Expression, Purification, and Characterization of Interleukin-11 Orthologues
Abstract
:1. Introduction
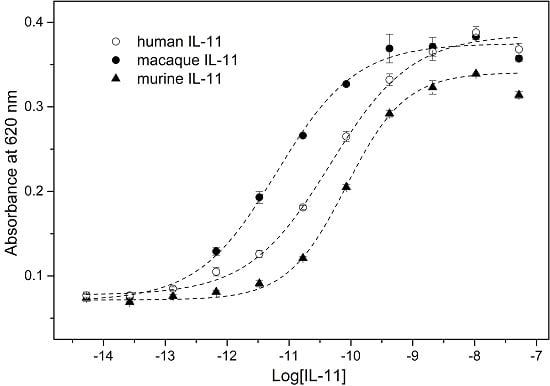
2. Results and Discussion
3. Experimental Section
3.1. Materials
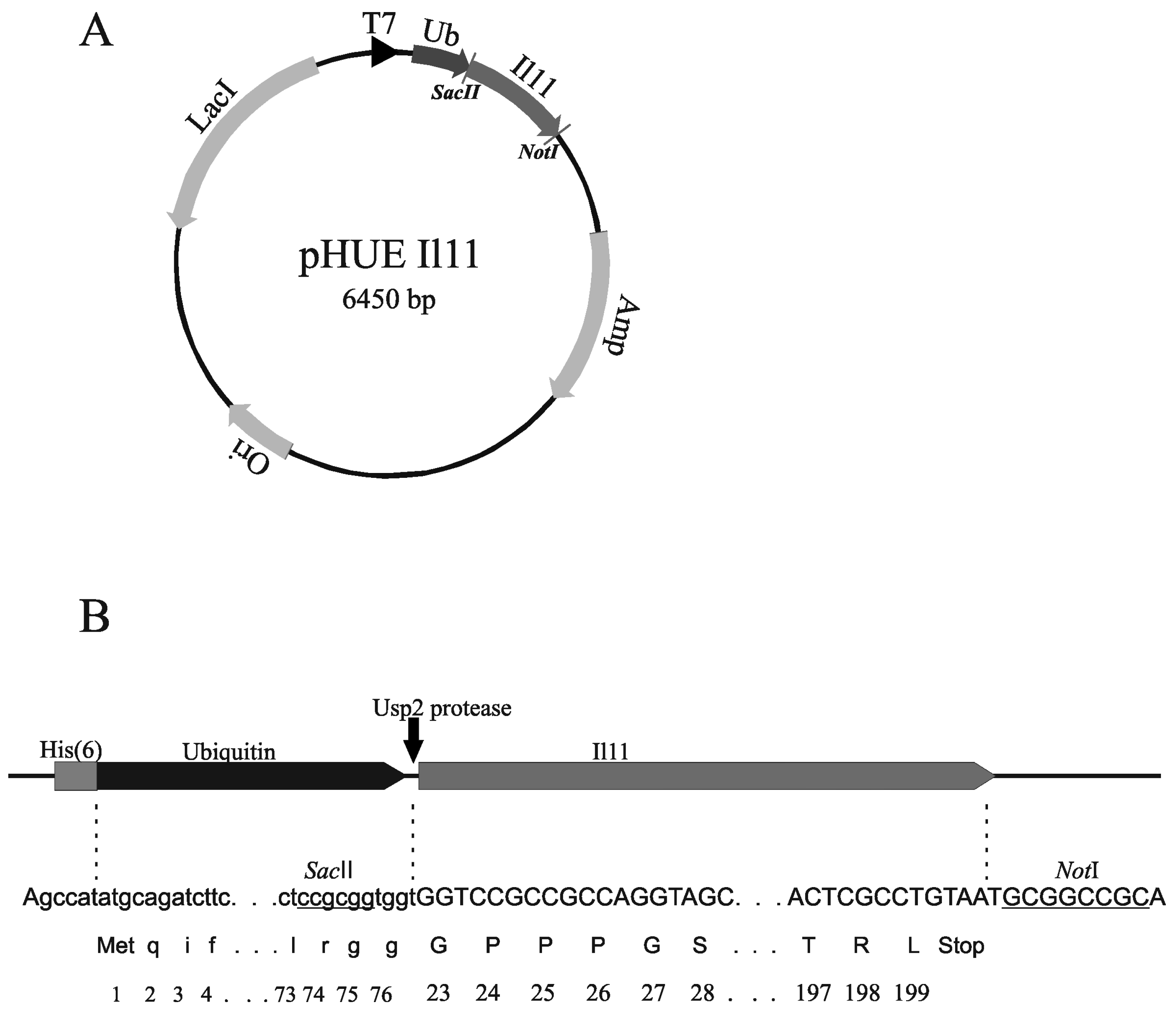
3.2. Design of Plasmids
3.3. Preparation of Recombinant IL-11 Samples
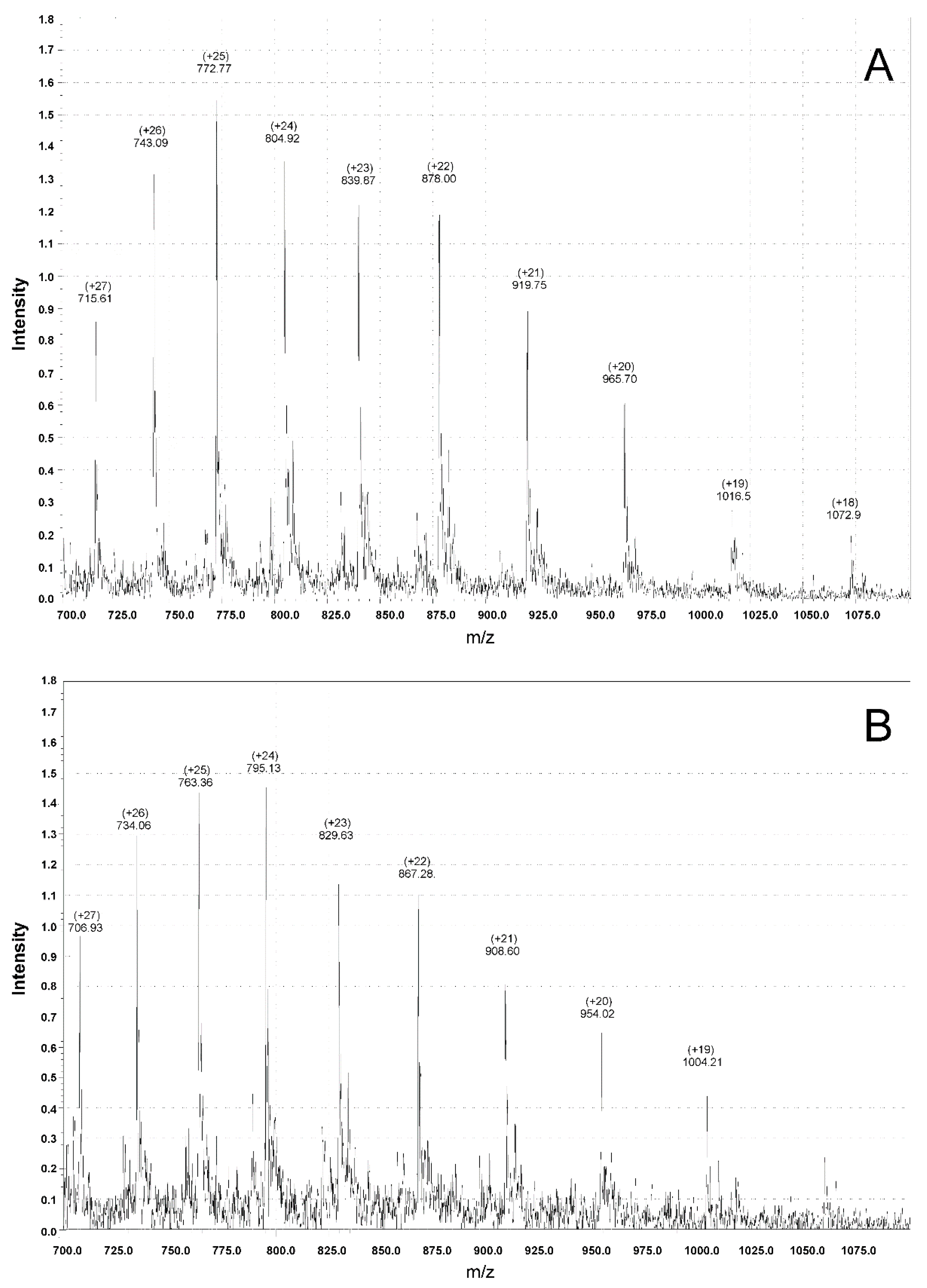
3.4. Mass Spectrometry Measurements
3.5. Protein Biotinylation
3.6. Surface Plasmon Resonance Studies
3.7. IL-11-Induced STAT3 Activation Assay
3.8. Per-residue Protein Disorder Predictions
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Putoczki, T.L.; Ernst, M. IL-11 signaling as a therapeutic target for cancer. Immunotherapy 2015, 7, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Garbers, C.; Scheller, J. Interleukin-6 and interleukin-11: Same same but different. Biol. Chem. 2013, 394, 1145–1161. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.R.; Bennett, F.; Calvetti, J.A.; Kelleher, K.; Wood, C.R.; O’Hara, R.M., Jr.; Leary, A.C.; Sibley, B.; Clark, S.C.; Williams, D.A.; et al. Molecular cloning of a cdna encoding interleukin 11, a stromal cell-derived lymphopoietic and hematopoietic cytokine. Proc. Natl. Acad. Sci. USA 1990, 87, 7512–7516. [Google Scholar] [CrossRef] [PubMed]
- Negahdaripour, M.; Nezafat, N.; Ghasemi, Y. A panoramic review and in silico analysis of IL-11 structure and function. Cytokine Growth Factor Rev. 2016, 32, 41–61. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. IL-6 and related cytokines as the critical lynchpins between inflammation and cancer. Semin. Immunol. 2014, 26, 54–74. [Google Scholar] [CrossRef] [PubMed]
- Schaper, F.; Rose-John, S. Interleukin-6: Biology, signaling and strategies of blockade. Cytokine Growth Factor Rev. 2015, 26, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Ogata, A.; Kishimoto, T. A new era for the treatment of inflammatory autoimmune diseases by interleukin-6 blockade strategy. Semin. Immunol. 2014, 26, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef] [PubMed]
- Yuzhalin, A.E.; Kutikhin, A.G. IL-6 family and cancer. In Interleukins in Cancer Biology; Academic Press: Amsterdam, The Netherlands, 2015; Chapter 5; pp. 117–146. [Google Scholar]
- Putoczki, T.L.; Thiem, S.; Loving, A.; Busutti, R.A.; Wilson, N.J.; Ziegler, P.K.; Nguyen, P.M.; Preaudet, A.; Farid, R.; Edwards, K.M.; et al. Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically. Cancer Cell 2013, 24, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, C.N.; Chand, A.; Putoczki, T.L.; Ernst, M. Emerging roles for IL-11 signaling in cancer development and progression: Focus on breast cancer. Cytokine Growth Factor Rev. 2015, 26, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Lewis, V.O. IL-11ralpha: A novel target for the treatment of osteosarcoma. Adv. Exp. Med. Biol. 2014, 804, 285–289. [Google Scholar] [PubMed]
- Lokau, J.; Nitz, R.; Agthe, M.; Monhasery, N.; Aparicio-Siegmund, S.; Schumacher, N.; Wolf, J.; Moller-Hackbarth, K.; Waetzig, G.H.; Grotzinger, J.; et al. Proteolytic cleavage governs interleukin-11 trans-signaling. Cell Rep. 2016, 14, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.H.; Zhu, Z.; Wakefield, M.R.; Xiao, H.; Bai, Q.; Fang, Y. The role of IL-11 in immunity and cancer. Cancer Lett. 2016, 373, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Cardo-Vila, M.; Marchio, S.; Sato, M.; Staquicini, F.I.; Smith, T.L.; Bronk, J.K.; Yin, G.S.; Zurita, A.J.; Sun, M.H.; Behrens, C.; et al. Interleukin-11 receptor is a candidate target for ligand-directed therapy in lung cancer analysis of clinical samples and BMTP-11 preclinical activity. Am. J. Pathol. 2016, 186, 2162–2170. [Google Scholar] [CrossRef] [PubMed]
- Curtis, D.J.; Hilton, D.J.; Roberts, B.; Murray, L.; Nicola, N.; Begley, C.G. Recombinant soluble interleukin-11 (IL-11) receptor alpha-chain can act as an IL-11 antagonist. Blood 1997, 90, 4403–4412. [Google Scholar] [PubMed]
- Du, X.X.; Williams, D.A. Interleukin-11: Review of molecular, cell biology, and clinical use. Blood 1997, 89, 3897–3908. [Google Scholar] [PubMed]
- Guk, K.D.; Kuprash, D.V. Interleukin-11, an IL-6 like cytokine. Mol. Biol. 2011, 45, 44–55. [Google Scholar]
- Permyakov, E.A.; Uversky, V.N.; Permyakov, S.E. Interleukin-11: A multifunctional cytokine with intrinsically disordered regions. Cell Biochem. Biophys. 2016, 74, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Putoczki, T.L.; Dobson, R.C.; Griffin, M.D. The structure of human interleukin-11 reveals receptor-binding site features and structural differences from interleukin-6. Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 2277–2285. [Google Scholar] [CrossRef] [PubMed]
- Van Snick, J. Interleukin-6: An overview. Annu. Rev. Immunol. 1990, 8, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Wilkin, J.M.; Boisteau, O.; Harmegnies, D.; Blanc, C.; Vandenbussche, P.; Montero-Julian, F.A.; Jacques, Y.; Content, J. Engineering and use of p-32-labeled human recombinant interleukin-11 for receptor binding studies. Eur. J. Biochem. 2002, 269, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Leon, A.; Wang, X.M.; Champion-Arnaud, P.; Sobczyk, A.; Pain, B.; Content, J.; Jacques, Y.; Valarche, I. Expression of a bioactive recombinant human interleukin-11 in chicken HD11 cell line. Cytokine 2005, 30, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.K.; Xu, Z.X.; Huang, W.D.; Ming, T.S.; Xie, W.; Xu, Y.; Zhang, X.G. Expression and purification of recombinant human interleukin-11 in Pichia pastoris. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 2001, 23, 127–131. [Google Scholar] [PubMed]
- Tang, J.; Xu, X.; Nie, X.; Mao, Q.; Gao, J. Prokaryotic expression, purification, and identification of recombinant human IL-11 protein. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi 2012, 29, 530–533. [Google Scholar] [PubMed]
- Farajnia, S.; Hassanpour, R.; Lotfipour, F. Cloning and expression of human IL-11 in E. coli. Pharm. Sci. 2010, 15, 353–359. [Google Scholar]
- Han, W.; Zhang, Y.; Yan, Z.; Shi, J. Construction of a new tumour necrosis factor fusion-protein expression vector for high-level expression of heterologous genes in Escherichia coli. Biotechnol. Appl. Biochem. 2003, 37, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Dan, G.; Gong, H.; Cao, L. Purification and characterization of recombinant truncated human interleukin-11 expressed as fusion protein in Escherichia coli. Biotechnol. Lett. 2005, 27, 905–910. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Huang, H. Preparation of rhIL-11 from fusion protein by using enterokinase. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2008, 16, 903–909. [Google Scholar] [PubMed]
- Zhang, Y.; Liu, C.H.; Liu, Y.L.; Tien, P. cDNA cloning, fusion expression in Escherichia coli and activity assay of hIL-11. Sheng Wu Gong Cheng Xue Bao 2000, 16, 353–356. [Google Scholar] [PubMed]
- Morris, J.C.; Neben, S.; Bennett, F.; Finnerty, H.; Long, A.; Beier, D.R.; Kovacic, S.; McCoy, J.M.; DiBlasio-Smith, E.; La Vallie, E.R.; et al. Molecular cloning and characterization of murine interleukin-11. Exp. Hematol. 1996, 24, 1369–1376. [Google Scholar] [PubMed]
- Kazakov, A.S.; Sokolov, A.S.; Rastrygina, V.A.; Solovyev, V.V.; Ismailov, R.G.; Mikhailov, R.V.; Ulitin, A.B.; Yakovenko, A.R.; Mirzabekov, T.A.; Permyakov, E.A.; et al. High-affinity interaction between interleukin-11 and S100P protein. Biochem. Biophys. Res. Commun. 2015, 468, 733–738. [Google Scholar] [CrossRef] [PubMed]
- Catanzariti, A.M.; Soboleva, T.A.; Jans, D.A.; Board, P.G.; Baker, R.T. An efficient system for high-level expression and easy purification of authentic recombinant proteins. Protein Sci. 2004, 13, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Baker, R.T.; Catanzariti, A.M.; Karunasekara, Y.; Soboleva, T.A.; Sharwood, R.; Whitney, S.; Board, P.G. Using deubiquitylating enzymes as research tools. Method Enzymol. 2005, 398, 540–554. [Google Scholar]
- Catic, A.; Misaghi, S.; Korbel, G.A.; Ploegh, H.L. Elad, a deubiquitinating protease expressed by E. coli. PLoS ONE 2007, 2, e381. [Google Scholar] [CrossRef] [PubMed]
- Hilton, D.J.; Hilton, A.A.; Raicevic, A.; Rakar, S.; Harrison-Smith, M.; Gough, N.M.; Begley, C.G.; Metcalf, D.; Nicola, N.A.; Willson, T.A. Cloning of a murine IL-11 receptor alpha-chain; requirement for gp130 for high affinity binding and signal transduction. EMBO J. 1994, 13, 4765–4775. [Google Scholar] [PubMed]
- Harmegnies, D.; Wang, X.M.; Vandenbussche, P.; Leon, A.; Vusio, P.; Grotzinger, J.; Jacques, Y.; Goormaghtigh, E.; Devreese, B.; Content, J. Characterization of a potent human interleukin-11 agonist. Biochem. J. 2003, 375, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.L.; Chen, Y.; Han, C.X.; Fu, D.Y.; Chen, H. Plasma interleukin-11 (IL-11) levels have diagnostic and prognostic roles in patients with pancreatic cancer. Tumor Biol. 2014, 35, 11467–11472. [Google Scholar] [CrossRef] [PubMed]
- Czupryn, M.; Bennett, F.; Dube, J.; Grant, K.; Scoble, H.; Sookdeo, H.; McCoy, J.M. Alanine-scanning mutagenesis of human interleukin-11: Identification of regions important for biological activity. Ann. N. Y. Acad. Sci. 1995, 762, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Babu, M.; Barbar, E.; Blackledge, M.; Bondos, S.E.; Dosztányi, Z.; Dyson, H.J.; Forman-Kay, J.; Fuxreiter, M.; Gsponer, J.; et al. What’s in a name? Why these proteins are intrinsically disordered. Intrinsically Disord. Proteins 2013, 1, e24157. [Google Scholar] [CrossRef]
- Dunker, A.K.; Obradovic, Z.; Romero, P.; Garner, E.C.; Brown, C.J. Intrinsic protein disorder in complete genomes. Genome Inform. Ser. Workshop Genome Inform. 2000, 11, 161–171. [Google Scholar] [PubMed]
- Ward, J.J.; Sodhi, J.S.; McGuffin, L.J.; Buxton, B.F.; Jones, D.T. Prediction and functional analysis of native disorder in proteins from the three kingdoms of life. J. Mol. Biol. 2004, 337, 635–645. [Google Scholar] [CrossRef] [PubMed]
- Tokuriki, N.; Oldfield, C.J.; Uversky, V.N.; Berezovsky, I.N.; Tawfik, D.S. Do viral proteins possess unique biophysical features? Trends Biochem. Sci. 2009, 34, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Williams, R.W.; Oldfield, C.J.; Dunker, A.K.; Uversky, V.N. Archaic chaos: Intrinsically disordered proteins in Archaea. BMC Syst. Biol. 2010, 4 (Suppl. 1), S1. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. The mysterious unfoldome: Structureless, underappreciated, yet vital part of any given proteome. J. Biomed. Biotechnol. 2010, 2010, 568068. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Dunker, A.K.; Uversky, V.N. Orderly order in protein intrinsic disorder distribution: Disorder in 3500 proteomes from viruses and the three domains of life. J. Biomol. Struct. Dyn. 2012, 30, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Daughdrill, G.W.; Pielak, G.J.; Uversky, V.N.; Cortese, M.S.; Dunker, A.K. Natively disordered proteins. In Handbook of Protein Folding; Buchner, J., Kiefhaber, T., Eds.; Wiley-VCH, Verlag GmbH & Co.: Weinheim, Germany, 2005; pp. 271–353. [Google Scholar]
- Dunker, A.K.; Brown, C.J.; Obradovic, Z. Identification and functions of usefully disordered proteins. Adv. Protein Chem. 2002, 62, 25–49. [Google Scholar] [PubMed]
- Dunker, A.K.; Brown, C.J.; Lawson, J.D.; Iakoucheva, L.M.; Obradovic, Z. Intrinsic disorder and protein function. Biochemistry 2002, 41, 6573–6582. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Cortese, M.S.; Romero, P.; Iakoucheva, L.M.; Uversky, V.N. Flexible nets. The roles of intrinsic disorder in protein interaction networks. FEBS J. 2005, 272, 5129–5148. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Garner, E.; Guilliot, S.; Romero, P.; Albrecht, K.; Hart, J.; Obradovic, Z.; Kissinger, C.; Villafranca, J.E. Protein disorder and the evolution of molecular recognition: Theory, predictions and observations. Pac. Symp. Biocomput. 1998, 473–484. [Google Scholar]
- Dunker, A.K.; Lawson, J.D.; Brown, C.J.; Williams, R.M.; Romero, P.; Oh, J.S.; Oldfield, C.J.; Campen, A.M.; Ratliff, C.M.; Hipps, K.W.; et al. Intrinsically disordered protein. J. Mol. Graph. Model. 2001, 19, 26–59. [Google Scholar] [CrossRef]
- Dyson, H.J.; Wright, P.E. Intrinsically unstructured proteins and their functions. Nat. Rev. Mol. Cell Biol. 2005, 6, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Wright, P.E.; Dyson, H.J. Intrinsically unstructured proteins: Re-assessing the protein structure-function paradigm. J. Mol. Biol. 1999, 293, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P. Intrinsically unstructured proteins. Trends Biochem. Sci. 2002, 27, 527–533. [Google Scholar] [CrossRef]
- Tompa, P. The interplay between structure and function in intrinsically unstructured proteins. FEBS Lett. 2005, 579, 3346–3354. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P.; Csermely, P. The role of structural disorder in the function of RNA and protein chaperones. FASEB J. 2004, 18, 1169–1175. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P.; Szasz, C.; Buday, L. Structural disorder throws new light on moonlighting. Trends Biochem. Sci. 2005, 30, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. Natively unfolded proteins: A point where biology waits for physics. Protein Sci. 2002, 11, 739–756. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N. What does it mean to be natively unfolded? Eur. J. Biochem. 2002, 269, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Gillespie, J.R.; Fink, A.L. Why are “natively unfolded″ proteins unstructured under physiologic conditions? Proteins 2000, 41, 415–427. [Google Scholar] [CrossRef]
- Uversky, V.N. Protein folding revisited. A polypeptide chain at the folding-misfolding-nonfolding cross-roads: Which way to go? Cell. Mol. Life Sci. 2003, 60, 1852–1871. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Vucetic, S.; Iakoucheva, L.M.; Oldfield, C.J.; Dunker, A.K.; Uversky, V.N.; Obradovic, Z. Functional anthology of intrinsic disorder. 1. Biological processes and functions of proteins with long disordered regions. J. Proteome Res. 2007, 6, 1882–1898. [Google Scholar] [CrossRef] [PubMed]
- Vucetic, S.; Xie, H.; Iakoucheva, L.M.; Oldfield, C.J.; Dunker, A.K.; Obradovic, Z.; Uversky, V.N. Functional anthology of intrinsic disorder. 2. Cellular components, domains, technical terms, developmental processes, and coding sequence diversities correlated with long disordered regions. J. Proteome Res. 2007, 6, 1899–1916. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Vucetic, S.; Iakoucheva, L.M.; Oldfield, C.J.; Dunker, A.K.; Obradovic, Z.; Uversky, V.N. Functional anthology of intrinsic disorder. 3. Ligands, post-translational modifications, and diseases associated with intrinsically disordered proteins. J. Proteome Res. 2007, 6, 1917–1932. [Google Scholar] [CrossRef] [PubMed]
- Uversky, V.N.; Oldfield, C.J.; Dunker, A.K. Showing your ID: Intrinsic disorder as an ID for recognition, regulation and cell signaling. J. Mol. Recognit. 2005, 18, 343–384. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Oldfield, C.J.; Meng, J.; Romero, P.; Uversky, V.N.; Dunker, A.K. Mining alpha-helix-forming molecular recognition features with cross species sequence alignments. Biochemistry 2007, 46, 13468–13477. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Silman, I.; Uversky, V.N.; Sussman, J.L. Function and structure of inherently disordered proteins. Curr. Opin. Struct. Biol. 2008, 18, 756–764. [Google Scholar] [CrossRef] [PubMed]
- Dunker, A.K.; Uversky, V.N. Signal transduction via unstructured protein conduits. Nat. Chem. Biol. 2008, 4, 229–230. [Google Scholar] [CrossRef] [PubMed]
- Mohan, A.; Oldfield, C.J.; Radivojac, P.; Vacic, V.; Cortese, M.S.; Dunker, A.K.; Uversky, V.N. Analysis of molecular recognition features (MoRFs). J. Mol. Biol. 2006, 362, 1043–1059. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, C.J.; Cheng, Y.; Cortese, M.S.; Romero, P.; Uversky, V.N.; Dunker, A.K. Coupled folding and binding with alpha-helix-forming molecular recognition elements. Biochemistry 2005, 44, 12454–12470. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Pace, C.N.; Vajdos, F.; Fee, L.; Grimsley, G.; Gray, T. How to measure and predict the molar absorption coefficient of a protein. Protein Sci. 1995, 4, 2411–2423. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.; Obradovic, Z.; Li, X.; Garner, E.C.; Brown, C.J.; Dunker, A.K. Sequence complexity of disordered protein. Proteins 2001, 42, 38–48. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the rIL-11 proteins from Homo sapiens, Macaca fascicularis, and Mus musculus are available from the authors.


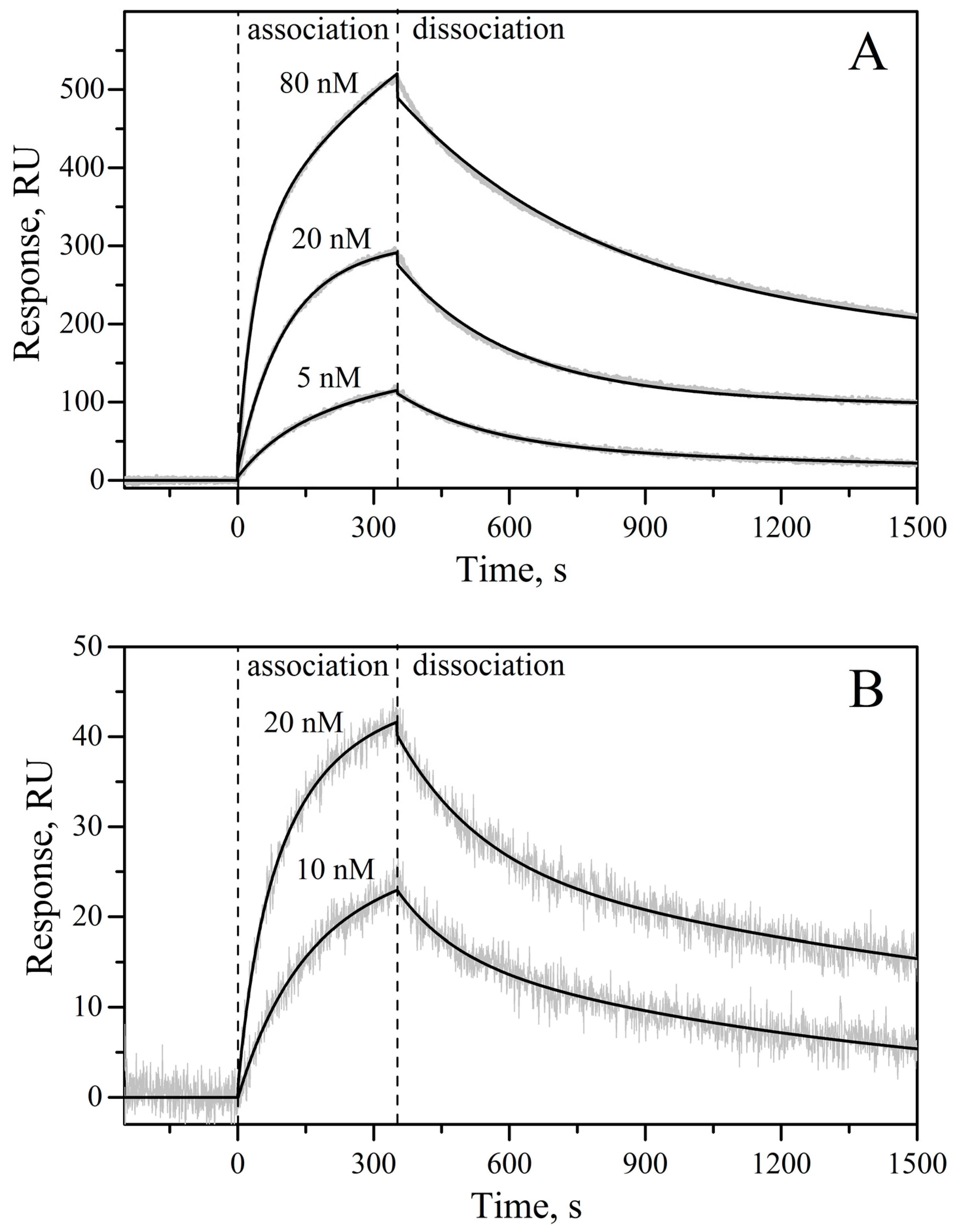
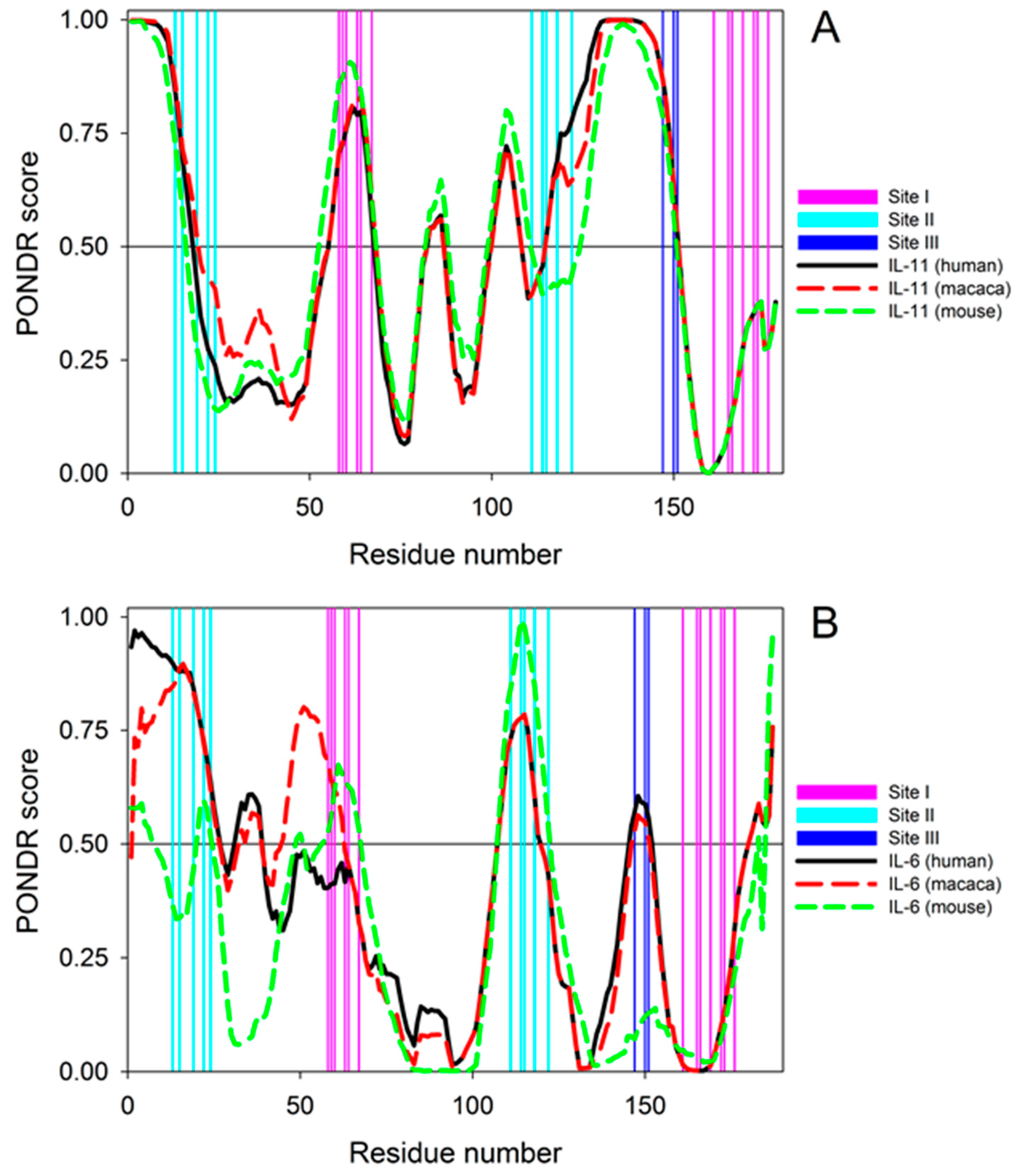
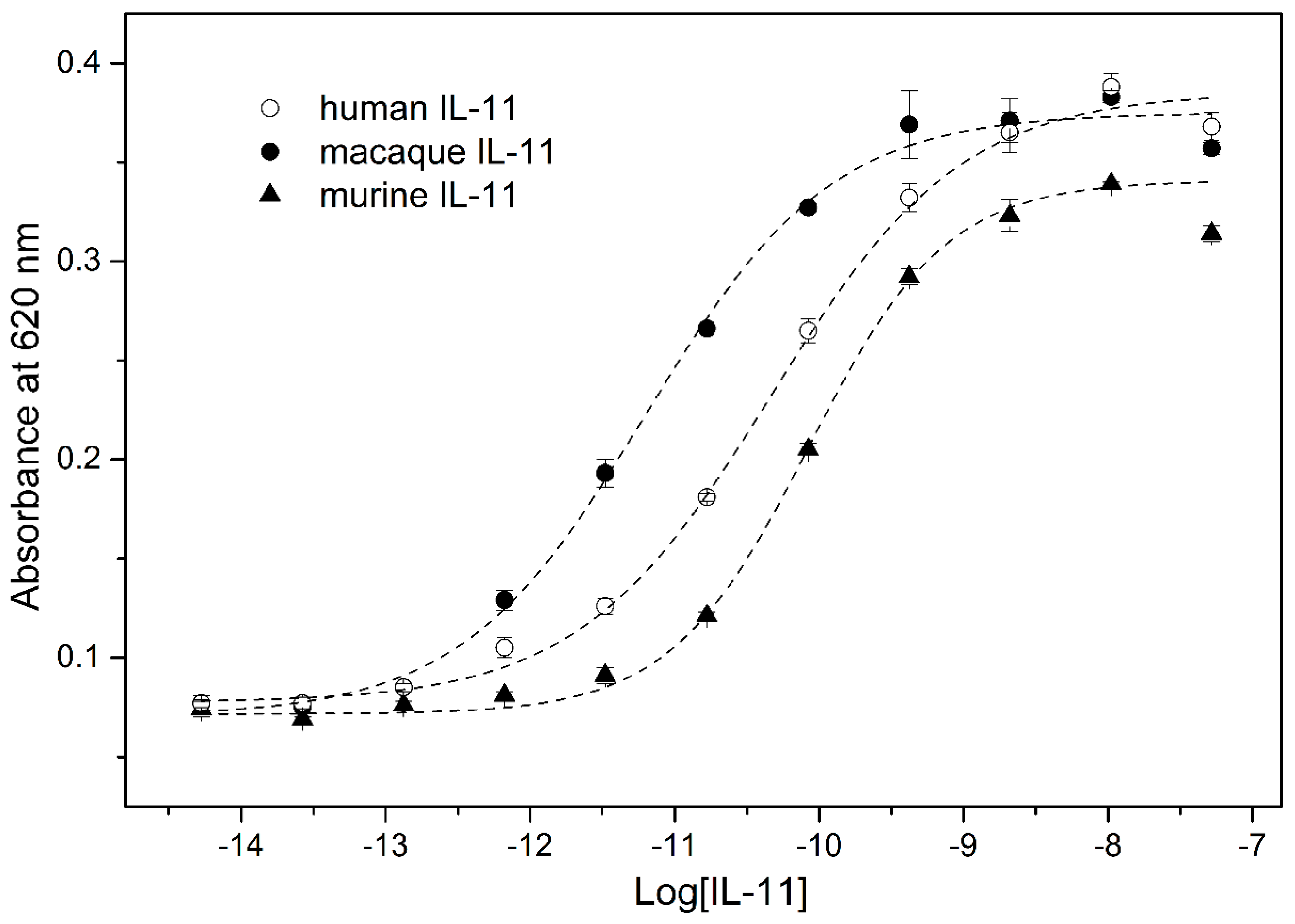
| rIL-11 Sample | kd1, s−1 | Kd1, nM | Rmax1 | kd2, s−1 | Kd2, nM | Rmax2 |
|---|---|---|---|---|---|---|
| macaque | (8.15 ± 0.03) × 10−4 | 4.2 ± 2.6 | 352 | (3.8 ± 1.4) × 10−3 | 8.9 ± 1.8 | 203 |
| mouse | (7.0 ± 2.5) × 10−4 | 2.2 ± 0.7 | 31 | (6.1 ± 1.3) × 10−3 | 14.6 ± 0.8 | 19 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolov, A.S.; Kazakov, A.S.; Solovyev, V.V.; Ismailov, R.G.; Uversky, V.N.; Lapteva, Y.S.; Mikhailov, R.V.; Pavlova, E.V.; Terletskaya, I.O.; Ermolina, L.V.; et al. Expression, Purification, and Characterization of Interleukin-11 Orthologues. Molecules 2016, 21, 1632. https://doi.org/10.3390/molecules21121632
Sokolov AS, Kazakov AS, Solovyev VV, Ismailov RG, Uversky VN, Lapteva YS, Mikhailov RV, Pavlova EV, Terletskaya IO, Ermolina LV, et al. Expression, Purification, and Characterization of Interleukin-11 Orthologues. Molecules. 2016; 21(12):1632. https://doi.org/10.3390/molecules21121632
Chicago/Turabian StyleSokolov, Andrei S., Alexei S. Kazakov, Valery V. Solovyev, Ramis G. Ismailov, Vladimir N. Uversky, Yulia S. Lapteva, Roman V. Mikhailov, Ekaterina V. Pavlova, Iana O. Terletskaya, Ludmila V. Ermolina, and et al. 2016. "Expression, Purification, and Characterization of Interleukin-11 Orthologues" Molecules 21, no. 12: 1632. https://doi.org/10.3390/molecules21121632
APA StyleSokolov, A. S., Kazakov, A. S., Solovyev, V. V., Ismailov, R. G., Uversky, V. N., Lapteva, Y. S., Mikhailov, R. V., Pavlova, E. V., Terletskaya, I. O., Ermolina, L. V., Permyakov, S. E., & Permyakov, E. A. (2016). Expression, Purification, and Characterization of Interleukin-11 Orthologues. Molecules, 21(12), 1632. https://doi.org/10.3390/molecules21121632