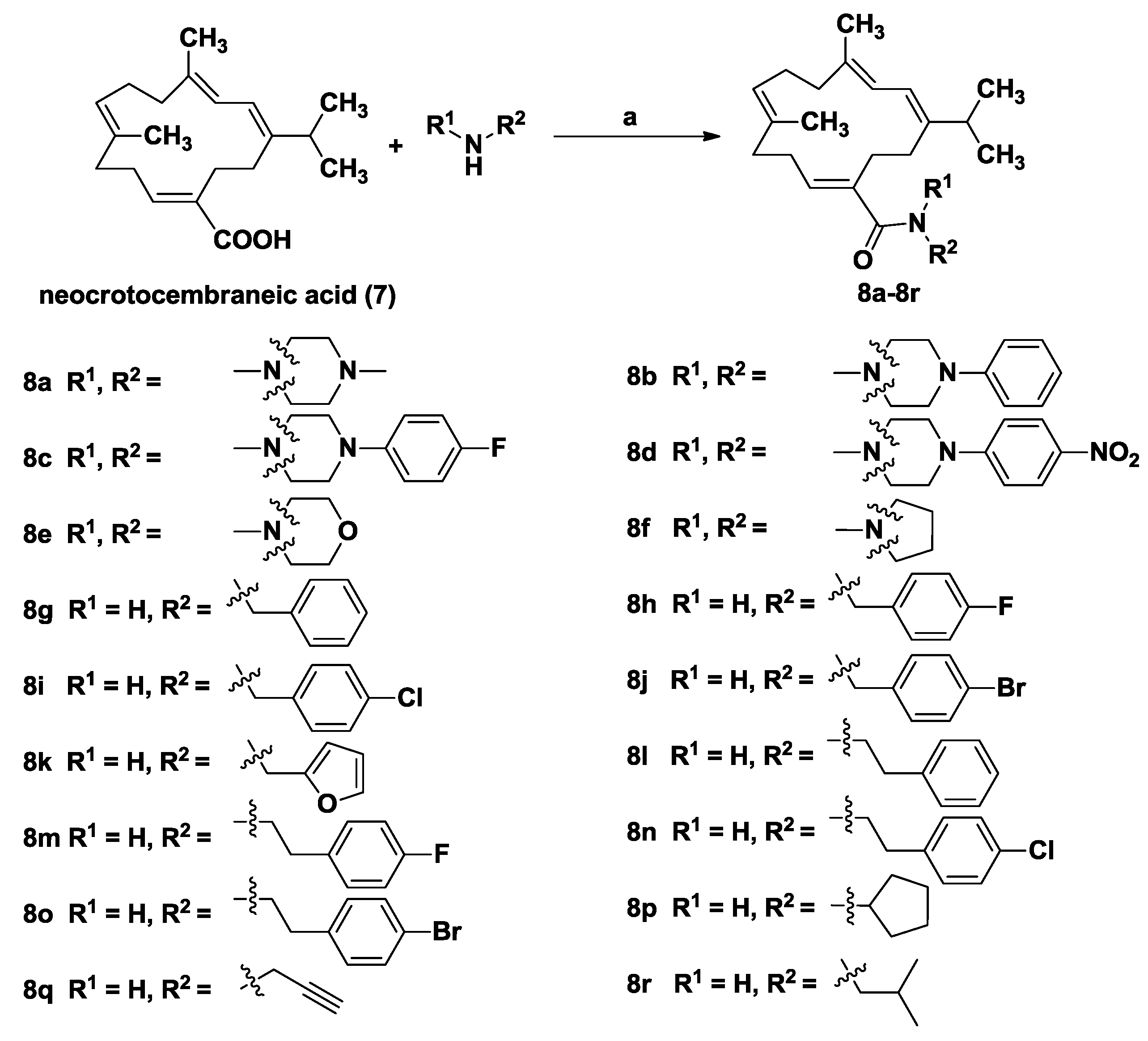
General Procedure for the Synthesis of Compounds 8a–8r
To a solution of neocrotocembraneic acid (150 mg, 0.50 mmol) in dry CH2Cl2 (10 mL) were added HOBt (81 mg, 0.60 mmol) and EDCI (115 mg, 0.60 mmol). The mixture was stirred at room temperature for 2 h, and then the corresponding amines (0.6 mmol 1.2 equiv.) were added. Upon completion, the reaction mixture was washed successively with 1M hydrochloric acid, saturated NaHCO3 and brine, then dried over Na2SO4 and concentrated in vacuo. The residue was purified by column chromatography to give the amide derivatives 8a–8r.
((1E,5E,9E,11E)-12-Isopropyl-5,9-dimethylcyclotetradeca-1,5,9,11-tetraen-1-yl)(4-methylpiperazin-1-yl)methanone (8a): Yield: 76%; Colorless oil; UV (MeOH) λmax (log ε) 250 (3.9); 1H-NMR (600 MHz, CDCl3) δ 6.02 (d, J = 11.2 Hz, 1H), 5.95 (d, J = 11.2 Hz, 1H), 5.45 (t, J = 7.7 Hz, 1H), 5.08 (t, J = 6.5 Hz, 1H), 3.64–3.49 (m,4H), 2.45 (t, J = 7.7 Hz, 2H), 2.35–2.31 (m, 5H), 2.31–2.24 (m, 7H), 2.22–2.19 (m, 2H), 2.15–2.12 (m, 4H), 1.73 (s, 3H), 1.72 (s, 3H), 1.00 (s, 3H), 0.99 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 171.7, 146.6, 135.6, 135.5, 134.7, 133.0, 126.9, 120.2, 118.7, 55.2, 46.2, 38.4, 37.6, 34.2, 28.6, 28.4, 28.3, 25.0, 22.2, 18.2, 18.0; IR νmax (film): 2955, 2925, 2853, 2789, 1623, 1458, 1427, 1291, 1144, 1002 cm−1; HRMS m/z calculated for C25H41N2O [M + H]+: 385.3219; found: 385.3219.
((1E,5E,9E,11E)-12-Isopropyl-5,9-dimethylcyclotetradeca-1,5,9,11-tetraen-1-yl)(4-phenylpiperazin-1-yl)methanone (8b): Yield: 66%; Colorless oil; UV (MeOH) λmax (log ε) 248 (4.3); 1H-NMR (600 MHz, CD3COCD3) δ 7.25–7.22 (m, 2H), 6.99 (dt, J = 7.8 Hz, 1.1Hz, 2H), 6.82 (tt, J = 7.2 Hz, 1.0Hz, 1H), 6.08 (d, J = 11.3 Hz, 1H), 6.06 (d, J = 11.3 Hz, 1H), 5.58 (t, J = 7.7 Hz, 1H), 5.18–5.15 (m, 1H), 3.68–3.64 (m, 4H), 3.16–3.12 (m, 4H), 2.51 (t, J = 7.6 Hz, 2H), 2.37–2.29 (m, 5H), 2.27 (t, J = 7.6 Hz, 2H), 2.23–2.19 (m, 4H), 1.79 (s, 3H), 1.74 (s, 3H), 1.01 (s, 3H), 1.00 (s, 3H); 13C-NMR (150 MHz, CD3COCD3) δ 170.6, 151.5, 145.7, 135.4, 134.9, 134.4, 132.9, 128.9, 127.0, 120.2, 119.6, 118.8, 116.3, 49.3, 38.3, 37.0, 33.5, 28.5, 27.7, 27.4, 24.7, 21.5, 17.4, 16.8; IR νmax (film): 3340, 2956, 2921, 2850, 1622, 1599, 1503, 1495, 1435, 1380, 1232, 1155 cm−1; HRMS m/z calculated for C30H43N2O [M + H]+: 447.3375; found: 447.3373.
(4-(4-Fluorophenyl)piperazin-1-yl)((1E,5E,9E,11E)-12-isopropyl-5,9-dimethylcyclotetradeca-1,5,9,11-tetraen-1-yl)methanone (8c): Yield: 71%; Colorless oil; UV (MeOH) λmax (log ε) 242 (4.4); 1H-NMR (600 MHz, CD3COCD3) δ 7.04–6.99 (m, 4H), 6.08 (d, J = 11.3 Hz, 1H), 6.05 (d, J = 11.3 Hz, 1H), 5.58 (t, J = 7.7 Hz, 1H), 5.18–5.14 (m, 1H), 3.68–3.62 (m, 4H), 3.11–3.05 (m, 4H), 2.51 (t, J = 7.6 Hz, 2H), 2.37–2.28 (m, 5H), 2.27 (t, J = 7.6 Hz, 2H), 2.23–2.18 (m, 4H), 1.78 (s, 3H), 1.73 (s, 3H), 1.01 (s, 3H), 1.00 (s, 3H); 13C-NMR (150 MHz, CD3COCD3) δ 171.5, 157.9 (d, J = 235.5 Hz), 149.2, 146.6, 136.3, 135.8, 135.2, 133.8, 127.8, 121.1, 119.6, 119.0 (d, J = 7.7 Hz), 116.1 (d, J = 22.3 Hz), 51.0, 39.2, 37.9, 34.3, 29.3, 28.5, 28.3, 25.6, 22.4, 18.2, 17.7; IR νmax (film): 2956, 2921, 2850, 1623, 1509, 1433, 1232 cm−1; HRMS m/z calculated for C30H42FN2O [M + H]+: 465.3281; found: 465.3283.
((1E,5E,9E,11E)-12-Isopropyl-5,9-dimethylcyclotetradeca-1,5,9,11-tetraen-1-yl)(4-(4-nitrophenyl)piperazin-1-yl)methanone (8d): Yield: 65%; Colorless oil; UV (MeOH) λmax (log ε) 240 (4.2); 1H-NMR (600 MHz, CD3COCD3) δ 8.12–8.09 (m, 2H), 7.08–7.05 (m, 2H), 6.11–6.06 (m, 2H), 5.63 (t, J = 7.7 Hz, 1H), 5.19–5.16 (m, 1H), 3.73–3.69 (m, 4H), 3.55–3.51 (m, 4H), 2.54 (t, J = 7.5 Hz, 2H), 2.38–2.30 (m, 5H), 2.29 (t, J = 7.6 Hz, 2H), 2.24–2.21 (m, 4H), 1.80 (s, 3H), 1.76 (s, 3H), 1.00 (s, 3H), 0.99 (s, 3H); 13C-NMR (150 MHz, CD3COCD3) δ 170.8, 154.9, 145.8, 138.2, 135.2, 135.1, 134.2, 133.5, 127.1, 125.5, 120.1, 118.7, 112.9, 46.7, 38.3, 36.9, 33.2, 28.6, 27.6, 27.1, 24.8, 21.6, 17.4, 16.7; IR νmax (film): 3435, 2935, 2919, 2847, 1647, 1595, 1496, 1430, 1318, 1237, 1112 cm−1; HRMS m/z calculated for C30H42N3O3 [M + H]+: 492.3226; found: 492.3233.
((1E,5E,9E,11E)-12-Isopropyl-5,9-dimethylcyclotetradeca-1,5,9,11-tetraen-1-yl)(morpholino)methanone (8e): Yield: 79%; Colorless oil; UV (MeOH) λmax (log ε) 243 (3.8); 1H-NMR (600 MHz, CDCl3) δ 6.03 (d, J = 11.3 Hz, 1H), 5.97 (d, J = 11.3 Hz, 1H), 5.46 (t, J = 7.7 Hz, 1H), 5.08–5.04 (m, 1H), 3.64–3.51 (m, 8H), 2.48 (t, J = 7.7 Hz, 2H), 2.36–2.28 (m, 3H), 2.28–2.24 (m, 2H), 2.22 (t, J = 7.7 Hz, 2H), 2.17–2.11 (m, 4H), 1.73 (s, 3H), 1.72 (s, 3H), 1.01 (s, 3H), 1.00 (s, 3H); 13C–NMR (150 MHz, CDCl3) δ 171.8, 146.5, 135.7, 135.1, 134.5, 133.7, 127.1, 120.1, 118.7, 67.1, 38.5, 37.5, 33.9, 28.7, 28.1, 28.0, 25.0, 22.3, 18.2, 17.8; IR νmax (film): 3446, 2957, 2922, 2851, 1622, 1457, 1435, 1273, 1115, 1032, 1021 cm−1; HRMS m/z calculated for C24H38NO2 [M + H]+: 372.2903; found: 372.2903.
((1E,5E,9E,11E)-12-Isopropyl-5,9-dimethylcyclotetradeca-1,5,9,11-tetraen-1-yl)(pyrrolidin-1-yl)methanone (8f): Yield: 70%; Colorless oil; UV (MeOH) λmax (log ε) 250 (4.1); 1H-NMR (600 MHz, CDCl3) δ 6.01 (s, 2H), 5.58 (t, J = 7.7 Hz, 1H), 5.09 (td, J = 6.6 Hz, 1.3Hz, 1H), 3.46–3.39 (m,4H), 2.51 (t, J = 7.7 Hz, 2H), 2.39–2.35 (m, 1H), 2.32–2.29 (m, 2H), 2.28–2.23 (m, 4H), 2.17–2.13 (m, 4H), 1.89–1.81 (m, 4H), 1.74 (s, 3H), 1.72 (s, 3H), 1.00 (s, 3H), 0.99 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 171.0, 147.0, 137.4, 135.4, 134.5, 133.5, 127.1, 120.4, 118.3, 38.5, 37.5, 33.6, 28.9, 28.2, 27.4, 25.0, 22.2, 18.2, 17.8; IR νmax (film): 3437, 2957, 2925, 2869, 1648, 1610, 1414, 1189 cm−1; HRMS m/z calculated for C24H38NO [M + H]+: 356.2953; found: 356.2953.
(1E,5E,9E,11E)-N-Benzyl-12-isopropyl-5,9-dimethylcyclotetradeca-1,5,9,11-tetraenecarboxamide (8g): Yield: 75%; Colorless oil; UV (MeOH) λmax (log ε) 240 (3.6); 1H-NMR (600 MHz, CDCl3) δ 7.35–7.32 (m, 2H), 7.27–7.25 (m, 3H), 6.01 (d, J = 11.3 Hz, 1H), 5.99 (dq, J = 11.3 Hz, 1.3 Hz, 1H), 5.88 (t, J = 7.5 Hz, 1H), 5.72 (t, J = 5.8 Hz, 1H), 5.04 (t, J = 6.5 Hz, 1H), 4.38 (d, J = 5.7 Hz, 2H), 2.52 (t, J = 7.0 Hz, 2H), 2.40–2.35 (m, 1H), 2.34–2.30 (m, 2H), 2.27–2.23 (m, 4H), 2.19–2.17 (m, 2H), 2.10–2.07 (m, 2H), 1.74 (s, 3H), 1.59 (s, 3H), 1.04 (s, 3H), 1.03 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 171.5, 146.3, 138.8, 138.8, 136.0, 134.9, 134.0, 128.8, 127.9, 127.6, 127.4, 119.9, 118.9, 43.8, 39.1, 37.1, 33.8, 29.7, 27.6, 26.4, 25.1, 22.3, 18.3, 17.1; IR νmax (film): 3353, 2959, 2925, 2872, 1648, 1525, 1445, 1363, 1272, 1081 cm−1; HRMS m/z calculated for C27H38NO [M + H]+: 392.2953; found: 392.2952.
(1E,5E,9E,11E)-N-(4-Fluorobenzyl)-12-isopropyl-5,9-dimethylcyclotetradeca-1,5,9,11-tetraenecarboxamide (8h): Yield: 72%; white solid; mp: 103.7–105.6 °C; UV (MeOH) λmax (log ε) 243 (4.2); 1H-NMR (600 MHz, CDCl3) δ 7.24–7.21 (m, 2H), 7.01 (t, J = 8.6 Hz, 2H), 6.01–5.96 (m, 2H), 5.86 (t, J = 7.5 Hz, 1H), 5.71 (t, J = 5.8 Hz, 1H), 5.04 (t, J = 6.7 Hz, 1H), 4.33 (d, J = 5.8 Hz, 2H), 2.51 (t, J = 7.0 Hz, 2H), 2.39–2.34 (m, 1H), 2.34–2.30 (m, 2H), 2.28–2.23 (m, 4H), 2.19–2.16 (m, 2H), 2.11–2.07 (m, 2H), 1.74 (s, 3H), 1.59 (s, 3H), 1.04 (s, 3H), 1.02 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 171.6, 162.2 (d, J = 245.4 Hz), 146.3, 138.7, 136.0, 135.1, 134.6 (d, J = 3.2 Hz), 133.9, 129.3 (d, J = 8.2 Hz), 127.9, 119.9, 118.9, 115.5 (d, J = 21.5 Hz), 43.1, 39.1, 37.1, 33.8, 29.7, 27.6, 26.3, 25.1, 22.3, 18.4, 17.1; IR νmax (film): 3370, 2954, 2927, 2868, 1654, 1621, 1510, 1448, 1379, 1222, 1156, 1026 cm−1; HRMS m/z calculated for C27H37FNO [M + H]+: 410.2859; found: 410.2859.
(1E,5E,9E,11E)-N-(4-Chlorobenzyl)-12-isopropyl-5,9-dimethylcyclotetradeca-1,5,9,11-tetraenecarboxamide (8i): Yield: 69%; white solid; mp: 107.3–109.3 °C; UV (MeOH) λmax (log ε) 242 (4.1); 1H-NMR (600 MHz, CDCl3) δ 7.30 (d, J = 8.4 Hz, 2H), 7.19 (d, J = 8.4 Hz, 2H), 6.01–5.97 (m, 2H), 5.85 (t, J = 7.5 Hz, 1H), 5.71 (t, J = 5.9 Hz, 1H), 5.04 (t, J = 6.6 Hz, 1H), 4.33 (d, J = 5.9 Hz, 2H), 2.51 (t, J = 7.0 Hz, 2H), 2.39–2.35 (m, 1H), 2.34–2.30 (m, 2H), 2.28–2.23 (m, 4H), 2.20–2.17 (m, 2H), 2.12–2.08 (m, 2H), 1.74 (s, 3H), 1.60 (s, 3H), 1.04 (s, 3H), 1.03 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 171.6, 146.4, 138.7, 137.4, 136.0, 135.1, 133.9, 133.2, 129.0, 128.9, 128.0, 120.0, 118.9, 43.1, 39.1, 37.2, 33.8, 29.7, 27.6, 26.4, 25.1, 22.3, 18.4, 17.1; IR νmax (film): 3355, 2955, 2922, 2852, 1653, 1614, 1525, 1490, 1091 cm−1; HRMS m/z calculated for C27H37ClNO [M + H]+: 426.2564; found: 426.2565.
(1E,5E,9E,11E)-N-(4-Bromobenzyl)-12-isopropyl-5,9-dimethylcyclotetradeca-1,5,9,11-tetraenecarboxamide (8j): Yield: 68%; Colorless oil; UV (MeOH) λmax (log ε) 244 (3.9); 1H-NMR (600 MHz, CDCl3) δ 7.44 (d, J = 8.4 Hz, 2H), 7.13 (d, J = 8.4 Hz, 2H), 6.01–5.96 (m, 2H), 5.85 (t, J = 7.5 Hz, 1H), 5.72 (t, J = 6.1 Hz, 1H), 5.04 (t, J = 6.8 Hz, 1H), 4.31 (d, J = 5.9 Hz, 2H), 2.51 (t, J = 7.0 Hz, 2H), 2.39–2.34 (m, 1H), 2.34–2.30 (m, 2H), 2.28–2.23 (m, 4H), 2.19–2.16 (m, 2H), 2.12–2.08 (m, 2H), 1.74 (s, 3H), 1.60 (s, 3H), 1.04 (s, 3H), 1.03 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 171.6, 146.3, 138.7, 138.0, 136.0, 135.1, 133.9, 131.8, 129.4, 128.0, 121.2, 120.0, 118.9, 43.2, 39.1, 37.2, 33.8, 29.7, 27.6, 26.4, 25.1, 22.3, 18.4, 17.1; IR νmax (film): 3353, 2958, 2926, 2869, 1732, 1658, 1622, 1515, 1487, 1456, 1265, 1071, 1011 cm−1; HRMS m/z calculated for C27H37BrNO [M + H]+: 470.2059; found: 470.2062.
(1E,5E,9E,11E)-N-(Furan-2-ylmethyl)-12-isopropyl-5,9-dimethylcyclotetradeca-1,5,9,11-tetraenecarboxamide (8k): Yield: 73%; Colorless oil; UV (MeOH) λmax (log ε) 241 (3.9); 1H-NMR (600 MHz, CD3COCD3) δ 7.44 (dd, J = 1.9 Hz, 0.9 Hz, 1H), 6.87 (s, 1H), 6.34 (dd, J = 3.2 Hz, 1.9 Hz, 1H), 6.20 (dd, J = 3.2 Hz, 0.9 Hz, 1H), 6.11 (t, J = 7.7 Hz, 1H), 6.02 (d, J = 11.1 Hz, 1H), 5.98 (dq, J = 11.1 Hz, 1.5 Hz, 1H), 5.14 (t, J = 6.8 Hz, 1H), 4.35 (d, J = 5.8 Hz, 2H), 2.46 (t, J = 7.6 Hz, 2H), 2.39–2.31 (m, 3H), 2.26–2.21 (m, 4H), 2.18–2.15 (m, 4H), 1.73 (s, 3H), 1.68 (s, 3H), 1.04 (s, 3H), 1.03 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 171.4, 151.8, 146.2, 142.1, 138.5, 136.2, 135.2, 133.9, 128.0, 119.8, 118.9, 110.6, 107.2, 39.1, 37.2, 37.0, 33.7, 29.7, 27.5, 26.2, 25.1, 22.3, 18.3, 17.0; IR νmax (film): 3376, 2958, 2926, 2870, 1726, 1661, 1628, 1520, 1507, 1448, 1379, 1190, 1148, 1012 cm−1; HRMS m/z calculated for C25H36NO2 [M + H]+: 382.2746; found: 382.2751.
(1E,5E,9E,11E)-12-Isopropyl-5,9-dimethyl-N-phenethylcyclotetradeca-1,5,9,11-tetraenecarboxamide (8l): Yield: 72%; white solid; mp: 109.5–111.6 °C; UV (MeOH) λmax (log ε) 249 (4.0); 1H-NMR (600 MHz, CDCl3) δ 7.33 (dd, J = 7.6 Hz, 7.4 Hz, 2H), 7.25 (t, J = 7.4 Hz, 1H), 7.19 (d, J = 7.6 Hz, 2H), 5.98 (d, J = 11.3 Hz, 1H), 5.91 (dq, J = 11.3 Hz, 1.6 Hz, 1H), 5.67 (t, J = 7.5 Hz, 1H), 5.38 (t, J = 6.1 Hz, 1H), 4.92 (t, J = 6.6 Hz, 1H), 3.44 (q, J = 6.6 Hz, 2H), 2.78 (t, J = 6.9 Hz, 2H), 2.45 (t, J = 7.0 Hz, 2H), 2.36–2.31 (m, 1H), 2.29–2.25 (m, 2H), 2.25–2.21 (m, 2H), 2.19 (t, J = 7.0 Hz, 2H), 2.15–2.12 (m, 2H), 2.07–2.03 (m, 2H), 1.70 (s, 3H), 1.65 (s, 3H), 1.02 (s, 3H), 1.01 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 171.4, 146.4, 139.4, 139.1, 135.7, 134.4, 133.9, 129.0, 128.8, 127.9, 126.6, 119.9, 118.8, 40.6, 39.2, 37.1, 36.1, 33.6, 29.8, 27.5, 26.2, 25.0, 22.4, 18.5, 17.1; IR νmax (film): 3356, 2955, 2921, 2851, 1658, 1630, 1521, 1454 cm−1; HRMS m/z calculated for C28H40NO [M + H]+: 406.3110; found: 406.3109.
(1E,5E,9E,11E)-N-(4-Fluorophenethyl)-12-isopropyl-5,9-dimethylcyclotetradeca-1,5,9,11-tetraenecarboxamide (8m): Yield: 58%; Colorless oil; UV (MeOH) λmax (log ε) 243 (4.1); 1H-NMR (600 MHz, CDCl3) δ 7.15 (dd, J = 8.4 Hz, 5.6 Hz, 2H), 7.01 (d, J = 8.4 Hz, 2H), 5.98 (d, J = 11.3 Hz, 1H), 5.92 (dq, J = 11.3 Hz, 1.6 Hz, 1H), 5.68 (t, J = 7.4 Hz, 1H), 5.39 (t, J = 6.1 Hz, 1H), 4.95 (td, J = 6.7 Hz, 1.6 Hz, 1H), 3.40 (q, J = 6.7 Hz, 2H), 2.75 (t, J = 7.0 Hz, 2H), 2.45 (t, J = 7.0 Hz, 2H), 2.36–2.31 (m, 1H), 2.30–2.22 (m, 4H), 2.20 (t, J = 7.0 Hz, 2H), 2.16–2.13 (m, 2H), 2.09–2.06 (m, 2H), 1.71 (s, 3H), 1.66 (s, 3H), 1.02 (s, 3H), 1.01 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 171.4, 161.7 (d, J = 244.4 Hz), 146.4, 139.0, 135.6, 135.0 (d, J = 3.3 Hz), 134.5, 133.9, 130.3 (d, J = 7.7 Hz), 127.8, 120.0, 118.8, 115.5 (d, J = 21.2 Hz), 40.7, 39.2, 37.1, 35.3, 33.6, 29.7, 27.5, 26.2, 25.0, 22.3, 18.4, 17.0; IR νmax (film): 3418, 3366, 2957, 2928, 2870, 1658, 1622, 1510, 1450, 1221 cm−1; HRMS m/z calculated for C28H39FNO [M + H]+: 424.3016; found: 424.3019.
(1E,5E,9E,11E)-N-(4-Chlorophenethyl)-12-isopropyl-5,9-dimethylcyclotetradeca-1,5,9,11-tetraenecarboxamide (8n): Yield: 55%; Colorless oil; UV (MeOH) λmax (log ε) 221 (4.0); 1H-NMR (600 MHz, CDCl3) δ 7.29 (d, J = 8.4 Hz, 2H), 7.12 (d, J = 8.4 Hz, 2H), 5.97 (d, J = 11.2 Hz, 1H), 5.92 (dq, J = 11.2 Hz, 1.6 Hz, 1H), 5.68 (t, J = 7.4 Hz, 1H), 5.37 (t, J = 6.1 Hz, 1H), 4.95 (t, J = 6.6 Hz, 1H), 3.40 (q, J = 6.7 Hz, 2H), 2.75 (t, J = 7.0 Hz, 2H), 2.45 (t, J = 7.0 Hz, 2H), 2.36–2.31 (m, 1H), 2.30–2.22 (m, 4H), 2.19 (t, J = 7.0 Hz, 2H), 2.16–2.13 (m, 2H), 2.09–2.06 (m, 2H), 1.71 (s, 3H), 1.66 (s, 3H), 1.02 (s, 3H), 1.00 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 171.4, 146.4, 139.0, 137.9, 135.6, 134.6, 133.9, 132.4, 130.3, 128.8, 127.8, 120.0, 118.8, 40.6, 39.2, 37.1, 35.5, 33.6, 29.7, 27.5, 26.2, 25.0, 22.3, 18.5, 17.0; IR νmax (film): 3367, 2958, 2925, 2853, 1714, 1649, 1517, 1492, 1457, 1368, 1090, 1015 cm−1; HRMS m/z calculated for C28H39ClNO [M + H]+: 440.2720; found: 440.2727.
(1E,5E,9E,11E)-N-(4-Bromophenethyl)-12-isopropyl-5,9-dimethylcyclotetradeca-1,5,9,11-tetraenecarboxamide (8o): Colorless oil; UV (MeOH) λmax (log ε) 242 (3.9); Yield: 62%; 1H-NMR (600 MHz, CDCl3) δ 7.44 (d, J = 8.4 Hz, 2H), 7.07 (d, J = 8.4 Hz, 2H), 5.97 (d, J = 11.3 Hz, 1H), 5.92 (dq, J = 11.3 Hz, 1.6 Hz, 1H), 5.68 (t, J = 7.4 Hz, 1H), 5.37 (t, J = 6.1 Hz, 1H), 4.95 (t, J = 6.6 Hz, 1H), 3.40 (q, J = 6.7 Hz, 2H), 2.74 (t, J = 7.0 Hz, 2H), 2.45 (t, J = 7.0 Hz, 2H), 2.36–2.31 (m, 1H), 2.30–2.23 (m, 4H), 2.20 (t, J = 7.0 Hz, 2H), 2.17–2.13 (m, 2H), 2.09–2.06 (m, 2H), 1.71 (s, 3H), 1.66 (s, 3H), 1.02 (s, 3H), 1.01 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 171.4, 146.4, 139.0, 138.4, 135.7, 134.6, 133.9, 131.8, 130.7, 127.9, 120.5, 120.0, 118.8, 40.5, 39.2, 37.1, 35.6, 33.6, 29.8, 27.5, 26.2, 25.0, 22.3, 18.5, 17.0; IR νmax (film): 3418, 2948, 2922, 2843, 1648, 1448, 1032, 1017 cm−1; HRMS m/z calculated for C28H39BrNO [M + H]+: 484.2215; found: 484.2208.
(1E,5E,9E,11E)-N-Cyclopentyl-12-isopropyl-5,9-dimethylcyclotetradeca-1,5,9,11-tetraenecarboxamide (8p): Yield: 80%; white solid; mp: 106.2–107.9 °C; UV (MeOH) λmax (log ε) 249 (4.2); 1H-NMR (600 MHz, CD3COCD3) δ 6.22 (d, J = 7.1 Hz, 1H), 6.05–6.01 (m, 3H), 5.16 (t, J = 6,8 Hz, 1H), 4.14–4.06 (m, 1H), 2.43 (t, J = 7.5 Hz, 2H), 2.39–2.33 (m, 1H), 2.32–2.28 (m, 2H), 2.27–2.24 (m, 2H), 2.22 (t, J = 7.6 Hz, 2H), 2.19–2.13 (m, 4H), 1.93–1.85 (m, 2H), 1.73 (s, 3H), 1.72 (s, 3H), 1.68–1.62 (m, 2H), 1.58–1.51 (m, 2H), 1.44–1.37 (m, 2H), 1.05 (s, 3H), 1.03 (s, 3H); 13C-NMR (150 MHz, CD3COCD3) δ 169.9, 146.5, 139.4, 135.7, 135.2, 134.0, 128.3, 121.1, 119.7, 51.8, 39.5, 38.0, 34.9, 33.4, 29.2, 27.3, 25.6, 24.5, 22.4, 18.3, 17.2; IR νmax (film): 3408, 2954, 2928, 2868, 1652, 1617, 1533, 1456, 1018 cm−1; HRMS m/z calculated for C25H40NO [M + H]+: 370.3110; found: 370.3099.
(1E,5E,9E,11E)-12-Isopropyl-5,9-dimethyl-N-(prop-2-yn-1-yl)cyclotetradeca-1,5,9,11-tetraenecarboxamide (8q): Yield: 48%; Colorless oil; UV (MeOH) λmax (log ε) 249 (3.9); 1H-NMR (600 MHz, CDCl3) δ 6.03 (d, J = 11.3 Hz, 1H), 5.98 (dq, J = 11.3 Hz, 1.5 Hz, 1H), 5.81 (t, J = 7.4 Hz, 1H), 5.46 (t, J = 5.0 Hz, 1H), 5.07 (t, J = 6.7 Hz, 1H), 3.93 (d, J = 5.2 Hz, 2.6 Hz, 2H), 2.49 (t, J = 6.8 Hz, 2H), 2.38–2.34 (m, 1H), 2.33–2.27 (m, 4H), 2.24 (t, J = 6.8 Hz, 2H), 2.22 (t, J = 2.6 Hz, 1H), 2.21–2.18 (m, 4H), 1.76 (s, 6H), 1.03 (s, 3H), 1.02 (s, 3H); 13C-NMR (150 MHz, CDCl3) δ 171.4, 146.2, 138.3, 136.2, 135.6, 133.7, 128.1, 119.9, 118.9, 80.1, 71.6, 39.1, 37.2, 33.5, 29.9, 29.7, 27.1, 26.0, 25.1, 22.3, 18.6, 16.9; IR νmax (film): 3357, 3311, 2955, 2920, 2851, 1658, 1631, 1507, 1448 cm−1; HRMS m/z calculated for C23H34NO [M + H]+: 340.2640; found: 340.2634.
(1E,5E,9E,11E)-N-Isobutyl-12-isopropyl-5,9-dimethylcyclotetradeca-1,5,9,11-tetraenecarboxamide (8r): Yield: 51%; Colorless oil; UV (MeOH) λmax (log ε) 241 (3.6); 1H-NMR (600 MHz, CDCl3) δ 6.03–5.99 (m, 2H), 5.80 (t, J = 7.4 Hz, 1H), 5.47 (t, J = 6.2 Hz, 1H), 5.07 (t, J = 6.8 Hz, 1H), 3.00 (t, J = 6.5 Hz, 2H), 2.47 (t, J = 7.1 Hz, 2H), 2.37–2.33 (m, 1H), 2.32–2.26 (m, 4H), 2.23 (t, J = 7.1 Hz, 2H), 2.19–2.14 (m, 5H), 1.74 (s, 3H), 1.72 (s, 3H), 1.03 (s, 3H), 1.02 (s, 3H), 0.90 (s, 3H), 0.89 (s, 3H); 13C-NMR (100 MHz, CDCl3) δ 171.5, 146.5, 139.3, 135.8, 134.2, 134.1, 127.9, 120.1, 118.9, 46.9, 39.2, 37.3, 33.8, 29.7, 28.8, 27.7, 26.4, 25.2, 22.3, 20.3, 18.5, 17.2; IR νmax (film): 3391, 2958, 2930, 2871, 1652, 1539, 1457, 1386, 1022 cm−1; HRMS m/z calculated for C24H40NO [M + H]+: 358.3110; found: 358.3103.