, J. Past, present, and future of cyclodextrin research. Pure Appl. Chem. 2004, 76, 1825–1845. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchêne, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharm. 2007, 329, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Das, S.K.; Rajabalaya, R.; David, S.; Gani, N.; Khanam, J.; Nanda, A. Cyclodextrins-The Molecular Container. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 1694–1720. [Google Scholar]
- Jin, L.; Liu, Q.; Sun, Z.; Ni, X.; Wei, M. Preparation of 5-Fluorouracil/β-Cyclodextrin Complex Intercalated in Layered Double Hydroxide and the Controlled Drug Release Properties. Ind. Eng. Chem. Res. 2010, 49, 11176–11181. [Google Scholar] [CrossRef]
- Kavitha, K.; Srinivasa Rao, A.; Nalini, C.N. An Investigation on Enhancement of Solubility of 5 Fluorouracil by Applying Complexation Technique-Characterization, Dissolution and Molecular-Modeling Studies. J. Appl. Pharm. Sci. 2013, 3, 162–166. [Google Scholar]
- Iacovino, R.; Caso, J.V.; Di Donato, C.; Malgieri, G.; Palmieri, M.; Russo, L.; Isernia, C. Cyclodextrins as Complexing Agents: Preparation and Applications. Curr. Org. Chem. 2016, 20. [Google Scholar] [CrossRef]
- Jozwiakowski, M.J.; Connors, K.A. Aqueous solubility behavior of three cyclodextrins. Carbohydr. Res. 1985, 143, 51–59. [Google Scholar] [CrossRef]
- Yamaguchi, R.; Perkins, G.; Hirota, K. Targeting cholesterol with β-cyclodextrin sensitizes cancer cells for apoptosis. FEBS Lett. 2015, 589, 4097–4105. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Shah, V.; Ghosh, A.; Zhang, Z.; Minko, T. Molecular inclusion complexes of β-cyclodextrin derivatives enhance aqueous solubility and cellular internalization of paclitaxel: Preformulation and in vitro assessments. J. Pharm. Pharmacol. 2015, 2, 8. [Google Scholar]
- Caso, J.V.; Russo, L.; Palmieri, M.; Malgieri, G.; Galdiero, S.; Falanga, A.; Isernia, C.; Iacovino, R. Investigating the inclusion properties of aromatic amino acids complexing beta‑cyclodextrins in model peptides. Amino Acids 2015, 47, 2215–2227. [Google Scholar] [CrossRef] [PubMed]
- Job, P. Formation and stability of inorganic complexes in solution. Ann. Chim. 1928, 9, 113–203. [Google Scholar]
- Rawat, S.; Jain, S.K. Solubility enhancement of celecoxib using β-cyclodextrin inclusion complexes. Eur. J. Pharm. Biopharm. 2004, 57, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Iacovino, R.; Caso, J.V.; Rapuano, F.; Russo, A.; Isidori, M.; Lavorgna, M.; Malgieri, G.; Isernia, C. Physicochemical characterization and cytotoxic activity evaluation of hydroxymethylferrocene:β-Cyclodextrin inclusion complex. Molecules 2012, 17, 6056–6070. [Google Scholar] [CrossRef] [PubMed]
- Fallon, L., III. The crystal and molecular structure of 5-fluorouracil. Acta Crystallogr. 1973, 29, 2549–2556. [Google Scholar] [CrossRef]
- Iacovino, R.; Rapuano, F.; Caso, J.V.; Russo, A.; Lavorgna, M.; Russo, C.; Isidori, M.; Russo, L.; Malgieri, G.; Isernia, C. β-Cyclodextrin Inclusion Complex to Improve Physicochemical Properties of Pipemidic Acid: Characterization and Bioactivity Evaluation. Int. J. Mol. Sci. 2013, 14, 13022–13041. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, D.W.; Venkatraman, V. Ultra-fast FFT protein docking ongraphics processors. Bioinformatics 2010, 26, 2398–2405. [Google Scholar] [CrossRef] [PubMed]
- Word, J.M.; Lovell, S.C.; Richardson, J.S.; Richardson, D.C. Asparagine and glutamine: Using hydrogen atom contacts in the choice of side-chain amide orientation. J. Mol. Biol. 1999, 285, 1735–1747. [Google Scholar] [CrossRef] [PubMed]
- Parrella, A.; Lavorgna, M.; Criscuolo, E.; Russo, C.; Isidori, M. Estrogenic activity and cytotoxicity of six anticancer drugs detected in water systems. Sci. Total Environ. 2014, 485–486, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Ono, N.; Arima, H.; Hirayama, F.; Uekama, K. A moderate interaction of maltosyl-α-cyclodextrin with Caco-2 cells in comparison with the parent cyclodextrin. Biol. Pharm. Bull. 2001, 24, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Róka, E.; Ujhelyi, Z.; Deli, M.; Bocsik, A.; Fenyvesi, É.; Szente, L.; Fenyvesi, F.; Vecsernyés, M.; Váradi, J.; Fehér, P.; et al. Evaluation of the cytotoxicity of α-cyclodextrin derivatives on the Caco-2 cell line and human erythrocytes. Molecules 2015, 20, 20269–20285. [Google Scholar] [CrossRef] [PubMed]
- Fenyvesi, F.; Reti-Nagy, K.; Bacso, Z.; Gutay-Toth, Z.; Malanga, M.; Fenyvesi, E.; Szente, L.; Varadi, J.; Ujhelyi, Z.; Feher, P.; et al. Fluorescently labeled methyl-β-cyclodextrin enters intestinal epithelial Caco-2 cells by fluid-phase endocytosis. PLoS ONE 2014, 9, e84856. [Google Scholar] [CrossRef] [PubMed]
- Gidwani, B.; Vyas, A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. BioMed Res. Int. 2015. [Google Scholar] [CrossRef] [PubMed]
- Christian, G.D. Acid-base Equilibria. In Analytical Chemistry, 6th ed.; Wiley: Singapore, 2004; pp. 253–254. [Google Scholar]
- Benesi, H.A.; Hildebrand, J.H. A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons. J. Am. Chem. Soc. 1949, 71, 2703–2707. [Google Scholar] [CrossRef]
- Connors, K.A. The stability of cyclodextrin complexes in solution. Chem. Rev. 1997, 97, 1325–1357. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y. Determination of binding stoichiometry by the continuous variation method: The Job plot. Methods Enzymol. 1982, 87, 509–525. [Google Scholar] [PubMed]
- Koradi, R.; Billeter, M.; Wüthrich, K. MOLMOL: A program for display and analysis of macromolecular structures. J. Mol. Graph. 1996, 14, 29–32. [Google Scholar] [CrossRef]
- DeLano, W.L. The PyMOL Molecular Graphics System; DeLano Scientific: San Carlos, CA, USA, 2002. [Google Scholar]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Baharum, Z.; Akim, A.; Taufiq-Yap, Y.H.; Hamid, R.A.; Kasran, R. In vitro antioxidant and antiproliferative activities of aethanolic plant part extracts of theobroma cacao. Molecules 2014, 19, 18317–18331. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
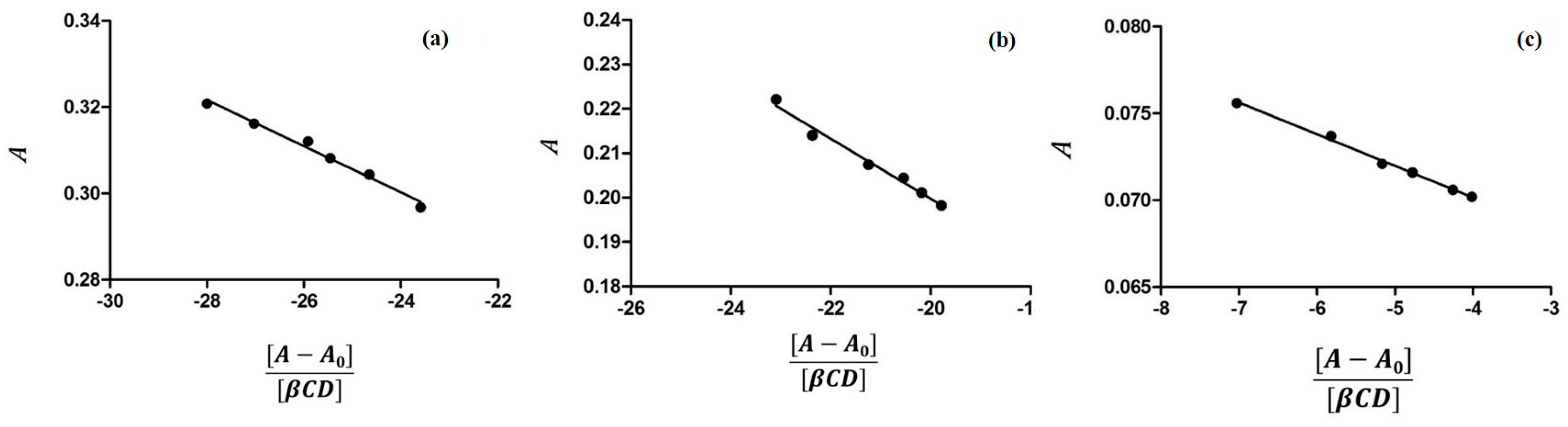
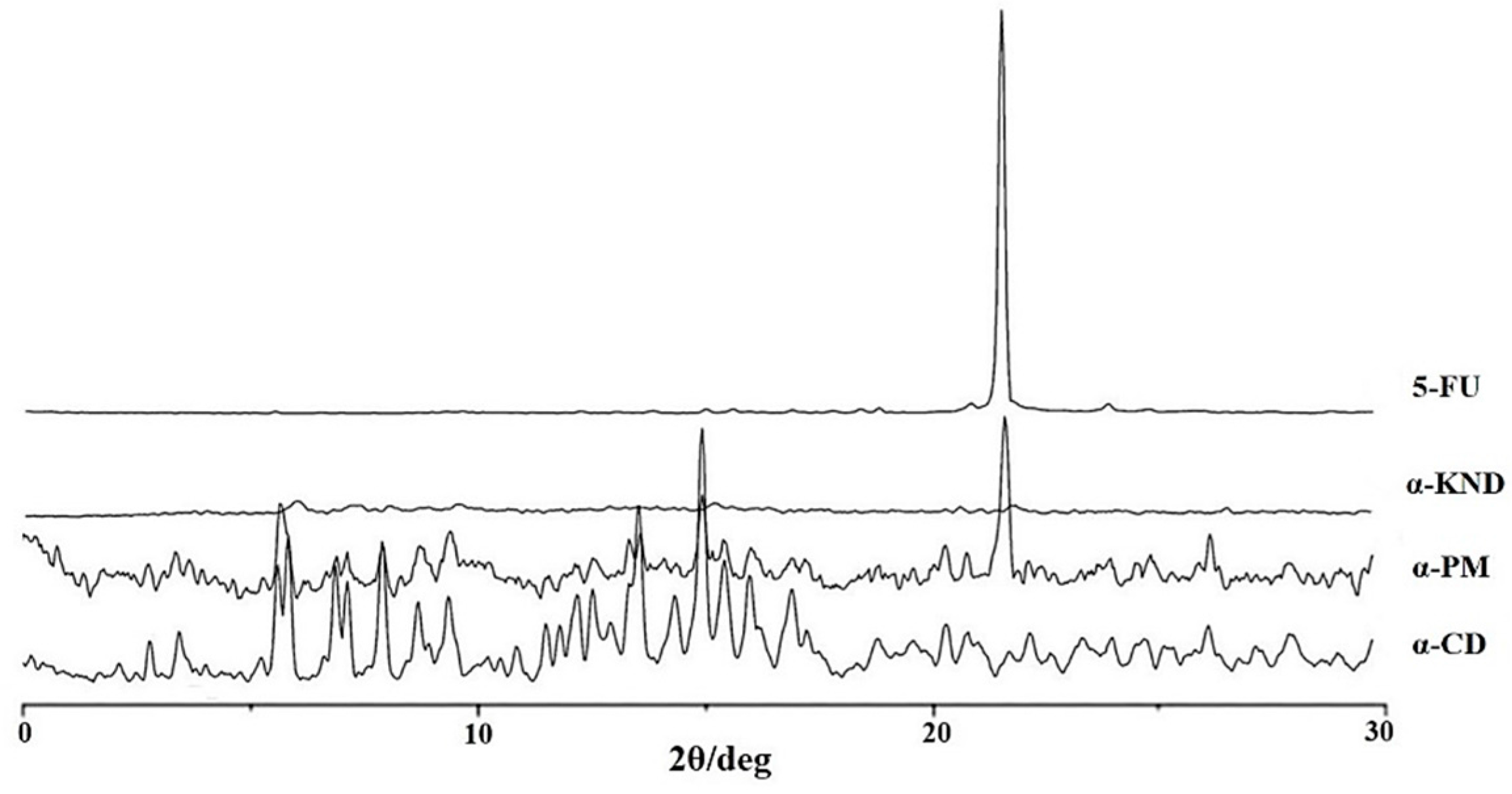
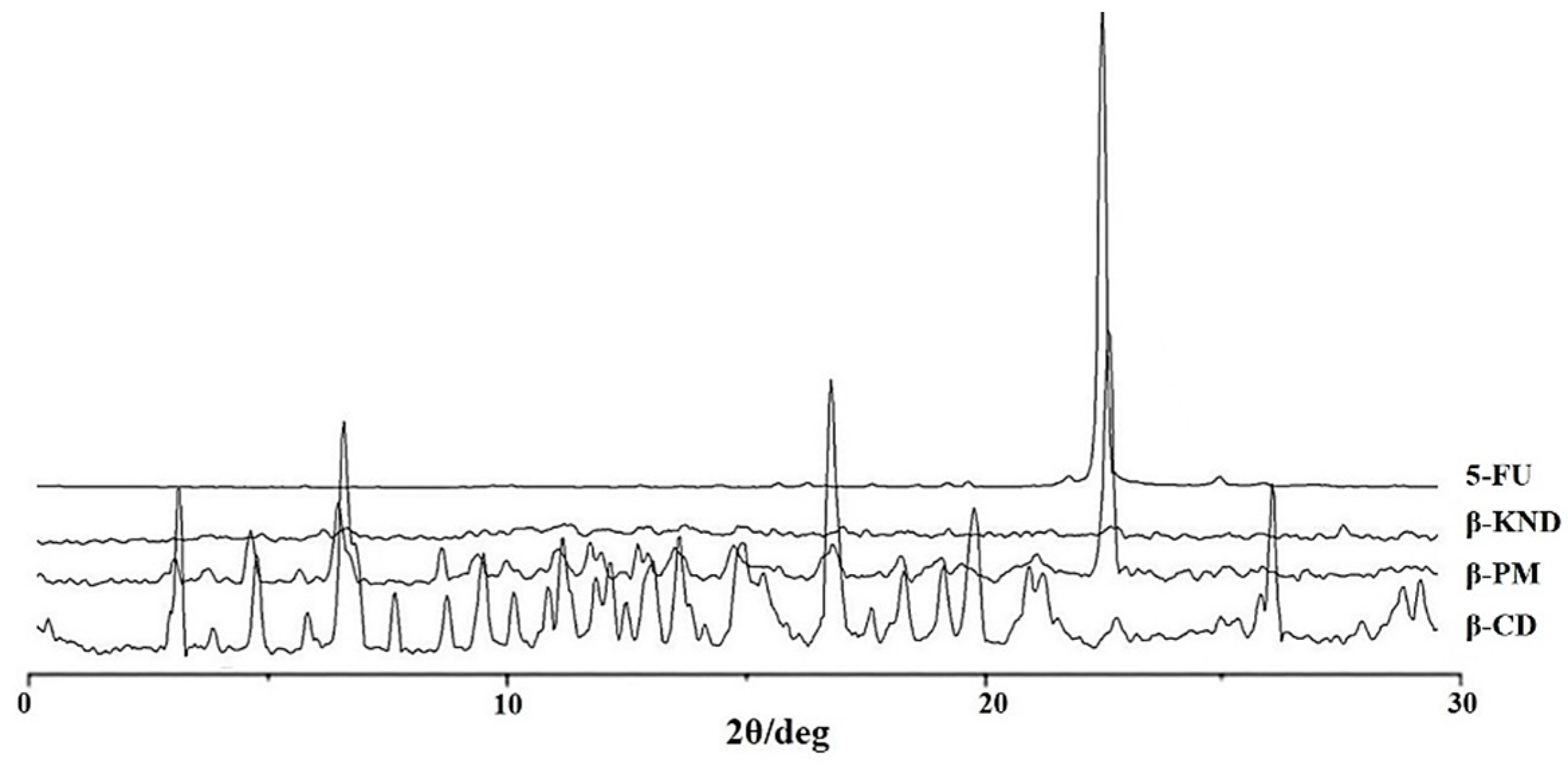
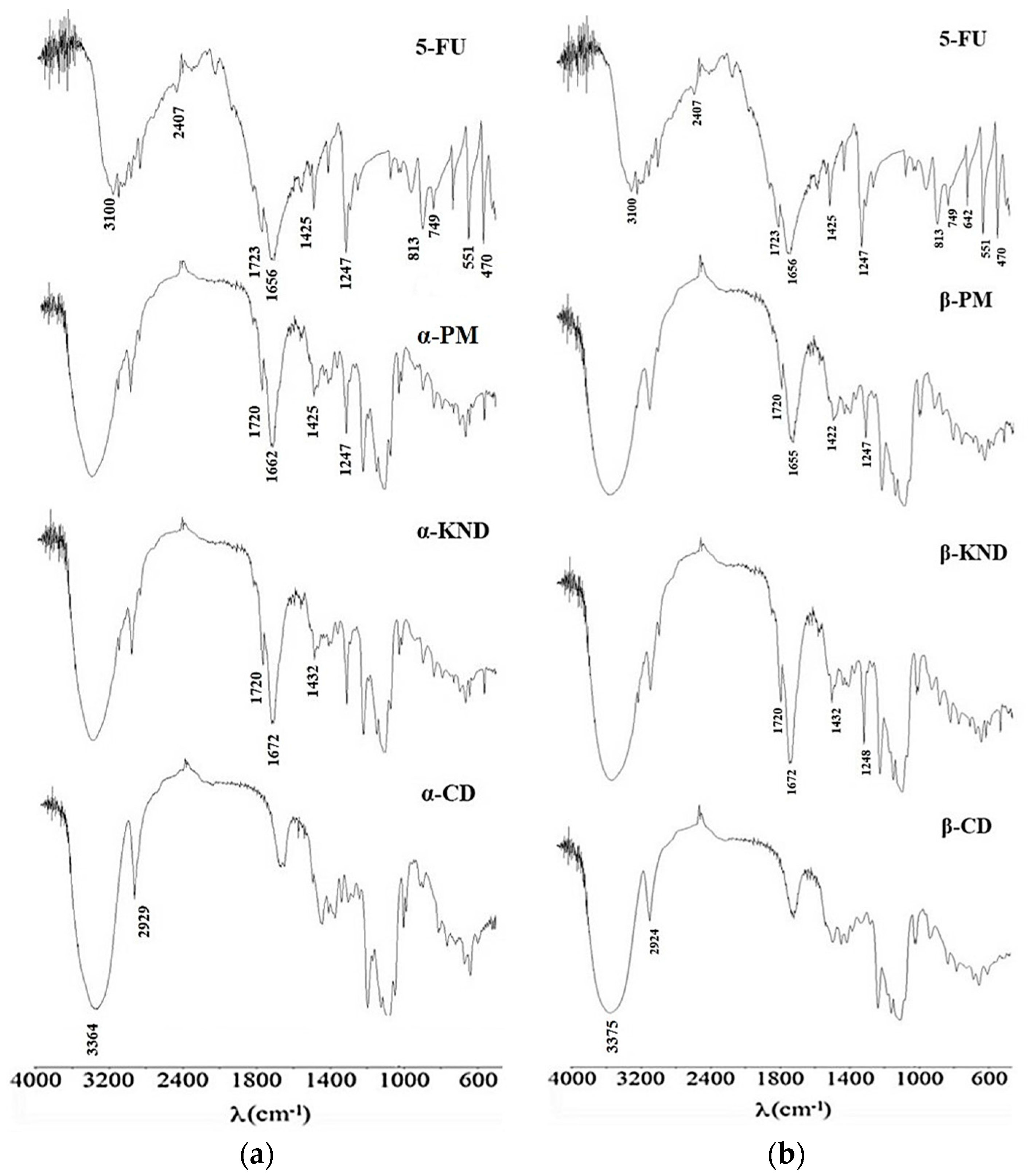

| pH | Kb (M−1) 5-FU:α-CD | Kb (M−1) 5-FU:β-CD |
|---|---|---|
| 4.3 | 74 | 187 |
| 6.8 | 127 | 148 |
| 9.8 | 563 | 549 |
| Compound | t (h) | 5-FU | α-KND | α-PM | β-KND | β-PM |
|---|---|---|---|---|---|---|
| MCF-7 | 24 h | 5 × 103 (3 × 103–8 × 103) | 312 (214–455) | >756 b | 199 (131–301) | >632 b |
| 48 h | 738 a (424–1286) | 301 (87–400) | >756 b | 85 (55–130) | 511 (222–1178) | |
| 72 h | 324 a (161–650) | 134 (84–216) | 463 (217–984) | 31 (18–55) | 309 (141–676) | |
| A-549 | 24 h | 3 × 103 (2 × 103–5 × 103) | 207 (116–371) | 419 (251–699) | 902 (375–1169) | 1 × 103 (0.8 × 103–2 × 103) |
| 48 h | 1 × 103 (0.6 × 103–2 × 103) | 111 (69–179) | 240 (137–423) | 334 (220–509) | 373 (140–648) | |
| 72 h | 200 (153–328) | 73 (54–99) | 85 (43–170) | 212 (108–417) | 255 (143–731) | |
| Hep-G2 | 24 h | 7 × 103 (2 × 103–24 × 103) | 328 (309–346) | 732 (678–790) | 514 (1531–722) | 609 (535–693) |
| 48 h | 2 × 103 (1 × 103–4 × 103) | 278 (251–307) | 528 (427–654) | 414 (178–987) | 493 (384–635) | |
| 72 h | 590 (380–930) | 225 (197–257) | 478 (290–786) | 94 (58–150) | 395 (298–523) | |
| Caco-2 | 24 h | 10 × 103 (4 × 103–26 × 103) | 700 (480–1010) | 920 (460–1810) | 600 (390–920) | 800 (440–1470) |
| 48 h | 1 × 103 (0.7 × 103–4 × 103) | 440 (226–760) | 580 (210–1610) | 400 (230–700) | 350 (140–910) | |
| 72 h | 327 (223–480) | 180 (140–240) | 470 (319–693) | 140 (100–190) | 327 (224–479) |
| Correlation Type | Shape Only |
|---|---|
| FFT Mode | 3D fast life |
| Post processing | MM Minimization |
| Receptor range angle | 180 |
| Ligand range angle | 180 |
| Twist range | 360 |
| Distance range | 40 |
| Docking main scan | 18 |
| Docking main search | 25 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Donato, C.; Lavorgna, M.; Fattorusso, R.; Isernia, C.; Isidori, M.; Malgieri, G.; Piscitelli, C.; Russo, C.; Russo, L.; Iacovino, R. Alpha- and Beta-Cyclodextrin Inclusion Complexes with 5-Fluorouracil: Characterization and Cytotoxic Activity Evaluation. Molecules 2016, 21, 1644. https://doi.org/10.3390/molecules21121644
Di Donato C, Lavorgna M, Fattorusso R, Isernia C, Isidori M, Malgieri G, Piscitelli C, Russo C, Russo L, Iacovino R. Alpha- and Beta-Cyclodextrin Inclusion Complexes with 5-Fluorouracil: Characterization and Cytotoxic Activity Evaluation. Molecules. 2016; 21(12):1644. https://doi.org/10.3390/molecules21121644
Chicago/Turabian StyleDi Donato, Cristina, Margherita Lavorgna, Roberto Fattorusso, Carla Isernia, Marina Isidori, Gaetano Malgieri, Concetta Piscitelli, Chiara Russo, Luigi Russo, and Rosa Iacovino. 2016. "Alpha- and Beta-Cyclodextrin Inclusion Complexes with 5-Fluorouracil: Characterization and Cytotoxic Activity Evaluation" Molecules 21, no. 12: 1644. https://doi.org/10.3390/molecules21121644
APA StyleDi Donato, C., Lavorgna, M., Fattorusso, R., Isernia, C., Isidori, M., Malgieri, G., Piscitelli, C., Russo, C., Russo, L., & Iacovino, R. (2016). Alpha- and Beta-Cyclodextrin Inclusion Complexes with 5-Fluorouracil: Characterization and Cytotoxic Activity Evaluation. Molecules, 21(12), 1644. https://doi.org/10.3390/molecules21121644






