Chemical Constituents of Phaius mishmensis
Abstract
:1. Introduction
2. Results and Discussion
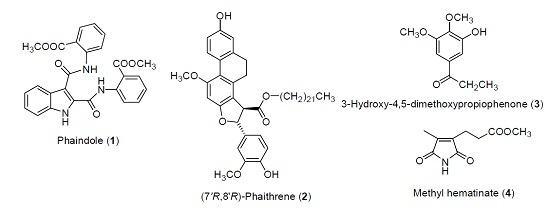
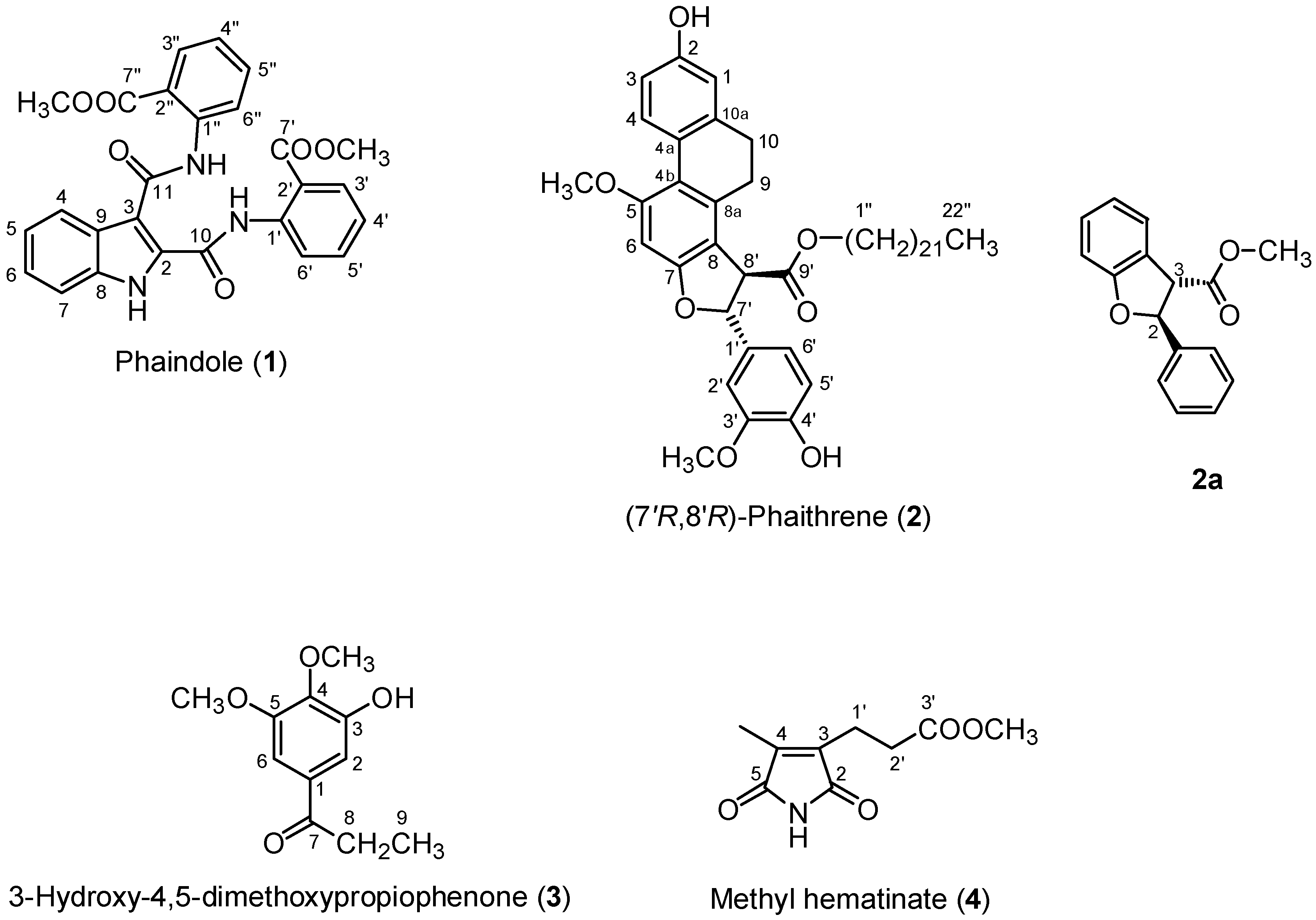
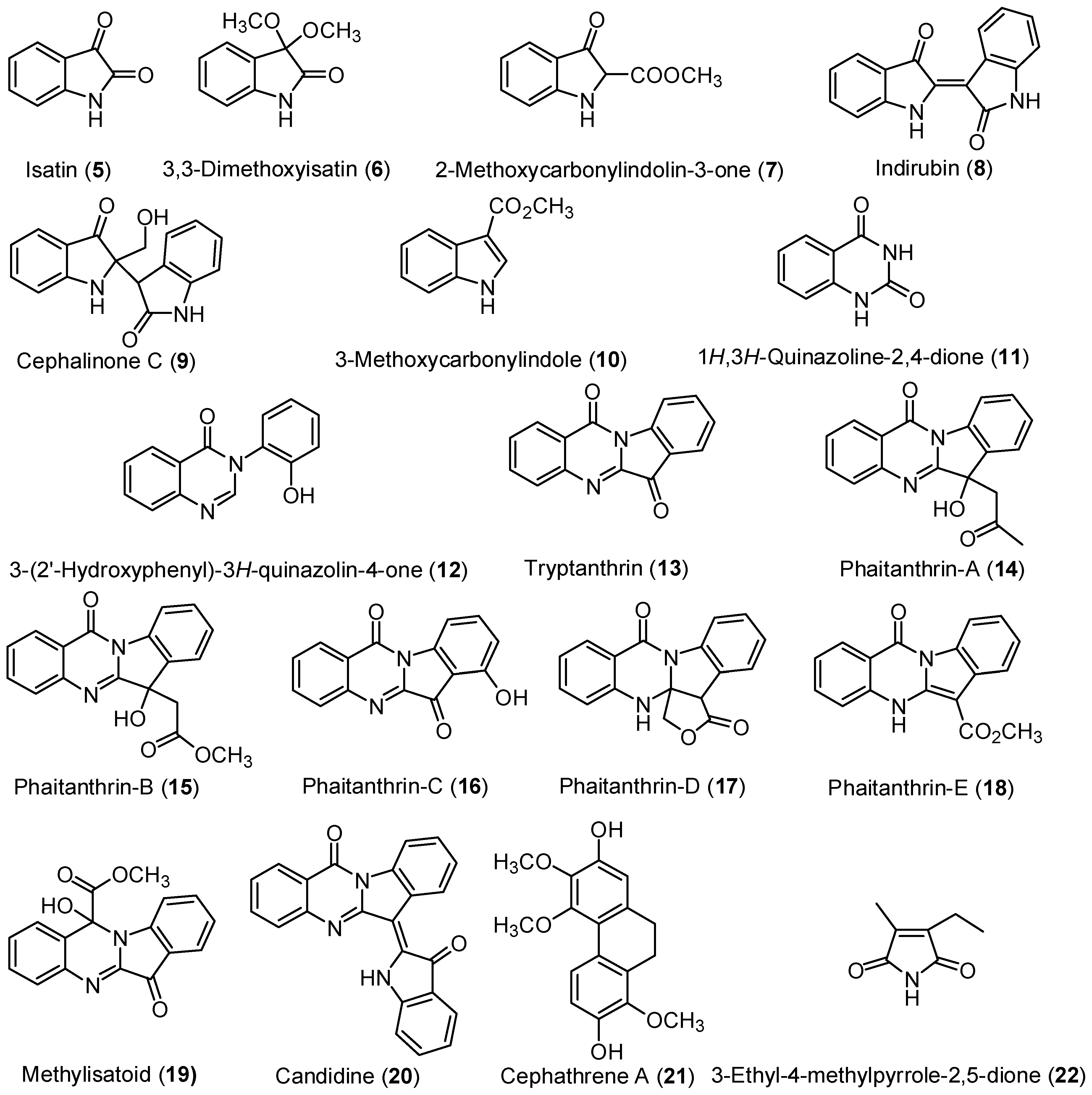
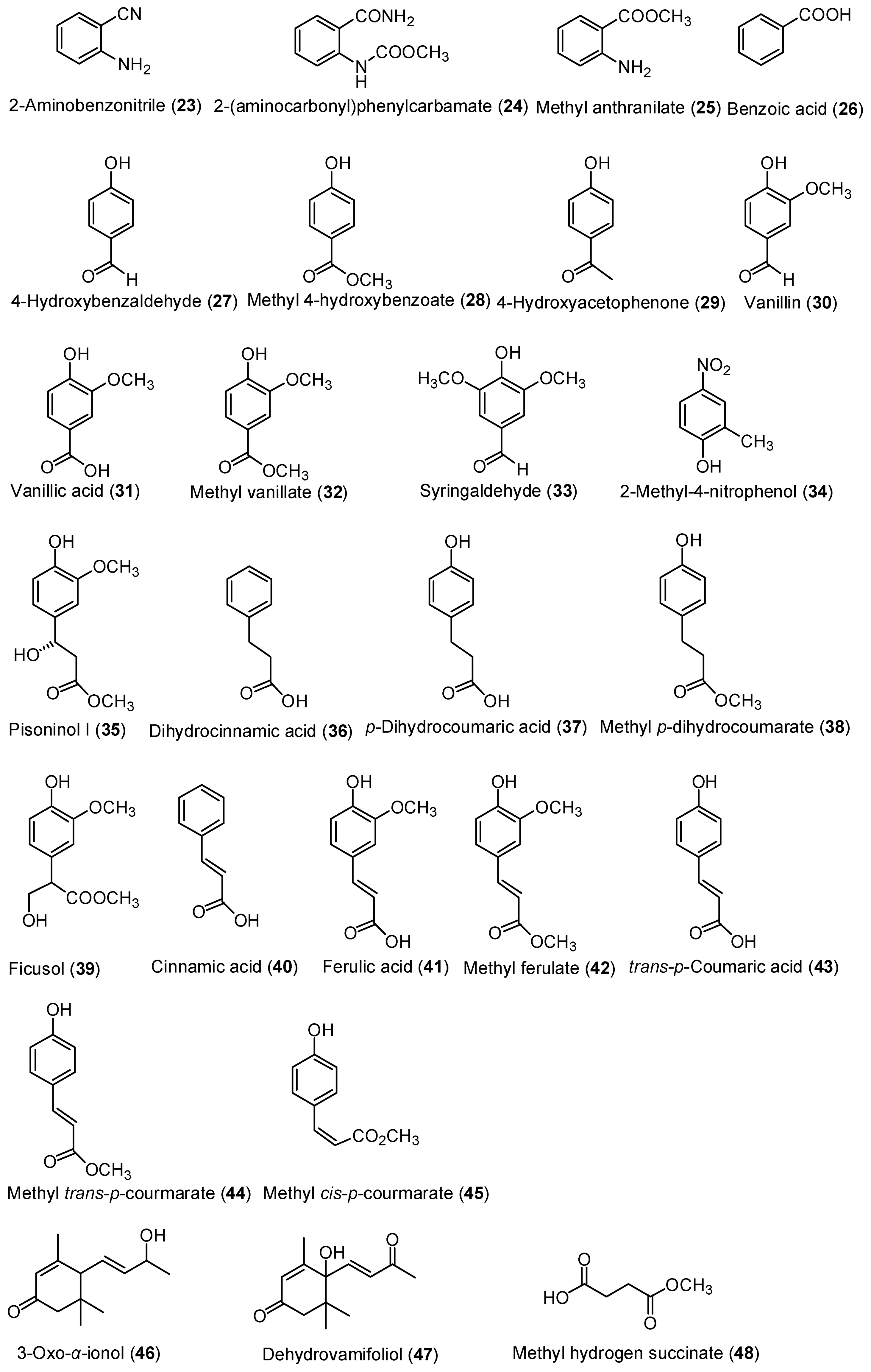
2.1. Isolation of Chemical Constituents of P. mishmensis
2.2. Cytotoxicity of Chemical Constituents of P. mishmensis
3. Materials and Methods
3.1. General
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Cytotoxicity Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Su, H.J. Flora of Taiwan, 2nd ed.; Editorial Committee of the Flora of Taiwan: Taipei, Taiwan, 2000; Volume 5, pp. 999–1001. [Google Scholar]
- Jao, C.W.; Lin, W.C.; Wu, Y.T.; Wu, P.L. Isolation, structure elucidation and synthesis of cytotoxic tryptanthrin analogues from Phaius mishmensis. J. Nat. Prod. 2008, 71, 1275–1279. [Google Scholar] [CrossRef] [PubMed]
- Juhasz, L.; Szilagyi, L.; Antus, S.; Visy, J.; Zsila, F.; Simonyi, M. New insight into the mechanism of hypervalent iodine oxidation of flavanones. Tetrahedron 2002, 58, 4261–4265. [Google Scholar] [CrossRef]
- Lightner, D.A.; Crandall, D.C. The photooxygenation of biliverdin. Tetrahedron Lett. 1973, 14, 953–956. [Google Scholar] [CrossRef]
- Zi, Y.; Cai, Z.J.; Wang, S.Y.; Ji, S.J. Synthesis of isatins by I2/TBHP mediated oxidation of indoles. Org. Lett. 2014, 16, 3094–3097. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Yao, W.; Jiang, C.; Lu, Y. Enantioselective N-alkylation of isatins and synthesis of chiral N-alkylated indoles. Chem. Commun. 2014, 50, 11354–11357. [Google Scholar] [CrossRef] [PubMed]
- Danieli, R.; Maccagnani, G.; Taddei, F. Synthesis, reduction, and proton magnetic resonance spectra of isatogen esters. Boll. Sci. Fac. Chim. Ind. Bologna 1968, 26, 45–60. [Google Scholar]
- Klock, C.; Jin, X.; Choi, K.; Khosla, C.; Madrid, P.B.; Spencer, A.; Raimundo, B.C.; Boardman, P.; Lanza, G.; Griffin, J.H. Acylideneoxoindoles: A new class of reversible inhibitors of human transglutaminase 2. Bioorg. Med. Chem. Lett. 2011, 21, 2692–2696. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.L.; Hsu, Y.L.; Jao, C.W. Indole Alkaloids from Cephalanceropsis gracilis. J. Nat. Prod. 2006, 69, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.F.; Hsu, Y.L.; Lee, C.Y.; Wu, C.H.; Wu, Y.C.; Chuang, T.H. Isolation and cytotoxicity evaluation of the chemical constituents from Cephalantheropsis gracilis. Int. J. Mol. Sci. 2015, 16, 3980–3989. [Google Scholar] [CrossRef] [PubMed]
- Jansen, R.; Sood, S.; Mohr, K.I.; Kunze, B.; Irschik, H.; Stadler, M.; Muller, R. Nannozinones and sorazinones, unprecedented pyrazinones from myxobacteria. J. Nat. Prod. 2014, 77, 2545–2552. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Ma, J.; Hu, J.; Song, J.; Zhang, Z.; Yang, G.; Han, B. Efficient synthesis of quinazoline-2,4(1H,3H)-diones from CO2 using ionic liquids as a dual solvent-catalyst at atmospheric pressure. Green Chem. 2014, 16, 221–225. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, G.; Ding, S.; Wu, X. Studies on chemical constituents in root of Isatis indigotica III. Zhongcaoyao 2002, 33, 97–99. [Google Scholar]
- Bishop, J.E.; Dagam, S.A.; Rapoport, H. Synthesis and characterization of monothiosuccinimides. J. Org. Chem. 1989, 54, 1876–1883. [Google Scholar] [CrossRef]
- Lu, G.P.; Bu, M.J.; Cai, C. Palladium-catalyzed cyanation of aryl bromides with malononitrile through carbon-nitrile bond cleavage mediated by copper. Synlett 2014, 25, 547–550. [Google Scholar]
- Baron, M.; Metay, E.; Lemaire, M.; Popowycz, F. Reduction of aromatic and aliphatic nitro groups to anilines and amines with hypophosphite associated with Pd/C. Green Chem. 2013, 156, 1006–1015. [Google Scholar] [CrossRef]
- Wu, P.L.; Wu, T.S.; He, C.X.; Su, C.H.; Lee, K.S. Constituents from the stems of Hibiscus taiwanensis. Chem. Pharm. Bull. 2005, 53, 56–59. [Google Scholar] [CrossRef] [PubMed]
- Chuang, T.H.; Chan, H.H.; Wu, T.S.; Li, C.F. Chemical constituents and biological studies of the leaves of Ggrevillea robusta. Molecules 2011, 16, 9331–9339. [Google Scholar] [CrossRef] [PubMed]
- Imoto, M.; Matsui, Y.; Takeda, M.; Tamaki, A.; Taniguchi, H.; Mizuno, K.; Ikeda, H. A probable hydrogen-bonded meisenheimer complex: An unusually high SNAr reactivity of nitroaniline derivatives with hydroxide ion in aqueous media. J. Org. Chem. 2011, 76, 6356–6361. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.T.; Peng, C.F.; Huang, H.Y.; Lin, C.H.; Chen, I.S.; Tsai, I.L. Chemical constituents and antitubercular activity of Formosan Pisonia umbellifera. Planta Med. 2011, 77, 736–741. [Google Scholar] [CrossRef] [PubMed]
- Kang, B. R.; Wang, J.; Li, H.; Li, Y.; Mei, Q.B.; Zhang, S.Q. Synthesis and antitumor activity evaluation of 2-arylisoquinoline-1,3(2H,4H)-diones in vitro and in vivo. Med. Chem. Res. 2014, 23, 1340–1349. [Google Scholar] [CrossRef]
- Jones, K.M.; Hillringhaus, T.; Klussmann, M. A singlet oxygen approach to oxaspirocycles. Tetrahedron Lett. 2013, 54, 3294–3297. [Google Scholar] [CrossRef]
- Lee, C.K.; Lu, C.K.; Kuo, Y.H.; Chen, J.Z.; Sun, G.Z. New prenylated flavones from the roots of Ficus Beecheyana. J. Chin. Chem. Soc. 2004, 51, 437–441. [Google Scholar] [CrossRef]
- Pirrung, M.C.; Chen, J.; Rowley, E.G.; McPhail, A.T. Mechanistic and stereochemical study of phenylpyruvate tautomerase. J. Am. Chem. Soc. 1993, 115, 7103–7110. [Google Scholar] [CrossRef]
- Oelschlaegel, S.; Pieper, L.; Staufenbiel, R.; Gruner, M.; Zeippert, L.; Pieper, B.; Koelling-Speer, I.; Speer, K. Floral markers of cornflower (Centaurea cyanus) honey and its peroxide antibacterial activity for an alternative treatment of digital dermatitis. J. Agric. Food Chem. 2012, 60, 11811–11820. [Google Scholar] [CrossRef] [PubMed]
- Schievano, E.; Morelato, E.; Facchin, C.; Mammi, S. Characterization of markers of botanical origin and other compounds extracted from unifloral honeys. J. Agric. Food Chem. 2013, 61, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Niwayama, S. Highly efficient selective monohydrolysis of symmetric diesters. J. Org. Chem. 2000, 65, 5834–5836. [Google Scholar] [CrossRef] [PubMed]
- Tryptanthrin (13) and phaitanthrin-A (14) showed significant cytotoxicity against MCF-7, NCI-H460, and SF-268 cell lines with IC50 values ranging from 9.0–43.9 μM that had been published in reference [2].
- Chuang, T.H.; Lee, S.J.; Yang, C.W.; Wu, P.L. Expedient synthesis and structure-activity relationships of phenanthroindolizidine and phenanthroquinolizidine alkaloids. Org. Biomol. Chem. 2006, 4, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds 1–17, 19–21, 23–34, 36–45, 47, and 48 are available from the authors.
| Position | 1 in CDCl3 (125 MHz/500 MHz) | 2 in CDCl3 (75 MHz/300 MHz) | 3 in CDCl3 (75 MHz/300 MHz) | 4 in CDCl3 (75 MHz/300 MHz) | ||||
|---|---|---|---|---|---|---|---|---|
| δC ppm | δH ppm (J in Hz) | δC ppm | δH ppm (J in Hz) | δC ppm | δH ppm (J in Hz) | δC ppm | δH ppm (J in Hz) | |
| 1 | 9.98 br s | 114.1 | 6.69 d (2.6) | 132.6 | 7.52 br s | |||
| 2 | 133.4 | 153.4 | 108.8 | 7.22 d (1.9) | 171.4 | |||
| 3 | 112.3 | 112.8 | 6.71 dd (8.2, 2.6) | 148.9 | 139.7 | |||
| 4 | 121.4 | 8.14 d (8.0) | 129.2 | 8.09 d (8.2) | 139.4 | 139.7 | ||
| 4a | 125.8 | |||||||
| 4b | 116.8 | |||||||
| 5 | 122.5 | 7.34 t (8.0) | 158.5 | 152.2 | 171.5 | |||
| 6 | 125.5 | 7.40 t (8.0) | 92.7 | 6.53 s | 103.7 | 7.16 d (1.9) | ||
| 7 | 112.5 | 7.52 d (8.0) | 159.0 | 199.8 | ||||
| 8 | 134.4 | 113.9 | 31.6 | 2.94 q (7.2) | ||||
| 8a | 136.8 | |||||||
| 9 | 125.7 | 27.0 | 2.66 m | 8.4 | 1.21 t (7.2) | |||
| 10 | 159.2 | 29.7 | 2.66 m | |||||
| 10a | 139.3 | |||||||
| 11 | 164.7 | |||||||
| 1′ | 137.9 | 132.8 | 19.3 | 2.71 m | ||||
| 2′ | 122.0 | 108.0 | 6.88 m | 31.7 | 2.61 m | |||
| 3′ | 130.9 | 7.98 d (8.0) | 146.7 | 172.5 | ||||
| 4′ | 124.6 | 7.22 t (8.0) | 145.8 | |||||
| 5′ | 133.0 | 7.56 t (8.0) | 114.5 | 6.88 m | ||||
| 6′ | 124.8 | 8.14 d (8.0) | 118.6 | 6.88 m | ||||
| 7′ | 167.1 | 87.4 | 5.93 d (5.4) | |||||
| 8′ | 56.0 | 4.21 d (5.4) | ||||||
| 9′ | 172.4 | |||||||
| 1″ | 141.2 | 65.7 | 4.15 t (6.9) | |||||
| 2″ | 116.0 | 28.6 | 1.62 quintet (6.9) | |||||
| 3″ | 131.0 | 8.07 d (8.0) | 25.8 | 1.25 m | ||||
| 4″ | 123.1 | 7.16 t (8.0) | 29.5 | |||||
| 5″ | 134.4 | 7.62 t (8.0) | ||||||
| 6″ | 121.6 | 8.86 d (8.0) | ||||||
| 7″ | 168.4 | |||||||
| 8″–19″ | ||||||||
| 20″ | 31.9 | |||||||
| 21″ | 22.7 | |||||||
| 22″ | 14.1 | 0.88 t (6.8) | ||||||
| 2-OH | 4.76 br s | |||||||
| 3-OH | 5.87 br s | |||||||
| 4-CH3 | 8.7 | 2.00 s | ||||||
| 4-OCH3 | 61.0 | 3.97 s | ||||||
| 5-OCH3 | 55.6 | 3.87 s * | 56.0 | 3.91 s | ||||
| 1′-NH | 12.55 s | |||||||
| 3′-OCH3 | 55.8 | 3.88 s * | 51.9 | 3.67 s | ||||
| 4′-OH | 5.63 br s | |||||||
| 7′-OCH3 | 52.2 | 3.83 s | ||||||
| 1″-NH | 11.85 s | |||||||
| 7″-OCH3 | 52.5 | 3.85 s | ||||||
| Extracts | % Inhibition at 20 μg/mL | ||
|---|---|---|---|
| MCF-7 1 | NCI-H460 2 | SF-268 3 | |
| n-hexane | 3 | 1 | 25 |
| CHCl3 | 2 | 1 | 4 |
| EtOAc | 1 | 1 | 1 |
| H2O | 134 | 90 | 114 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jao, C.-W.; Hung, T.-H.; Chang, C.-F.; Chuang, T.-H. Chemical Constituents of Phaius mishmensis. Molecules 2016, 21, 1605. https://doi.org/10.3390/molecules21111605
Jao C-W, Hung T-H, Chang C-F, Chuang T-H. Chemical Constituents of Phaius mishmensis. Molecules. 2016; 21(11):1605. https://doi.org/10.3390/molecules21111605
Chicago/Turabian StyleJao, Chen-Wei, Tzu-Heng Hung, Chi-Fen Chang, and Ta-Hsien Chuang. 2016. "Chemical Constituents of Phaius mishmensis" Molecules 21, no. 11: 1605. https://doi.org/10.3390/molecules21111605
APA StyleJao, C.-W., Hung, T.-H., Chang, C.-F., & Chuang, T.-H. (2016). Chemical Constituents of Phaius mishmensis. Molecules, 21(11), 1605. https://doi.org/10.3390/molecules21111605