Ultrasound-Assisted Extraction, Centrifugation and Ultrafiltration: Multistage Process for Polyphenol Recovery from Purple Sweet Potatoes
Abstract
:1. Introduction
2. Results and Discussion
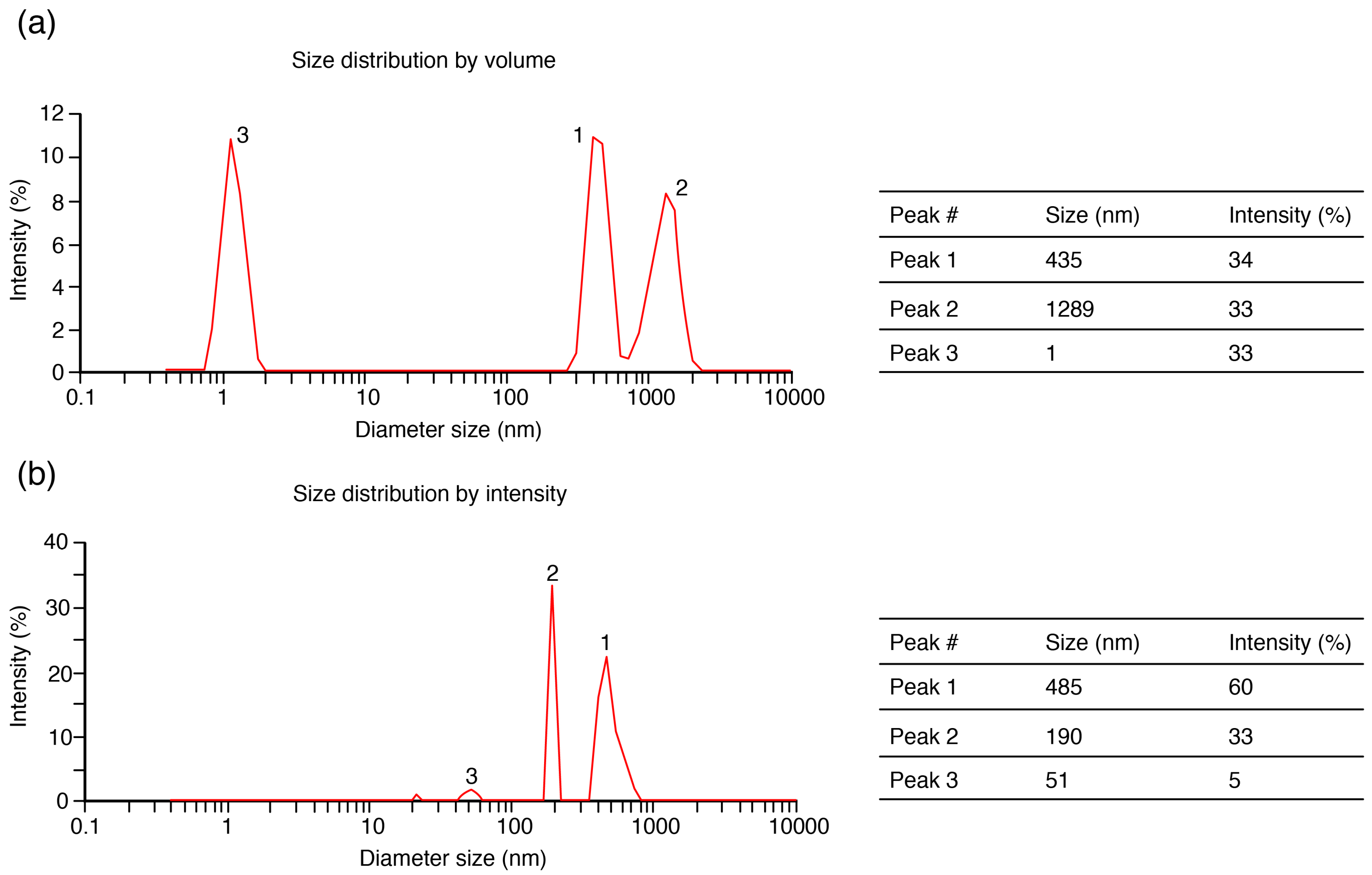
2.1. Impact of Centrifugation Pre-Treatment on UF Efficiency
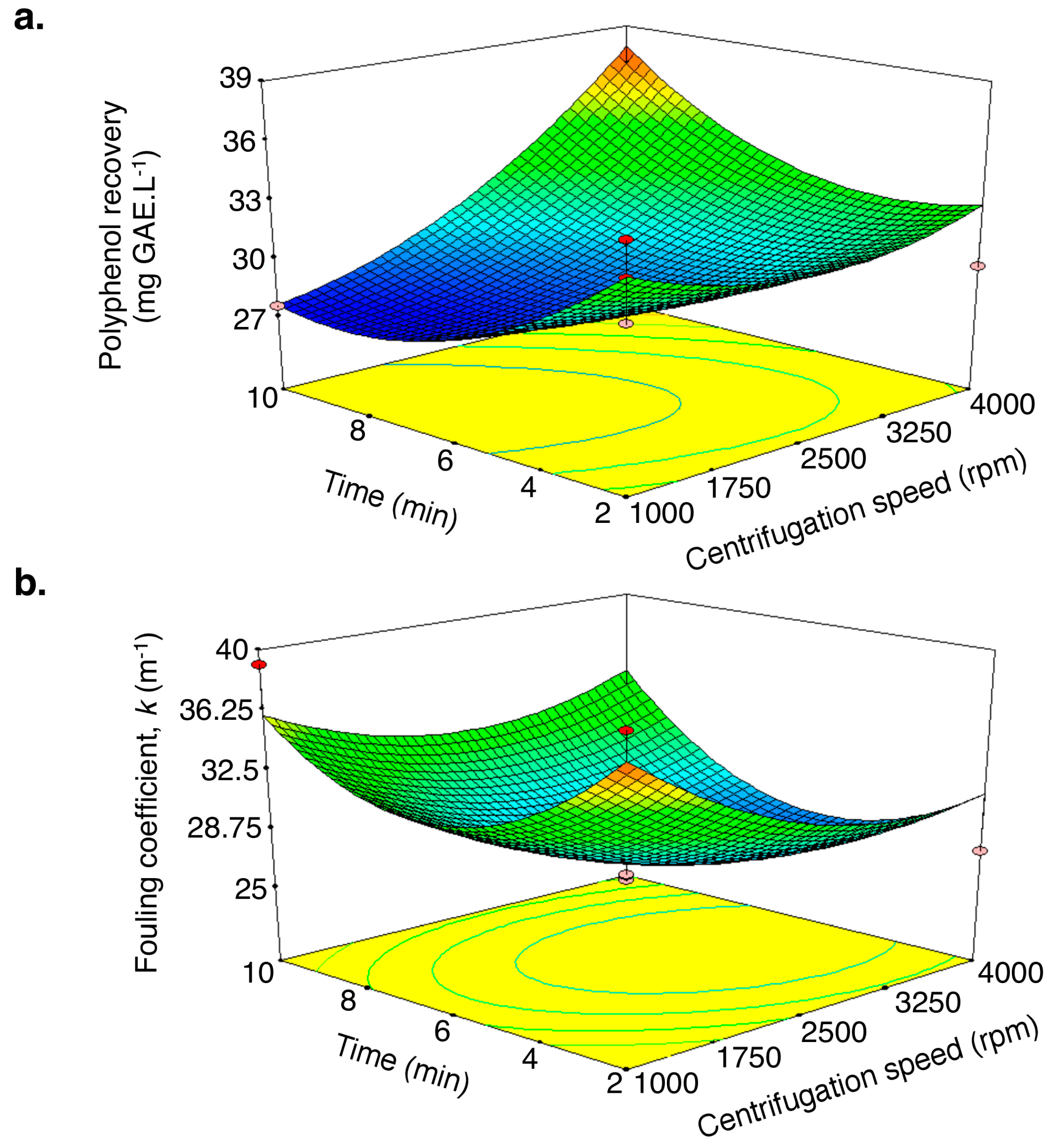
2.2. Optimization of the Centrifugation Conditions
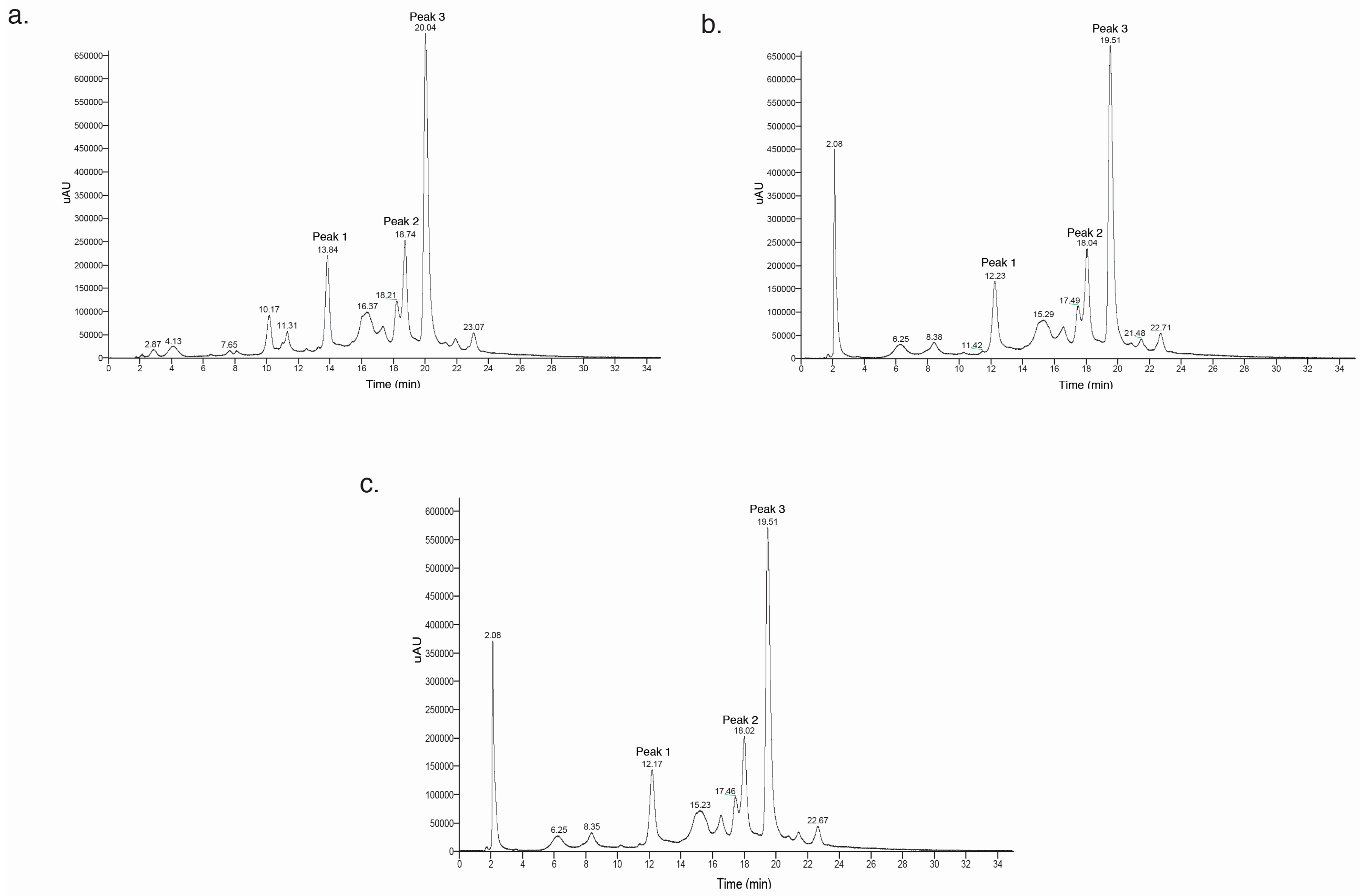
2.3. Anthocyanin Identification and HPLC-DAD-ESI-MS2 Profiles
3. Materials and Methods
3.1. Samples
3.2. PSP Extract Preparation
3.3. Centrifugation of PSP Extract
3.4. Filtration of PSP Juice
3.5. Membrane Fouling Analysis with Exponential Model
3.6. Compound Analyses
3.6.1. Polyphenol Analysis
HPLC-DAD-ESI-MS2 Anthocyanin Analysis
Anthocyanin Analysis
Total Phenolic Compounds
3.6.2. Total Protein Content
3.7. Particle Size Distribution
3.8. Centrifugation Study by Experimental Design
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sun, W.; Zhang, M.; Chen, H.; Zheng, D.; Fang, Z. Effects of deodorization on the physicochemical index and volatile compounds of purple sweet potato anthocyanins (PSPAs). LWT-Food Sci. Technol. 2016, 68, 265–272. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Choi, J.H.; Choi, J.M.; Chung, Y.C.; Jeong, H.G. Protective mechanisms of anthocyanins from purple sweet potato against tert-butyl hydroperoxide-induced hepatotoxicity. Food Chem. Toxicol. 2011, 49, 2081–2089. [Google Scholar] [CrossRef] [PubMed]
- Bovell-Benjamin, A.C. Sweet potato: A review of its past, present, and future role in human nutrition. Adv. Food Nutr. Res. 2007, 52, 1–59. [Google Scholar] [PubMed]
- Zhu, Z.; Guan, Q.; Guo, Y.; He, J.; Liu, G.; Li, S.; Barba, F.J.; Jaffrin, M.Y. Green ultrasound-assisted extraction of anthocyanin and phenolic compounds from purple sweet potato using response surface methodology. Int. Agrophys. 2016, 30, 113–122. [Google Scholar] [CrossRef]
- Wang, S.-M.; Yu, D.-J.; Song, K.B. Quality characteristics of purple sweet potato (Ipomoea batatas) slices dehydrated by the addition of maltodextrin. Hortic. Environ. Biotechnol. 2011, 52, 435–441. [Google Scholar] [CrossRef]
- Galanakis, C.M.; Schieber, A. Recovery and utilization of valuable compounds from food processing by-products. Food Res. Int. 2014, 65, 299–484. [Google Scholar] [CrossRef]
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Galanakis, C.M.; Brnčić, M.; Orlien, V.; Trujillo, F.J.; Mawson, R.; Knoerzer, K.; Tiwari, B.K.; Barba, F.J. Clean recovery of antioxidant compounds from plant foods, by-products and algae assisted by ultrasounds processing. Modeling approaches to optimize processing conditions. Trends Food Sci. Technol. 2015, 42, 134–149. [Google Scholar] [CrossRef]
- Galanakis, C.M. Emerging technologies for the production of nutraceuticals from agricultural by-products: A viewpoint of opportunities and challenges. Food Bioprod. Process. 2013, 91, 575–579. [Google Scholar] [CrossRef]
- Kobus, Z. Dry matter extraction from valerian roots (Valeriana officinalis L.) with the help of pulsed acoustic field. Int. Agrophys. 2008, 1, 133–137. [Google Scholar]
- Lagnika, C.; Zhang, M.; Nsor-Atindana, J.; Tounkara, F. Extension of mushroom shelf-life by ultrasound treatment combined with high pressure argon. Int. Agrophys. 2014, 28, 39–47. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.; Guan, Q.; He, J.; Liu, G.; Li, S.; Ding, L.; Jaffrin, M.Y. Purification of purple sweet potato extract by dead-end filtration and investigation of membrane fouling mechanism. Food Bioprocess Technol. 2015, 8, 1680–1689. [Google Scholar] [CrossRef]
- Zhu, Z.; Luo, J.; Ding, L.; Bals, O.; Jaffrin, M.Y.; Vorobiev, E. Chicory juice clarification by membrane filtration using rotating disk module. J. Food Eng. 2013, 115, 264–271. [Google Scholar] [CrossRef]
- Gökmen, V.; Çetinkaya, Ö. Effect of pretreatment with gelatin and bentonite on permeate flux and fouling layer resistance during apple juice ultrafiltration. J. Food Eng. 2007, 80, 300–305. [Google Scholar] [CrossRef]
- Dahdouh, L.; Delalonde, M.; Ricci, J.; Servent, A.; Dornier, M.; Wisniewski, C. Size-cartography of orange juices foulant particles: Contribution to a better control of fouling during microfiltration. J. Membr. Sci. 2016, 509, 164–172. [Google Scholar] [CrossRef]
- Loginov, M.; Loginova, K.; Lebovka, N.; Vorobiev, E. Comparison of dead-end ultrafiltration behaviour and filtrate quality of sugar beet juices obtained by conventional and “cold” PEF-assisted diffusion. J. Membr. Sci. 2011, 377, 273–283. [Google Scholar] [CrossRef]
- Loginov, M.; Larue, O.; Lebovka, N.; Vorobiev, E. Fluidity of highly concentrated kaolin suspensions: Influence of particle concentration and presence of dispersant. Colloids Surf. Physicochem. Eng. Asp. 2008, 325, 64–71. [Google Scholar] [CrossRef]
- Loginov, M.; Lebovka, N.; Vorobiev, E. Multistage centrifugation method for determination of filtration and consolidation properties of mineral and biological suspensions using the analytical photocentrifuge. Chem. Eng. Sci. 2014, 107, 277–289. [Google Scholar] [CrossRef]
- Hu, Y.-Y.; Zheng, P.; He, Y.-Z.; Sheng, G.-P. Response surface optimization for determination of pesticide multiresidues by matrix solid-phase dispersion and gas chromatography. J. Chromatogr. A 2005, 1098, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Pinzauti, S.; Gratteri, P.; Furlanetto, S.; Mura, P.; Dreassi, E.; Phan-Tan-Luu, R. Experimental design in the development of voltammetric method for the assay of omeprazole. J. Pharm. Biomed. Anal. 1996, 14, 881–889. [Google Scholar] [CrossRef]
- Zhu, Z.; Ladeg, S.; Ding, L.; Bals, O.; Moulai-Mostefa, N.; Jaffrin, M.Y.; Vorobiev, E. Study of rotating disk assisted dead-end filtration of chicory juice and its performance optimization. Ind. Crops Prod. 2014, 53, 154–162. [Google Scholar] [CrossRef]
- Zhu, Z.; Guan, Q.; Koubaa, M.; Barba, F.J.; Roohinejad, S.; Cravotto, G.; Yang, X.; Li, S.; He, J. HPLC-DAD-ESI-MS2 analytical profile of extracts obtained from purple sweet potato after green ultrasound-assisted extraction. Food Chem. 2017, 215, 391–400. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Deng, L.; Chen, J.; Zhou, S.; Liu, S.; Fu, Y.; Yang, C.; Liao, Z.; Chen, M. An analytical pipeline to compare and characterise the anthocyanin antioxidant activities of purple sweet potato cultivars. Food Chem. 2016, 194, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Kim, J.B.; Cho, S.M.; Chung, M.N.; Lee, Y.M.; Chu, S.M.; Che, J.H.; Kim, S.N.; Kim, S.Y.; Cho, Y.S.; et al. Anthocyanin changes in the Korean purple-fleshed sweet potato, Shinzami, as affected by steaming and baking. Food Chem. 2012, 130, 966–972. [Google Scholar] [CrossRef]
- Cai, Z.; Qu, Z.; Lan, Y.; Zhao, S.; Ma, X.; Wan, Q.; Jing, P.; Li, P. Conventional, ultrasound-assisted, and accelerated-solvent extractions of anthocyanins from purple sweet potatoes. Food Chem. 2016, 197, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Zhang, Y.; Zheng, Z.; Qu, Z.; Liu, M.; Zhu, S.; Liu, S.; Wang, M.; Qu, L. Identification and thermal stability of purple-fleshed sweet potato anthocyanins in aqueous solutions with various pH values and fruit juices. Food Chem. 2013, 136, 1429–1434. [Google Scholar] [CrossRef] [PubMed]
- Lapornik, B.; Prošek, M.; Golc Wondra, A. Comparison of extracts prepared from plant by-products using different solvents and extraction time. J. Food Eng. 2005, 71, 214–222. [Google Scholar] [CrossRef]
- De la Garza, F.; Boulton, R. The modeling of wine filtrations. Am. J. Enol. Vitic. 1984, 35, 189–195. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available from the authors.



| Source | Sum of Squares | df | Mean Square | F-Value | p-Value (Prob > F) | |
|---|---|---|---|---|---|---|
| Polyphenol recovery a | ||||||
| Model | 135.09 | 5 | 27.02 | 4.62 | 0.0349 | Significant |
| Residual | 40.94 | 7 | 5.85 | |||
| Lack of fit | 32.64 | 3 | 10.88 | 5.24 | 0.0718 | Not significant |
| Pure error | 8.3 | 4 | 2.08 | |||
| Fouling coefficient b | ||||||
| Model | 262.73 | 5 | 52.55 | 4.85 | 0.0309 | Significant |
| Residual | 75.77 | 7 | 10.82 | |||
| Lack of fit | 61.83 | 3 | 20.61 | 5.91 | 0.0594 | Not significant |
| Pure error | 13.94 | 4 | 3.49 | |||
| Peak | m/z | Anthocyanins | Normalized Peak Area | |||
|---|---|---|---|---|---|---|
| [M]+ | Fragment Ions | S1 | S2 | S3 | ||
| 1 | 963 | 801; 463; 301 | Peonidin 3-feruloyl sophoroside-5-glucoside | 1 | 1 | 0.88 |
| 2 | 1069 | 907; 463; 301 | Peonidin 3-caffeoyl-p-hydroxybenzoyl sophoroside-5-glucoside | 1 | 1 | 0.96 |
| 3 | 1125 | 963; 463; 301 | Peonidin 3-caffeoyl-feruloyl sophoroside-5-glucoside | 1 | 1 | 0.86 |
| Independent Variables | Symbol | Levels | |||
|---|---|---|---|---|---|
| Actual | Coded | −1 | 0 | 1 | |
| Speed (rpm) | X1 | x1 | 1000 | 2500 | 4000 |
| (75× g) | (467× g) | (1195× g) | |||
| Time (min) | X2 | x2 | 2 | 6 | 10 |
| Run | Independent Variables | Response Variables | ||
|---|---|---|---|---|
| Centrifugation Speed (rpm) X1(x1) | Centrifugation Time (min) X2(x2) | Polyphenol Recovery (%) | Fouling Coefficient (m−1) | |
| 1 | 2500 (0) | 6 (0) | 29 | 25.78 |
| 2 | 2500 (0) | 6 (0) | 31 | 25.67 |
| 3 | 2500 (0) | 6 (0) | 27.3 | 25.39 |
| 4 | 1000 (−1) | 2 (−1) | 31.3 | 39.48 |
| 5 | 2500 (−1) | 0.3 (−1.414) | 37 | 38.05 |
| 6 | 4621 (+1.414) | 6 (0) | 38.1 | 33.67 |
| 7 | 379 (−1.414) | 6 (0) | 29.8 | 31.71 |
| 8 | 2500 (0) | 12 (+1.414) | 33.5 | 37.45 |
| 9 | 4000 (+1) | 2 (−1) | 29.6 | 27.34 |
| 10 | 1000 (−1) | 10 (+1) | 27.5 | 39.07 |
| 11 | 2500 (0) | 6 (0) | 27.8 | 29.45 |
| 12 | 2500 (0) | 6 (0) | 28.3 | 28.45 |
| 13 | 4000 (+1) | 10 (+1) | 36.8 | 32.62 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, Z.; Jiang, T.; He, J.; Barba, F.J.; Cravotto, G.; Koubaa, M. Ultrasound-Assisted Extraction, Centrifugation and Ultrafiltration: Multistage Process for Polyphenol Recovery from Purple Sweet Potatoes. Molecules 2016, 21, 1584. https://doi.org/10.3390/molecules21111584
Zhu Z, Jiang T, He J, Barba FJ, Cravotto G, Koubaa M. Ultrasound-Assisted Extraction, Centrifugation and Ultrafiltration: Multistage Process for Polyphenol Recovery from Purple Sweet Potatoes. Molecules. 2016; 21(11):1584. https://doi.org/10.3390/molecules21111584
Chicago/Turabian StyleZhu, Zhenzhou, Tian Jiang, Jingren He, Francisco J. Barba, Giancarlo Cravotto, and Mohamed Koubaa. 2016. "Ultrasound-Assisted Extraction, Centrifugation and Ultrafiltration: Multistage Process for Polyphenol Recovery from Purple Sweet Potatoes" Molecules 21, no. 11: 1584. https://doi.org/10.3390/molecules21111584
APA StyleZhu, Z., Jiang, T., He, J., Barba, F. J., Cravotto, G., & Koubaa, M. (2016). Ultrasound-Assisted Extraction, Centrifugation and Ultrafiltration: Multistage Process for Polyphenol Recovery from Purple Sweet Potatoes. Molecules, 21(11), 1584. https://doi.org/10.3390/molecules21111584









