Sugar Composition Analysis of Fuzi Polysaccharides by HPLC-MSn and Their Protective Effects on Schwann Cells Exposed to High Glucose
Abstract
:1. Introduction
2. Results
2.1. The MS Analysis of FPS
2.1.1. The Fragmentation Regulations of PMP-Labeled Monosaccharides
2.1.2. Monosaccharide Composition Analysis of the Hydrolyzed FPS
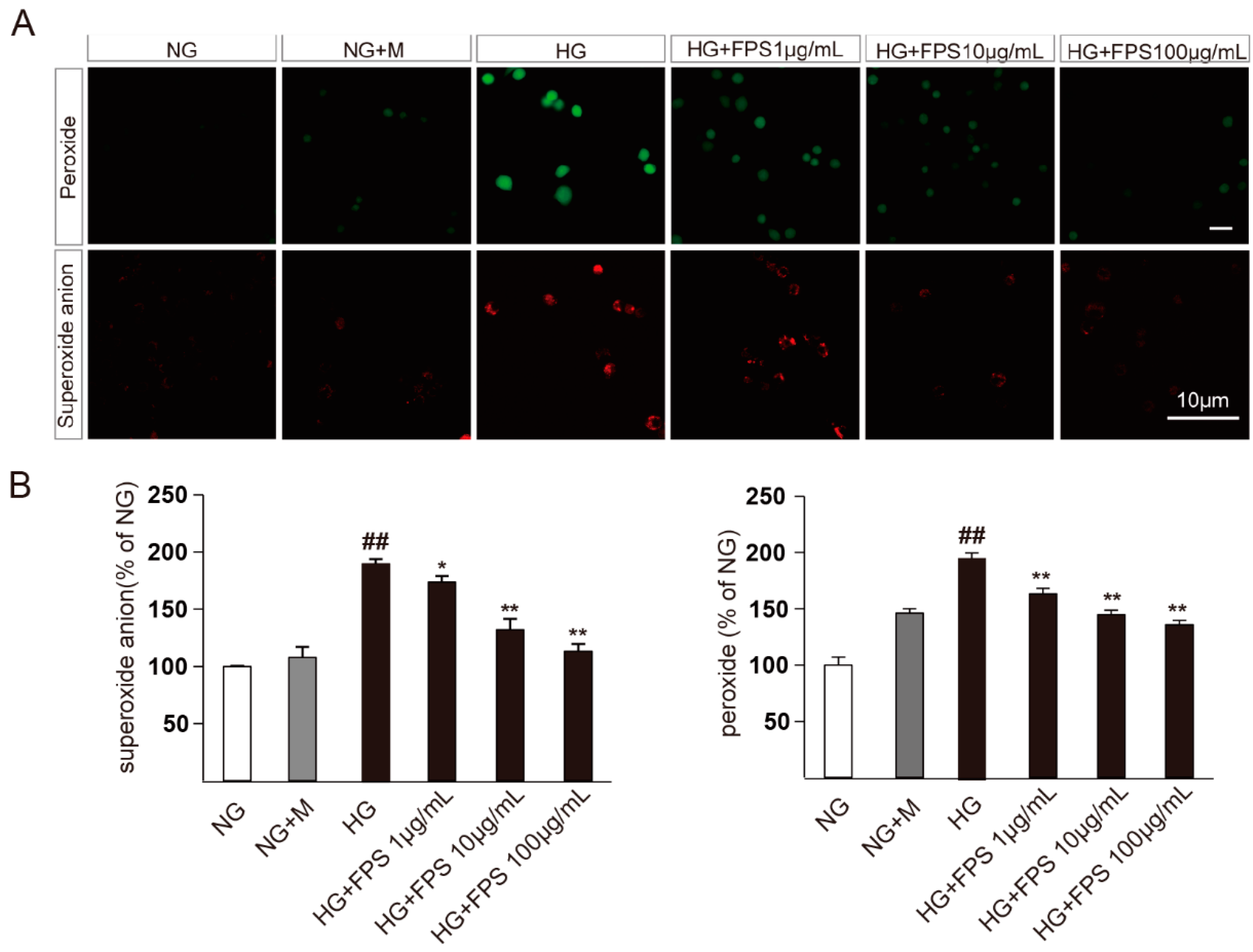
2.2. Evaluation of the Effects of FPS on Reactive Oxygen Species in High Glucose-Stimulated RSC96 Cells
2.3. Evaluation of the Effects of FPS on Apoptosis in High Glucose-Stimulated RSC96 Cells
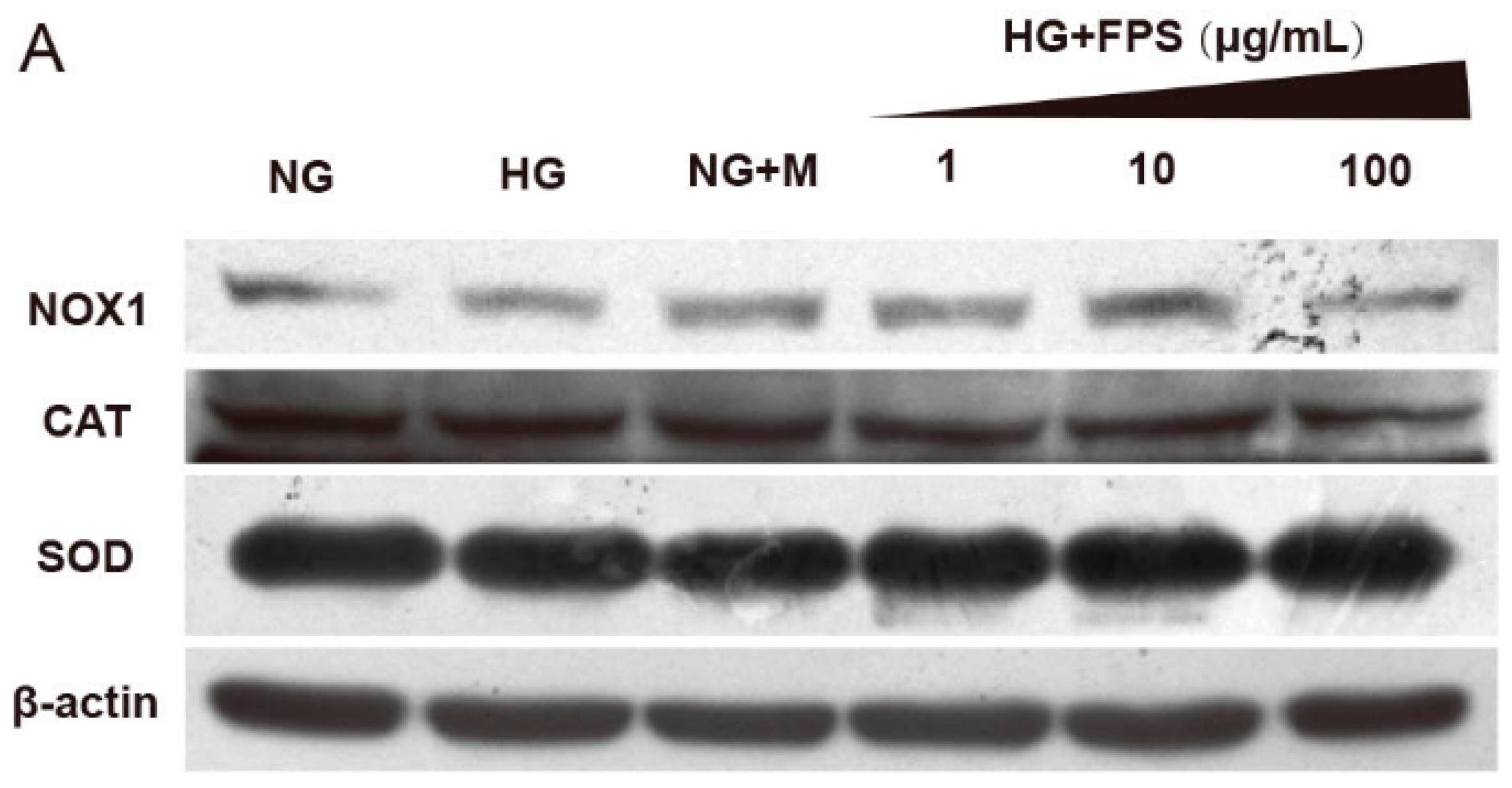
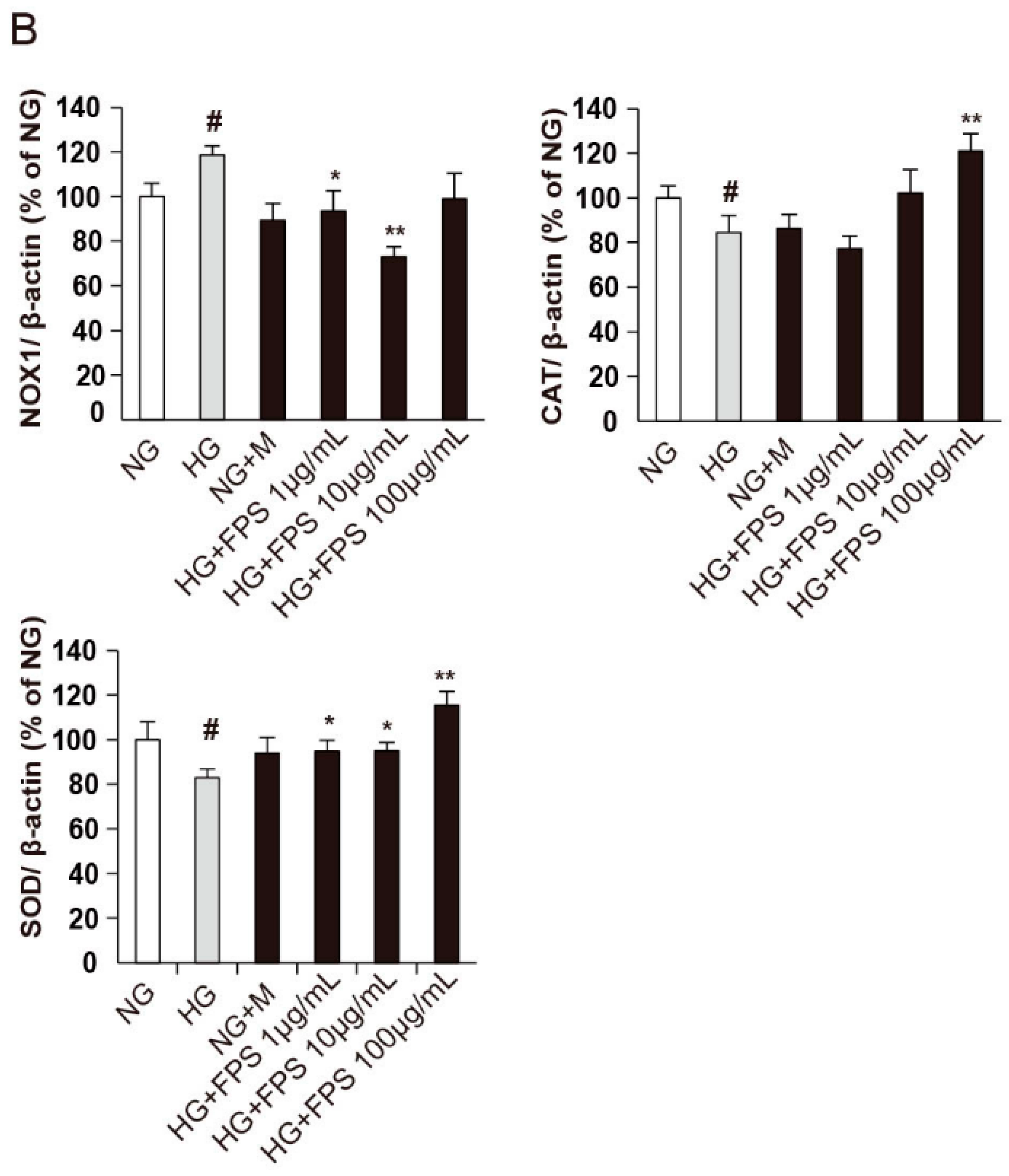
2.4. Evaluation of the Effects of on the Expression of Antioxidant Enzymes and Nox1
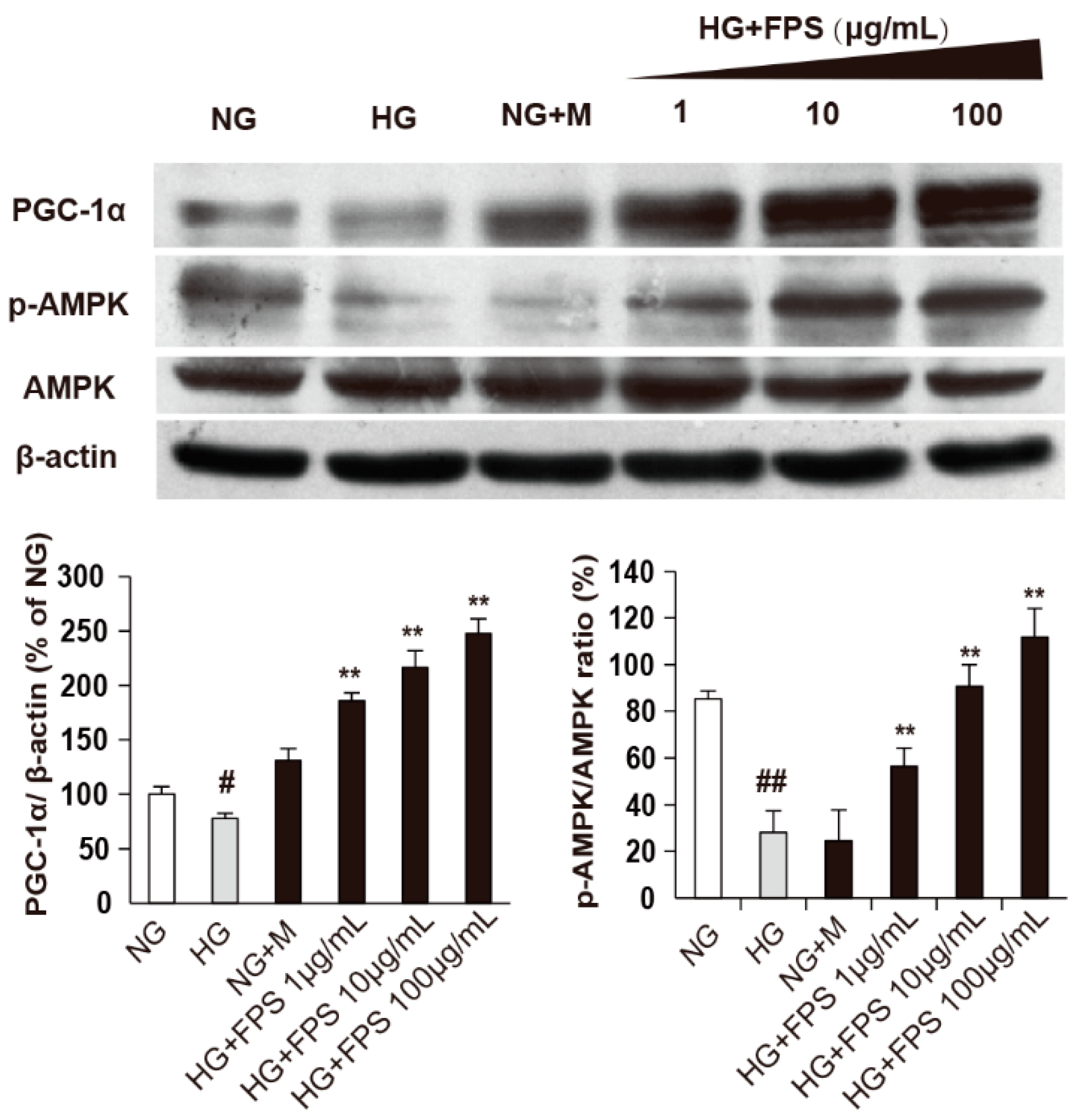
2.5. The Effects of FPS on AMPK-PGC-1α Pathway
3. Experimental Section
3.1. Materials and Reagents
3.2. Chemistry
3.2.1. Extraction of the Polysaccharides from Fuzi
3.2.2. The Monosaccharide Composition Analysis of FPS
Acid Hydrolysis of the Polysaccharides
Derivatization Treatment
Chromatography and Mass Spectrometry Conditions
3.3. Biological Effectiveness
3.3.1. Cell Cultivation and Treatment Allocation
3.3.2. Detection of the Level of Active Oxygen Species (ROS)
3.3.3. Measurement of Cell Apoptosis Rate
3.4. Mechanism
Determination of the Protein Expression Levels Related Oxidative Stress
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Callaghan, B.C.; Cheng, H.T.; Stables, C.L.; Smith, A.L.; Feldman, E.L. Diabetic neuropathy: Clinical manifestations and current treatments. Lancet Neurol. 2012, 11, 521–534. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Farmer, K.L.; Li, C.; Dobrowsky, R.T. Diabetic peripheral neuropathy: Should a chaperone accompany our therapeutic approach? Pharmacol. Rev. 2012, 64, 880–900. [Google Scholar] [CrossRef] [PubMed]
- Stavniichuk, R.; Drel, V.R.; Shevalye, H.; Maksimchyk, Y.; Kuchmerovska, T.M.; Nadler, J.L.; Obrosova, I.G. Baicalein alleviates diabetic peripheral neuropathy through inhibition of oxidative-nitrosative stress and p38 mapk activation. Exp. Neurol. 2011, 230, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Z.; Ye, R.; He, Y.; Li, Y.; Qiu, X. Protective effect of jiaweibugan decoction against diabetic peripheral neuropathy. Neural Regen. Res. 2013, 8, 1113–1121. [Google Scholar] [PubMed]
- Ziegler, D.; Nowak, H.; Kempler, P.; Vargha, P.; Low, P.A. Treatment of symptomatic diabetic polyneuropathy with the antioxidant α-lipoic acid: A meta-analysis. Diabet. Med. 2004, 21, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, H.C.; Höke, A. Schwann cells as a therapeutic target for peripheral neuropathies. CNS Neurol. Disord. Drug Targets 2010, 9, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Cinci, L.; Corti, F.; Di Cesare Mannelli, L.; Micheli, L.; Zanardelli, M.; Ghelardini, C. Oxidative, metabolic, and apoptotic responses of schwann cells to high glucose levels. J. Biochem. Mol. Toxicol. 2015, 29, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, J.M.; Zochodne, D.W. Impaired peripheral nerve regeneration in diabetes mellitus. J. Peripher. Nerv. Syst. 2005, 10, 144–157. [Google Scholar] [CrossRef] [PubMed]
- Askwith, T.; Zeng, W.; Eggo, M.C.; Stevens, M.J. Taurine reduces nitrosative stress and nitric oxide synthase expression in high glucose-exposed human schwann cells. Exp. Neurol. 2012, 233, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Sango, K.; Yanagisawa, H.; Takaku, S.; Kawakami, E.; Watabe, K. Immortalized adult rodent schwann cells as in vitro models to study diabetic neuropathy. Exp. Diabetes Res. 2011, 2011. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Xue, B.; Li, X.; Liu, H. Puerarin prevents high glucose-induced apoptosis of schwann cells by inhibiting oxidative stress. Neural Regen. Res. 2012, 7, 2583–2591. [Google Scholar] [PubMed]
- Zhou, Y.-K.; Liang, Z.; Guo, Y.; Zhang, H.-T.; Wang, K.-H. High glucose upregulates cyp24a1 expression which attenuates the ability of 1,25(OH)2D3 to increase ngf secretion in a rat schwann cell line RSC96. Mol. Cell. Endocrinol. 2015, 404, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-Q.; Chen, Y.-Y.; Wang, X.; Li, X.-J.; Xue, B.; Qu, L.; Zhang, T.-T.; Mu, Y.-M.; Lu, J.-M. The protective effect of alpha lipoic acid on schwann cells exposed to constant or intermittent high glucose. Biochem. Pharmacol. 2012, 84, 961–973. [Google Scholar] [CrossRef] [PubMed]
- Xu, C. A Research on the Pathological Changes and Transmission Rules of DPN Diseases Treated with Classical Prescriptions. Ph.D. Thesis, Guangzhou University of Chinese Medicine, Guangzhou, China, 2012. [Google Scholar]
- Hu, Y.; Wang, T. The clinical observation of mahuangfuzixixin decoction for treatment of type 2 diabetic peripheral neuropathy. J. Liaoning Coll. Tradit. Chin. Med. 2005, 7, 588. [Google Scholar]
- Liu, H.; Xu, Z. Clinical research of modified fuzi decoctionon diabetic peripheral neuropathy. J. Emerg. Tradit. Chin. Med. 2013, 22, 51–52. [Google Scholar]
- Han, J.; Tan, P.; Li, Z.; Wu, Y.; Li, C.; Wang, Y.; Wang, B.; Zhao, S.; Liu, Y. Fuzi attenuates diabetic neuropathy in rats and protects schwann cells from apoptosis induced by high glucose. PLoS ONE 2014, 9, e86539. [Google Scholar] [CrossRef] [PubMed]
- Honda, S.; Akao, E.; Suzuki, S.; Okuda, M.; Kakehi, K.; Nakamura, J. High-performance liquid chromatography of reducing carbohydrates as strongly ultraviolet-absorbing and electrochemically sensitive 1-phenyl-3-methyl5-pyrazolone derivatives. Anal. Biochem. 1989, 180, 351–357. [Google Scholar] [CrossRef]
- Shen, X.; Perreault, H. Characterization of carbohydrates using a combination of derivatization, high-performance liquid chromatography and mass spectrometry. J. Chromatogr. A 1998, 811, 47–59. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Z.; Huang, L.; Bai, Q.; Jia, J. An improved pmp derivatization method for analyzing monosaccharide composition. Chemical J. Chin. Univ. 2006, 27, 1456–1458. [Google Scholar]
- Lu, Y.; Bu, H.; Yang, L.; Li, X.; Li, F. Comparasion of polysaccharides in parent root, daughter root and rootlet of aconitum carmichaeli. China J. Chin. Mater. Med. 2011, 36, 1154–1157. [Google Scholar]
- Wu, X.; Jiang, W.; Lu, J.; Yu, Y.; Wu, B. Analysis of the monosaccharide composition of water-soluble polysaccharides from sargassum fusiforme by high performance liquid chromatography/electrospray ionisation mass spectrometry. Food Chem. 2014, 145, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhao, Y.; Wang, Q.; Wang, H.; Mei, Q. Analysis of the monosaccharide components in angelica polysaccharides by high performance liquid chromatography. Anal. Sci. 2005, 21, 1177–1180. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.I.; Griendling, K.K. Nox proteins in signal transduction. Free Radic. Biol. Med. 2009, 47, 1239–1253. [Google Scholar] [CrossRef] [PubMed]
- Velázquez, I.; Plaud, M.; Wojna, V.; Skolasky, R.; Laspiur, J.P.; Meléndez, L.M. Antioxidant enzyme dysfunction in monocytes and csf of hispanic women with hiv-associated cognitive impairment. J. Neuroimmunol. 2009, 206, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Chandrasekaran, K.; Inoue, T.; Muragundla, A.; Russell, J.W. Pgc-1α regulation of mitochondrial degeneration in experimental diabetic neuropathy. Neurobiol. Dis. 2014, 64, 118–130. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W. Suppression of reactive oxygen species and neurodegeneration by the pgc-1 transcriptional coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Yang, S.J. Amp-activated protein kinase mediates activity-dependent regulation of peroxisome proliferator-activated receptor γ coactivator-1α and nuclear respiratory factor 1 expression in rat visual cortical neurons. Neuroscience 2010, 169, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S.; Mount, P.; Hill, R.; Levidiotis, V.; Katsis, F.; Stapleton, D.; Kemp, B.E.; Power, D.A. Regulation of the energy sensor amp-activated protein kinase in the kidney by dietary salt intake and osmolality. Am. J. Physiol.-Ren. Physiol. 2005, 288, F578–F586. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Brozinick, J.T.; Valladares, O.; Bucan, M.; Birnbaum, M.J. A role for amp-activated protein kinase in contraction-and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell 2001, 7, 1085–1094. [Google Scholar] [CrossRef]
- Ye, Q.; Zhang, D.; Guo, L. Research on polysaccharide extraction and purification process of fuzi. Pharm. Clin. Chin. Mater. Med. 2013, 2, 29–31. [Google Scholar]
- Askwith, T.; Zeng, W.; Eggo, M.C.; Stevens, M.J. Oxidative stress and dysregulation of the taurine transporter in high-glucose-exposed human schwann cells: Implications for pathogenesis of diabetic neuropathy. Am. J. Physiol.-Endocrinol. Metab. 2009, 297, E620–E628. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Tang, L.; Zhou, X.; Wang, T.; Kou, Z.; Wang, Z. A review on phytochemistry and pharmacological activities of the processed lateral root of aconitum carmichaelii debeaux. J. Ethnopharmacol. 2015, 160, 173–193. [Google Scholar] [CrossRef] [PubMed]
- Bedard, K.; Krause, K.H. The nox family of ros-generating nadph oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Cameron, K.O.; Kurumbail, R.G. Recent progress in the identification of adenosine monophosphate-activated protein kinase (ampk) activators. Bioorg. Med. Chem. Lett. 2016, 26, 5139–5148. [Google Scholar] [CrossRef] [PubMed]
- Sid, B.; Verrax, J.; Calderon, P.B. Role of ampk activation in oxidative cell damage: Implications for alcohol-induced liver disease. Biochem. Pharmacol. 2013, 86, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Detaille, D.; Guigas, B.; Chauvin, C.; Batandier, C.; Fontaine, E.; Wiernsperger, N.; Leverve, X. Metformin prevents high-glucose-induced endothelial cell death through a mitochondrial permeability transition-dependent process. Diabetes 2005, 54, 2179–2187. [Google Scholar] [CrossRef] [PubMed]
- Ido, Y.; Carling, D.; Ruderman, N. Hyperglycemia-induced apoptosis in human umbilical vein endothelial cells: Inhibition by the amp-activated protein kinase activation. Diabetes 2002, 51, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Viollet, B.; Mounier, R.; Leclerc, J.; Yazigi, A.; Foretz, M.; Andreelli, F. Targeting amp-activated protein kinase as a novel therapeutic approach for the treatment of metabolic disorders. Diabetes Metab. 2007, 33, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xie, N.; Cao, L.; Zhao, X.; Liu, X.; Jiang, H.; Chi, Z. Adenosine monophosphate-activated protein kinase and peroxisome proliferator-activated receptor gamma coactivator 1alpha signaling provides neuroprotection in status epilepticus in rats. Neurosci. Lett. 2011, 500, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.B.; Wu, Y.T.; Wu, T.P.; Wei, Y.H. Role of ampk-mediated adaptive responses in human cells with mitochondrial dysfunction to oxidative stress. Biochim. Biophys. Acta 2014, 1840, 1331–1344. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds FPS are available from the authors.
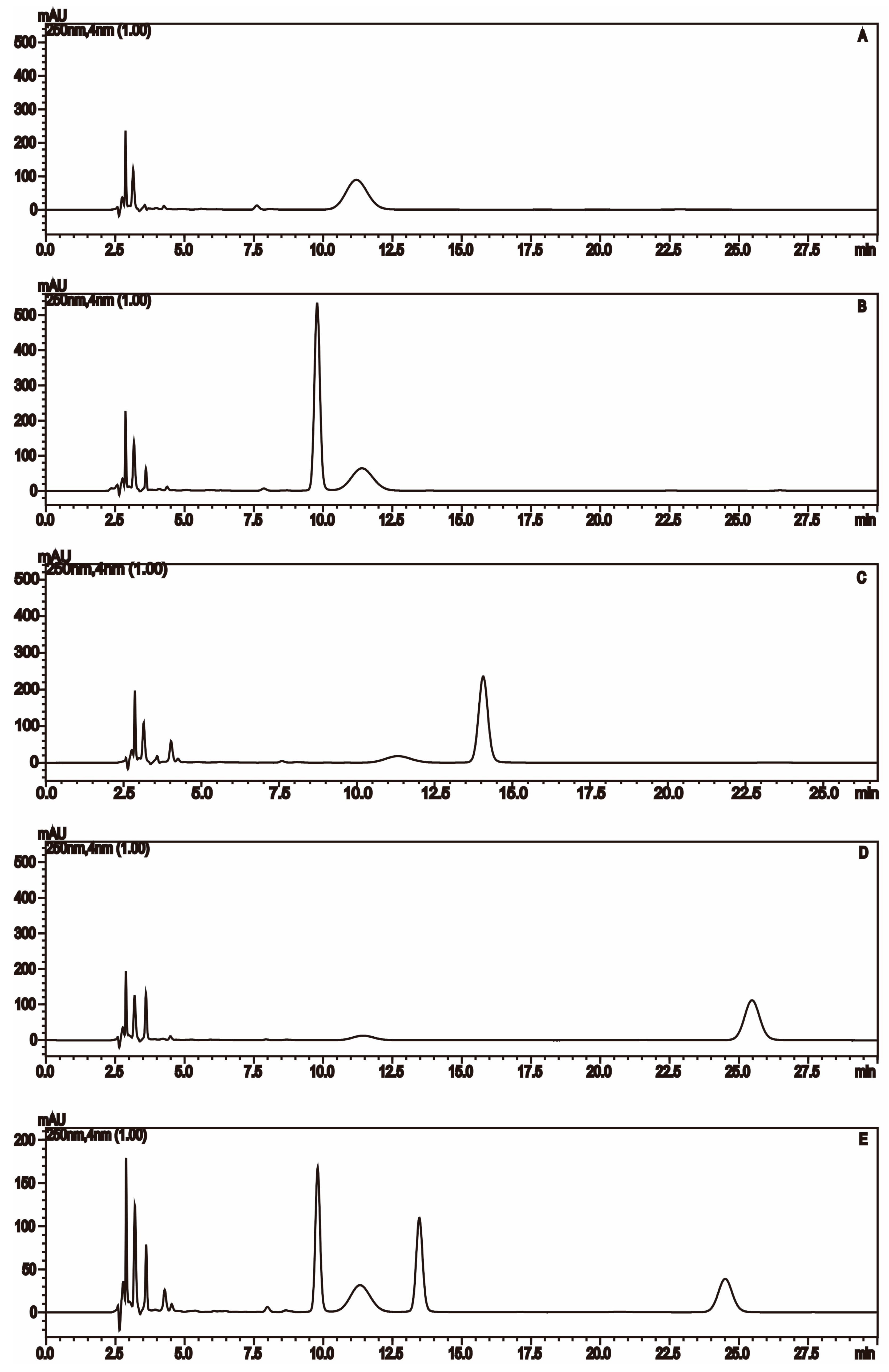
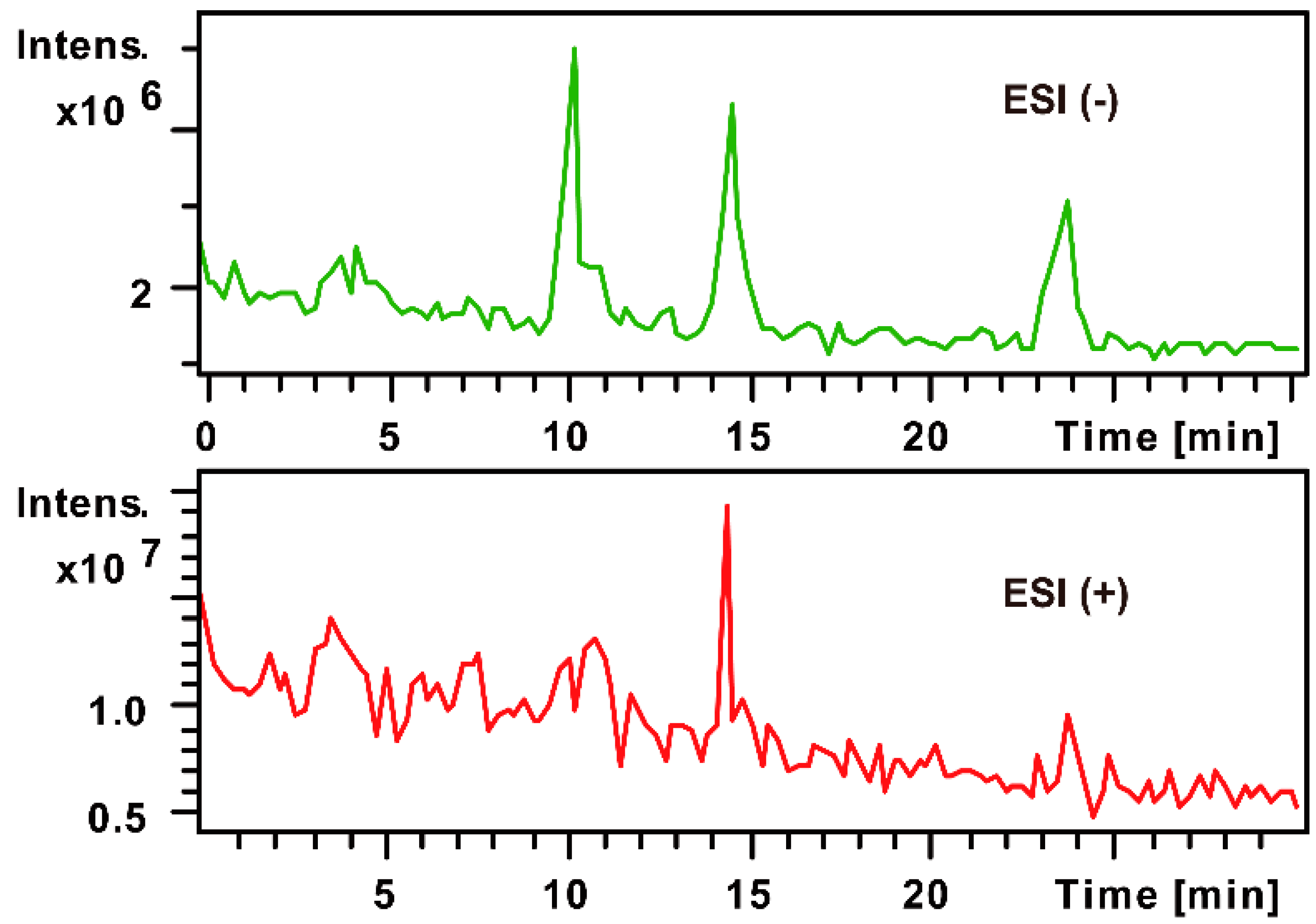
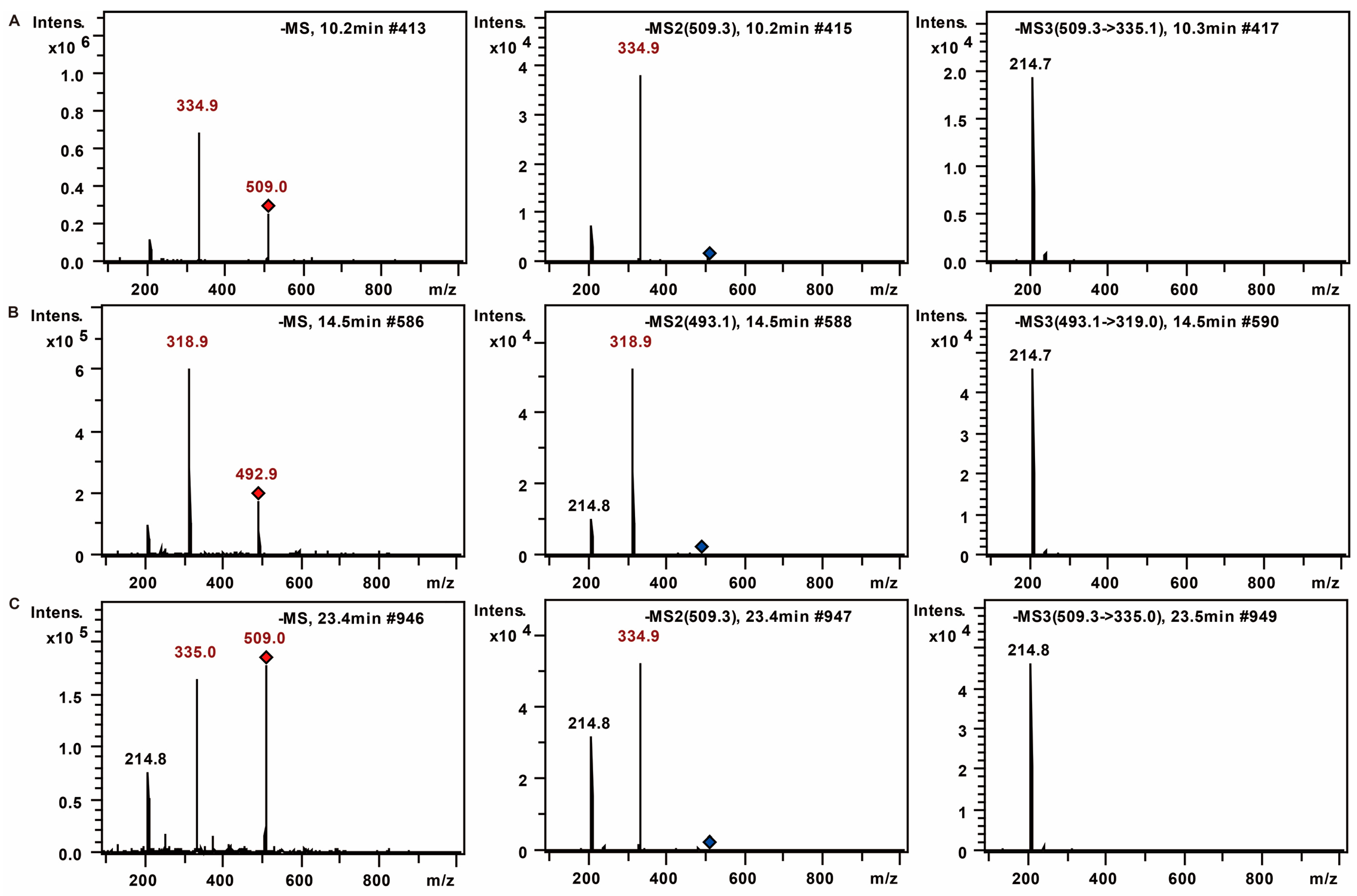
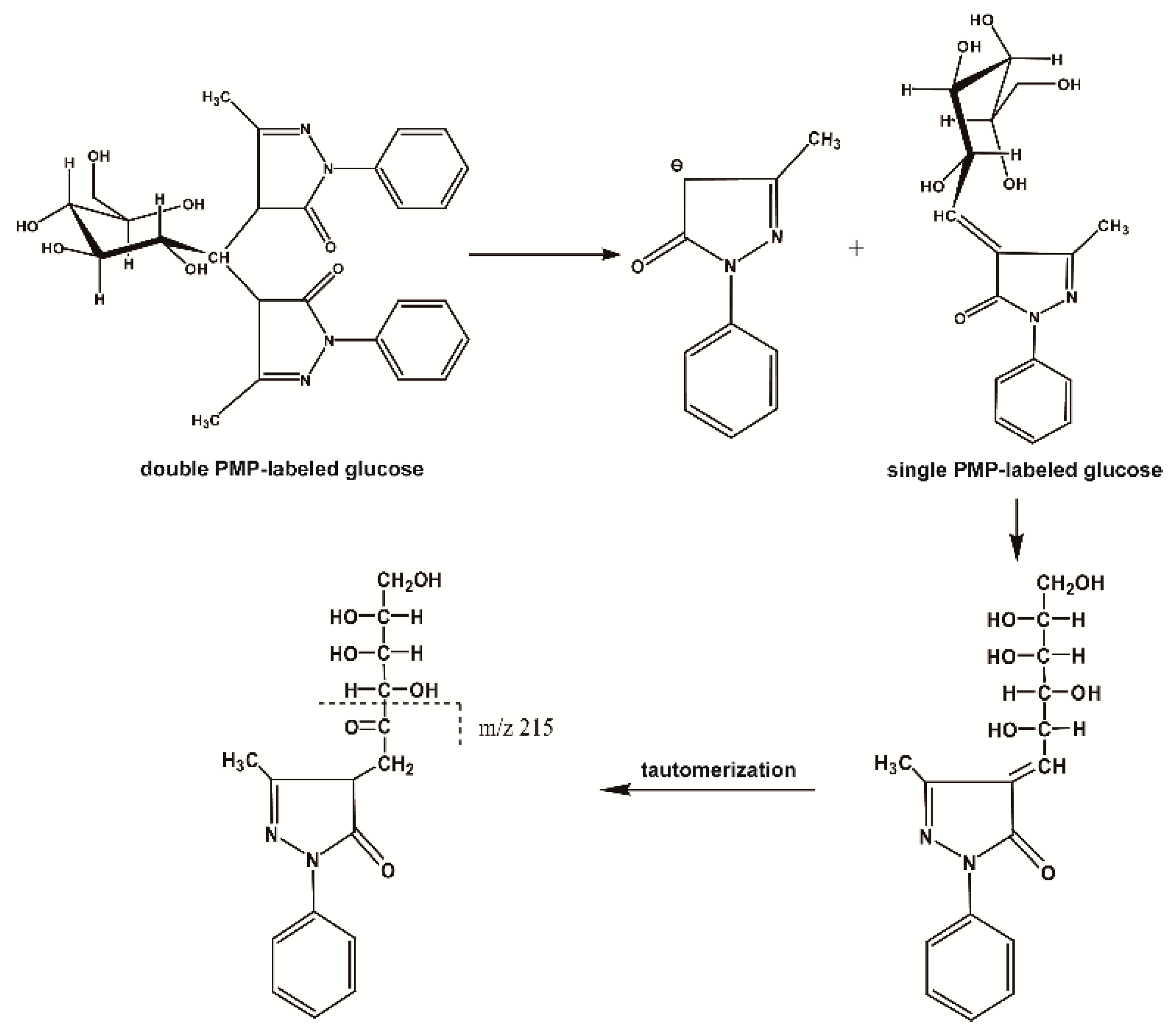
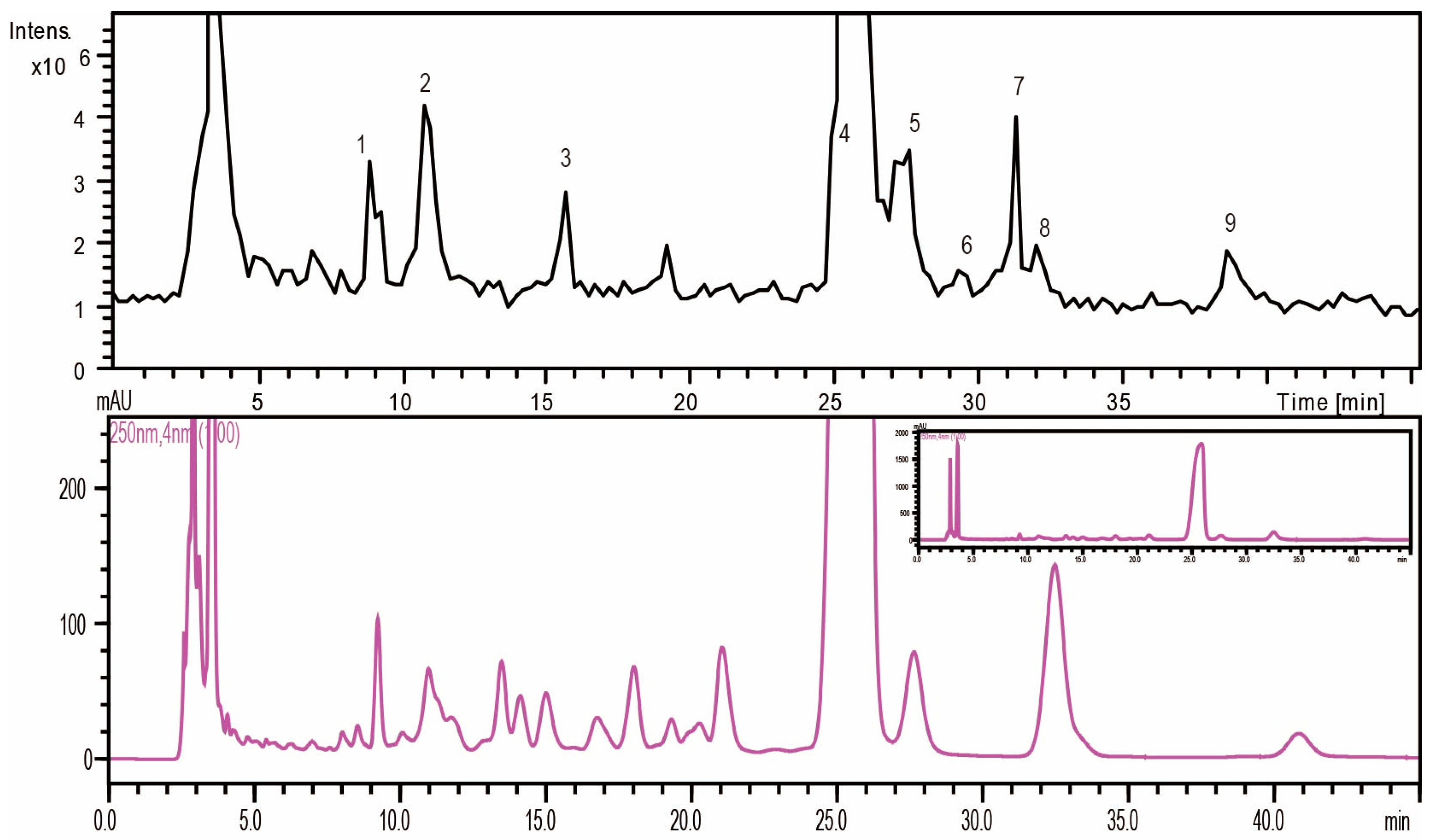

| No. | tR (min) | MS | MS2 | MS3 | Mw | Saccharides |
|---|---|---|---|---|---|---|
| 1 | 9 | 508.2 | 334.1 | 213.8 | 180 | Unknown (C6H12O6) |
| 2 | 10.8 | 509 | 335.1 | 214.9 | 180 | Mannose (C6H12O6) |
| 3 | 15.8 | 493.2 | 319 | 214.8 | 164 | Rhamnose (C6H12O5) |
| 4 | 24.9 | 509.2 | 335 | 214.7 | 180 | Glucose (C6H12O6) |
| 5 | 27.4 | 509.3 | 335 | 214.8 | 180 | Galactose (C6H12O6) |
| 6 | 29.4 | 509.3 | 335.1 | 214.7 | 180 | Unknown (C6H12O6) |
| 7 | 31 | 479.2 | 305 | 214.7 | 150 | Xylose (C5H10O5) |
| 8 | 31.9 | 479.2 | 305 | 214.8 | 150 | Arabinose (C5H10O5) |
| 9 | 38.5 | 493.3 | 319.1 | 214.7 | 164 | Fucose (C6H12O5) |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.-B.; Wang, J.-L.; Yuan, J.; Quan, Q.-H.; Ji, R.-F.; Tan, P.; Han, J.; Liu, Y.-G. Sugar Composition Analysis of Fuzi Polysaccharides by HPLC-MSn and Their Protective Effects on Schwann Cells Exposed to High Glucose. Molecules 2016, 21, 1496. https://doi.org/10.3390/molecules21111496
Wang B-B, Wang J-L, Yuan J, Quan Q-H, Ji R-F, Tan P, Han J, Liu Y-G. Sugar Composition Analysis of Fuzi Polysaccharides by HPLC-MSn and Their Protective Effects on Schwann Cells Exposed to High Glucose. Molecules. 2016; 21(11):1496. https://doi.org/10.3390/molecules21111496
Chicago/Turabian StyleWang, Bei-Bei, Jia-Li Wang, Jiang Yuan, Qing-Hua Quan, Rui-Fang Ji, Peng Tan, Jing Han, and Yong-Gang Liu. 2016. "Sugar Composition Analysis of Fuzi Polysaccharides by HPLC-MSn and Their Protective Effects on Schwann Cells Exposed to High Glucose" Molecules 21, no. 11: 1496. https://doi.org/10.3390/molecules21111496
APA StyleWang, B.-B., Wang, J.-L., Yuan, J., Quan, Q.-H., Ji, R.-F., Tan, P., Han, J., & Liu, Y.-G. (2016). Sugar Composition Analysis of Fuzi Polysaccharides by HPLC-MSn and Their Protective Effects on Schwann Cells Exposed to High Glucose. Molecules, 21(11), 1496. https://doi.org/10.3390/molecules21111496






