Towards Water Soluble Mitochondria-Targeting Theranostic Osmium(II) Triazole-Based Complexes
Abstract
:1. Introduction
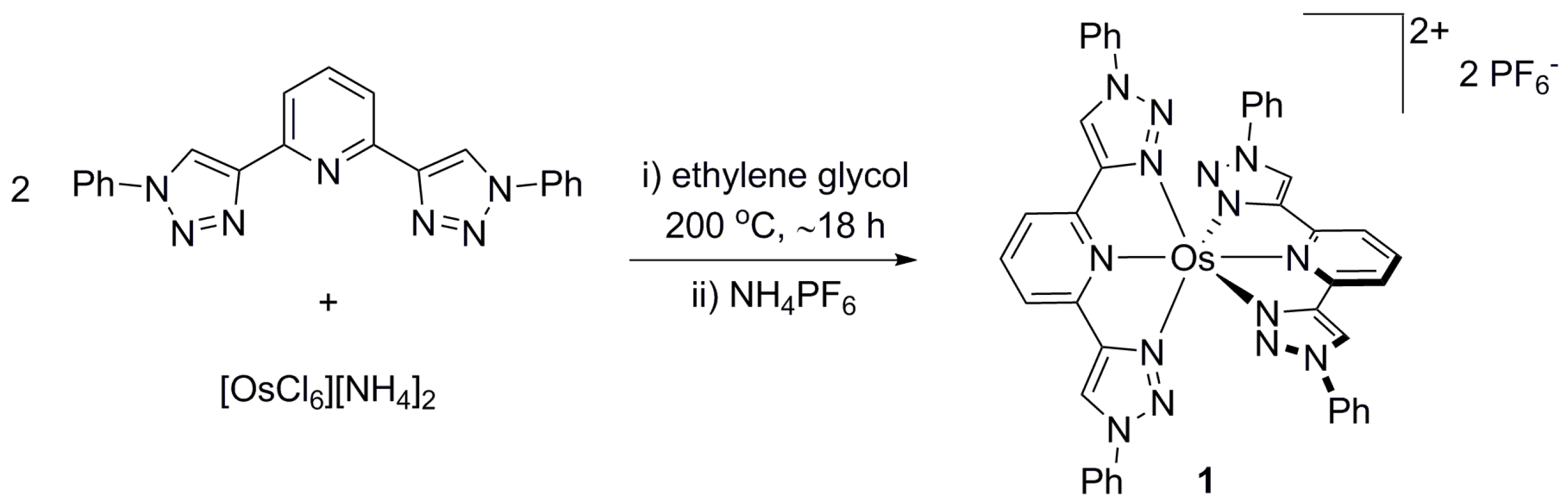
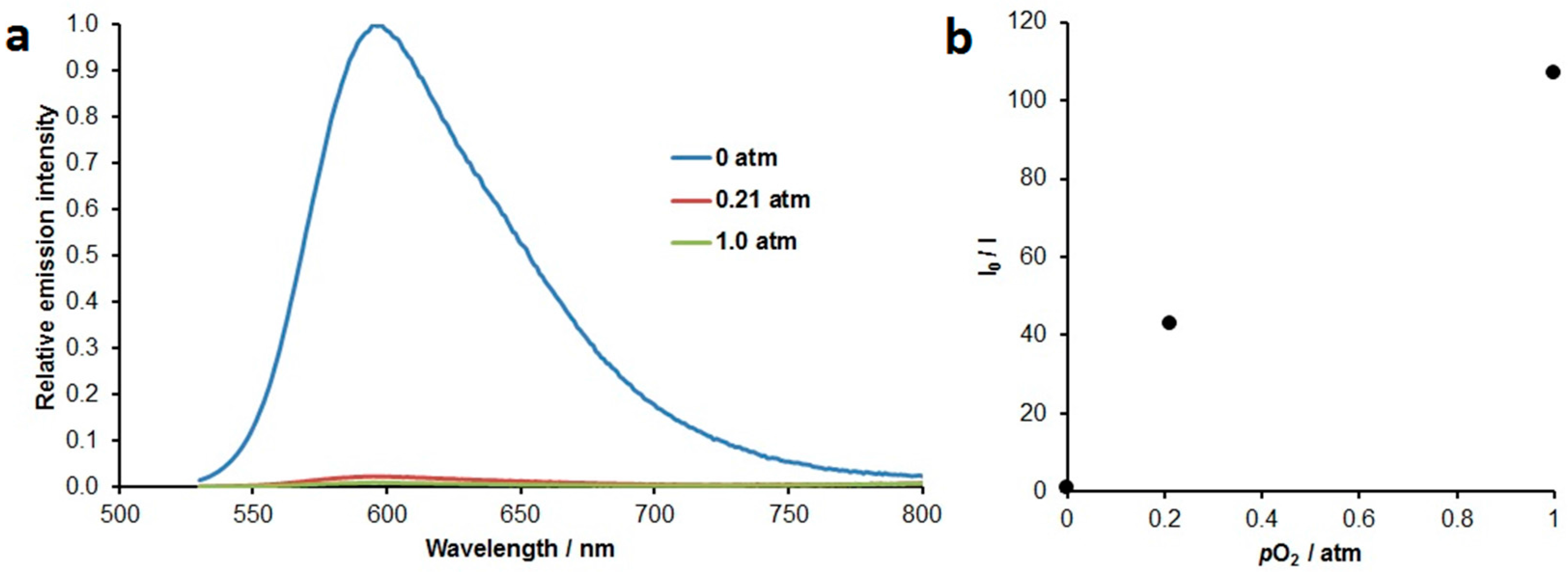
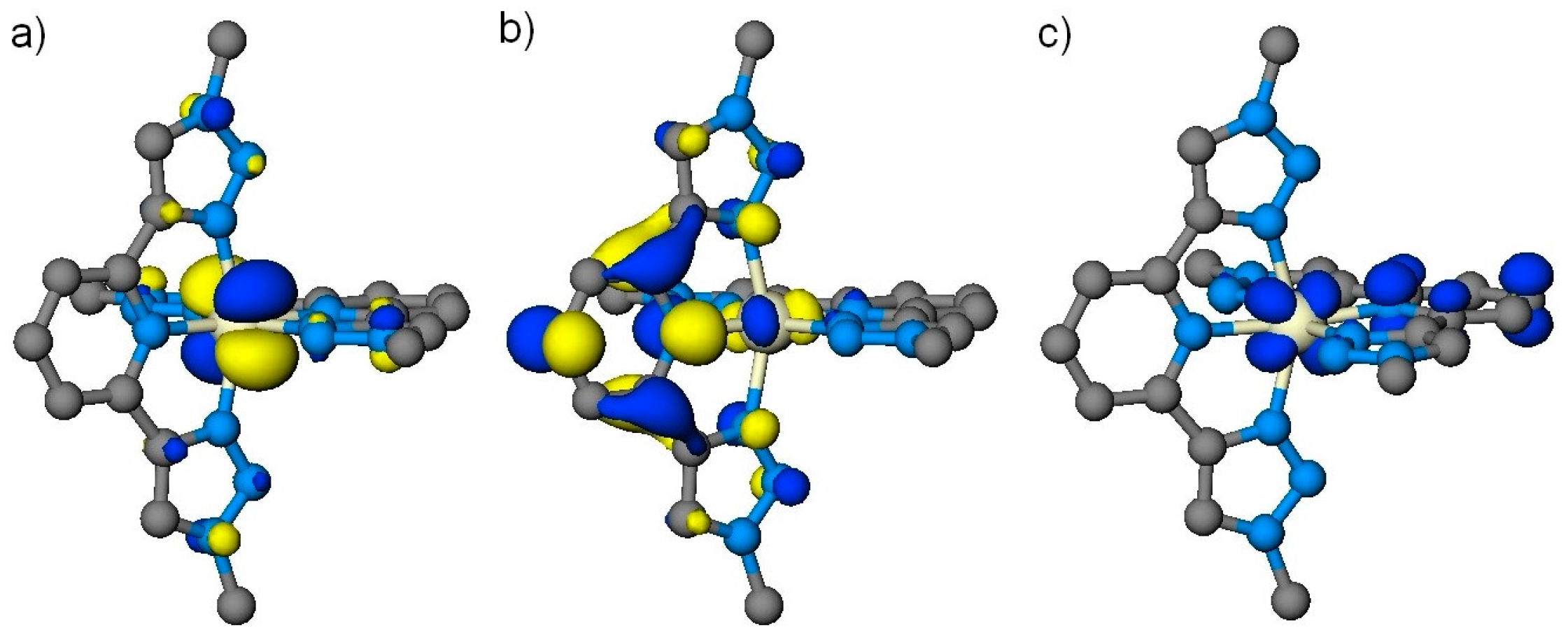
2. Results & Discussion
3. Conclusions
4. Experimental Section
4.1. General Methods
4.2. Synthesis of [Os(btzpy)2][PF6]2 (1)
4.3. Synthesis of [Os(btzpy)2]Cl2 (1Cl)
4.4. Computational Details
4.5. Cell Culture
4.6. Luminescence Imaging and Colocalisation Studies
4.7. Cell Viability Assay-MTT
4.8. Clonogenic Survival
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Baba, A.I.; Shaw, J.R.; Simon, J.A.; Thummel, R.P.; Schmehl, R.H. The photophysical behavior of d6 complexes having nearly isoenergetic MLCT and ligand localized excited states. Coord. Chem. Rev. 1998, 171, 43–59. [Google Scholar] [CrossRef]
- Balzani, V.; Ceroni, P.; Juris, A. Photochemistry and Photophysics: Concepts, Research, Applications; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Happ, B.; Winter, A.; Hager, M.D.; Schubert, U.S. Photogenerated avenues in macromolecules containing Re(I), Ru(II), Os(II), and Ir(III) metal complexes of pyridine-based ligands. Chem. Soc. Rev. 2012, 41, 2222–2255. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, D.; Shankar, K.; Vaidya, S.; Schmehl, R.H. Photochemistry and Photophysics of Coordination Compounds: Osmium. In Photochemistry and Photophysics of Coordination Compounds; Balzani, V., Campagna, S., Eds.; Springer: Berlin, Germany, 2007; Volume 281, pp. 101–142. [Google Scholar]
- Costa, R.D.; Ortí, E.; Bolink, H.J. Recent advances in light-emitting electrochemical cells. Pure Appl. Chem. 2011, 83, 2115–2128. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.D.; McMorran, D.A. “Click-triazole” coordination chemistry: Exploiting 1,4-disubstituted-1,2,3-triazoles as ligands. In Click Triazoles; Košmrljc, J., Ed.; Springer: Berlin, Germany, 2012; Volume 28, pp. 31–83. [Google Scholar]
- Elliott, P.I.P. Organometallic complexes with 1,2,3-triazole-derived ligands. In Organometallic Chemistry; RSC Publishing: Cambridge, UK, 2014; Volume 39, p. 1. [Google Scholar]
- Kumar, S.V.; Scottwell, S.O.; Waugh, E.; McAdam, C.J.; Hanton, L.R.; Brooks, H.J.L.; Crowley, J.D. Antimicrobial Properties of Tris(homoleptic) Ruthenium(II) 2-Pyridyl-1,2,3-triazole “Click” Complexes against Pathogenic Bacteria, Including Methicillin-Resistant Staphylococcus aureus (MRSA). Inorg. Chem. 2016, 55, 9767–9777. [Google Scholar] [CrossRef] [PubMed]
- Preston, D.; Barnsley, J.E.; Gordon, K.C.; Crowley, J.D. Controlled Formation of Heteroleptic [Pd2(La)2(Lb)2]4+ Cages. J. Am. Chem. Soc. 2016, 138, 10578–10585. [Google Scholar] [CrossRef] [PubMed]
- Beyer, B.; Ulbricht, C.; Escudero, D.; Friebe, C.; Winter, A.; Gonzalez, L.; Schubert, U.S. Phenyl-1H-[1,2,3]triazoles as New Cyclometalating Ligands for Iridium(III) Complexes. Organometallics 2009, 28, 5478. [Google Scholar] [CrossRef]
- Happ, B.; Escudero, D.; Hager, M.D.; Friebe, C.; Winter, A.; Goerls, H.; Altuntas, E.; Gonzalez, L.; Schubert, U.S. N-Heterocyclic Donor- and Acceptor-Type Ligands Based on 2-(1H-1,2,3 Triazol-4-yl)pyridines and Their Ruthenium(II) Complexes. J. Org. Chem. 2010, 75, 4025–4038. [Google Scholar] [CrossRef] [PubMed]
- Happ, B.; Friebe, C.; Winter, A.; Hager, M.D.; Hoogenboom, R.; Schubert, U.S. 2-(1H-1,2,3-Triazol-4-yl)-Pyridine Ligands as Alternatives to 2,2′-Bipyridines in Ruthenium(II) Complexes. Chem. Asian J. 2009, 4, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Ladouceur, S.; Fortin, D.; Zysman-Colman, E. Enhanced Luminescent Iridium(III) Complexes Bearing Aryltriazole Cyclometallated Ligands. Inorg. Chem. 2011, 50, 11514–11526. [Google Scholar] [CrossRef] [PubMed]
- Ladouceur, S.; Zysman-Colman, E. A Comprehensive Survey of Cationic Iridium(III) Complexes Bearing Nontraditional Ligand Chelation Motifs. Eur. J. Inorg. Chem. 2013, 2985–3007. [Google Scholar] [CrossRef]
- Mydlak, M.; Bizzarri, C.; Hartmann, D.; Sarfert, W.; Schmid, G.; de Cola, L. Positively Charged Iridium(III) Triazole Derivatives as Blue Emitters for Light-Emitting Electrochemical Cells. Adv. Funct. Mater. 2010, 20, 1812–1820. [Google Scholar] [CrossRef]
- Obata, M.; Kitamura, A.; Mori, A.; Kameyama, C.; Czaplewska, J.A.; Tanaka, R.; Kinoshita, I.; Kusumoto, T.; Hashimoto, H.; Harada, M.; et al. Syntheses, structural characterization and photophysical properties of 4-(2-pyridyl)-1,2,3-triazole rhenium(I) complexes. Dalton Trans. 2008, 25, 3292–3300. [Google Scholar] [CrossRef] [PubMed]
- Orselli, E.; Albuquerque, R.Q.; Fransen, P.M.; Froehlich, R.; Janssen, H.M.; de Cola, L. 1,2,3-Triazolyl-pyridine derivatives as chelating ligands for blue iridium(III) complexes. Photophysics and electroluminescent devices. J. Mater. Chem. 2008, 18, 4579–4590. [Google Scholar] [CrossRef]
- Ross, D.A.W.; Scattergood, P.A.; Babaei, A.; Pertegás, A.; Bolink, H.J.; Elliott, P.I.P. Luminescent osmium(II) bi-1,2,3-triazol-4-yl complexes: Photophysical characterisation and application in light-emitting electrochemical cells. Dalton Trans. 2016, 45, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Schulze, B.; Friebe, C.; Hager, M.D.; Winter, A.; Hoogenboom, R.; Goerls, H.; Schubert, U.S. 2,2′:6′,2′′-Terpyridine meets 2,6-bis(1H-1,2,3-triazol-4-yl)pyridine: Tuning the electro-optical properties of ruthenium(II) complexes. Dalton Trans. 2009, 5, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Swanick, K.N.; Ladouceur, S.; Zysman-Colman, E.; Ding, Z. Bright electrochemiluminescence of iridium(III) complexes. Chem. Commun. 2012, 48, 3179–3181. [Google Scholar] [CrossRef] [PubMed]
- Swanick, K.N.; Ladouceur, S.; Zysman-Colman, E.; Ding, Z. Self-Enhanced Electrochemiluminescence of an Iridium(III) Complex: Mechanistic Insight. Angew. Chem. Int. Ed. 2012, 51, 11079–11082. [Google Scholar] [CrossRef] [PubMed]
- Uppal, B.S.; Booth, R.K.; Ali, N.; Lockwood, C.; Rice, C.R.; Elliott, P.I.P. Synthesis and characterisation of luminescent rhenium tricarbonyl complexes with axially coordinated 1,2,3-triazole ligands. Dalton Trans. 2011, 40, 7610–7616. [Google Scholar] [CrossRef] [PubMed]
- Welby, C.E.; Gilmartin, L.; Marriott, R.R.; Zahid, A.; Rice, C.R.; Gibson, E.A.; Elliott, P.I.P. Luminescent biscyclometalated arylpyridine iridium(III) complexes with 4,4′-bi-1,2,3-triazolyl ancillary ligands. Dalton Trans. 2013, 42, 13527–13536. [Google Scholar] [CrossRef] [PubMed]
- Zanarini, S.; Felici, M.; Valenti, G.; Marcaccio, M.; Prodi, L.; Bonacchi, S.; Contreras-Carballada, P.; Williams, R.M.; Feiters, M.C.; Nolte, R.J.M.; et al. Green and Blue Electrochemically Generated Chemiluminescence from Click Chemistry-Customizable Iridium Complexes. Chem. Eur. J. 2011, 17, 4640–4647. [Google Scholar] [CrossRef] [PubMed]
- Baggaley, E.; Weinstein, J.A.; Williams, J.A.G. Lighting the way to see inside the live cell with luminescent transition metal complexes. Coord. Chem. Rev. 2012, 256, 1762–1785. [Google Scholar] [CrossRef]
- Holmlin, R.E.; Yao, J.A.; Barton, J.K. Dipyridophenazine complexes of Os(II) as red-emitting DNA probes: Synthesis, characterization, and photophysical properties. Inorg. Chem. 1999, 38, 174–189. [Google Scholar] [CrossRef]
- Lo, K.K.W. Luminescent Rhenium(I) and Iridium(III) Polypyridine complexes as biological probes, imaging reagents, and photocytotoxic agents. Acc. Chem. Res. 2015, 48, 2985–2995. [Google Scholar] [CrossRef] [PubMed]
- Baggaley, E.; Botchway, S.W.; Haycock, J.W.; Morris, H.; Sazanovich, I.V.; Williams, J.A.G.; Weinstein, J.A. Long-lived metal complexes open up microsecond lifetime imaging microscopy under multiphoton excitation: From FLIM to PLIM and beyond. Chem. Sci. 2014, 5, 879–886. [Google Scholar] [CrossRef]
- Baggaley, E.; Weinstein, J.A.; Williams, J.A.G. Time-resolved emission imaging microscopy using phosphorescent metal complexes: Taking FLIM and PLIM to new lengths. In Structure and Bonding; Lo, K.K.-W., Ed.; Springer: Berlin, Germany, 2015; Volume 165, pp. 205–256. [Google Scholar]
- Jahn, K.; Buschmann, V.; Hille, C. Simultaneous fluorescence and phosphorescence lifetime imaging microscopy in living cells. Sci. Rep. 2015, 5, 14334. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Knight, T.E.; Stewart, D.J.; Brennaman, M.K.; Meyer, T.J. Rigid medium effects on photophysical properties of MLCT excited states of polypyridyl Os(II) complexes in polymerized poly(ethylene glycol)dimethacrylate monoliths. J. Phys. Chem. A 2014, 118, 10326–10332. [Google Scholar] [CrossRef] [PubMed]
- Kober, E.M.; Meyer, T.J. Concerning the absorption spectra of the ions M(bpy)32+ (M = Fe, Ru, Os; bpy = 2,2-bipyridine). Inorg. Chem. 1982, 21, 3967–3977. [Google Scholar] [CrossRef]
- Lumpkin, R.S.; Kober, E.M.; Worl, L.A.; Murtaza, Z.; Meyer, T.J. Metal-to-ligand charge-transfer (MLCT) photochemistry. Experimental evidence for the participation of a higher lying MLCT state in polypyridyl complexes of ruthenium(II) and osmium(II). J. Phys. Chem. 1990, 94, 239–243. [Google Scholar] [CrossRef]
- Scattergood, P.A.; Ross, D.A.W.; Rice, C.R.; Elliott, P.I.P. Labilizing the photoinert: Extraordinarily facile photochemical ligand ejection in an [Os(N^N)3]2+ complex. Angew. Chem. Int. Ed. 2016, 55, 10697–10701. [Google Scholar] [CrossRef] [PubMed]
- Stacey, O.J.; Pope, S.J.A. New avenues in the design and potential application of metal complexes for photodynamic therapy. RSC Adv. 2013, 3, 25550–25564. [Google Scholar] [CrossRef]
- Allampally, N.K.; Daniliuc, C.G.; Strassert, C.A.; de Cola, L. Tuning the structural and photophysical properties of cationic Pt(II) complexes bearing neutral bis(triazolyl)pyridine ligands. Inorg. Chem. 2015, 54, 1588–1596. [Google Scholar] [CrossRef] [PubMed]
- Crowley, J.D.; Bandeen, P.H.; Hanton, L.R. A one pot multi-component CuAAC “click” approach to bidentate and tridentate pyridyl-1,2,3-triazole ligands: Synthesis, X-ray structures and copper(II) and silver(I) complexes. Polyhedron 2010, 29, 70–83. [Google Scholar] [CrossRef]
- Constable, E.C.; Thompson, A.M.W.C. Pendant-functionalised ligands for metallosupramolecular assemblies; ruthenium(II) and osmium(II) complexes of 4′-(4-pyridyl)-2,2′:6′,2″-terpyridine. J. Chem. Soc. Dalton Trans. 1994, 9, 1409–1418. [Google Scholar] [CrossRef]
- Fortage, J.; Dupeyre, G.; Tuyèras, F.; Marvaud, V.; Ochsenbein, P.; Ciofini, I.; Hromadová, M.; Pospísil, L.; Arrigo, A.; Trovato, E.; et al. Molecular dyads of ruthenium(II)- or osmium(II)-bis(terpyridine) chromophores and expanded pyridinium acceptors: Equilibration between MLCT and charge-separated excited states. Inorg. Chem. 2013, 52, 11944–11955. [Google Scholar] [CrossRef] [PubMed]
- Shao, J.Y.; Zhong, Y.W. Monometallic osmium(II) complexes with bis(N -methylbenzimidazolyl)benzene or -pyridine: A comparison study with ruthenium(II) analogues. Inorg. Chem. 2013, 52, 6464–6472. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huffman, J.C.; Flood, A.H. Can terdentate 2,6-bis(1,2,3-triazol-4-yl)pyridines form stable coordination compounds? Chem. Commun. 2007, 26, 2692–2694. [Google Scholar] [CrossRef] [PubMed]
- Wadman, S.H.; Lutz, M.; Tooke, D.M.; Spek, A.L.; Hartl, F.; Havenith, R.W.A.; van Klink, G.P.M.; van Koten, G. Consequences of N, C, N′- and C, N, N′-coordination modes on electronic and photophysical properties of cyclometalated aryl ruthenium(II) complexes. Inorg. Chem. 2009, 48, 1887–1900. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.W.; Zhong, Y.W.; Yoshikawa, S.; Shao, J.Y.; Masaoka, S.; Sakai, K.; Yao, J.; Haga, M.A. Tuning of redox potentials by introducing a cyclometalated bond to bis-tridentate ruthenium(II) complexes bearing bis(N -methylbenzimidazolyl) benzene or -pyridine ligands. Inorg. Chem. 2012, 51, 890–899. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Kobayashi, A.; Kaneko, S.; Takehira, K.; Yoshihara, T.; Ishida, H.; Shiina, Y.; Oishi, S.; Tobita, S. Reevaluation of absolute luminescence quantum yields of standard solutions using a spectrometer with an integrating sphere and a back-thinned CCD detector. Phys. Chem. Chem. Phys. 2009, 11, 9850–9860. [Google Scholar] [CrossRef] [PubMed]
- Sauvage, J.P.; Collin, J.P.; Chambron, J.C.; Guillerez, S.; Coudret, C.; Balzani, V.; Barigelletti, F.; de Cola, L.; Flamigni, L. Ruthenium(II) and osmium(II) bis(terpyridine) complexes in covalently-linked multicomponent systems: Synthesis, electrochemical behavior, absorption spectra, and photochemical and photophysical properties. Chem. Rev. 1994, 94, 993–1019. [Google Scholar] [CrossRef]
- Abrahamsson, M.; Wolpher, H.; Johansson, O.; Larsson, J.; Kritikos, M.; Eriksson, L.; Norrby, P.O.; Bergquist, J.; Sun, L.; Åkermark, B.; et al. A new strategy for the improvement of photophysical properties in ruthenium(II) polypyridyl complexes. Synthesis and photophysical and electrochemical characterization of six mononuclear ruthenium(II) bisterpyridine-type complexes. Inorg. Chem. 2005, 44, 3215–3225. [Google Scholar] [CrossRef] [PubMed]
- Kreitner, C.; Heinze, K. Excited state decay of cyclometalated polypyridine ruthenium complexes: Insight from theory and experiment. Dalton Trans. 2016, 45, 13631–13647. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pápai, M.; Møller, K.B.; Zhang, J.; Canton, S.E. Characterizing the solvated structure of photoexcited [Os(terpy)2]2+ with X-ray transient absorption spectroscopy and DFT calculations. Molecules 2016, 21, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Scattergood, P.A.; Delor, M.; Sazanovich, I.V.; Bouganov, O.V.; Tikhomirov, S.A.; Stasheuski, A.S.; Parker, A.W.; Greetham, G.M.; Towrie, M.; Davies, E.S.; et al. Electron transfer dynamics and excited state branching in a charge-transfer platinum(II) donor-bridge-acceptor assembly. Dalton Trans. 2014, 43, 17677–17693. [Google Scholar] [CrossRef] [PubMed]
- Abrahamse, H.; Hamblin, M.R. New photosensitizers for photodynamic therapy. Biochem. J. 2016, 473, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Hamann, T.W.; Gstrein, F.; Brunschwig, B.S.; Lewis, N.S. Measurement of the free-energy dependence of interfacial charge-transfer rate constants using ZnO/H2O semiconductor/liquid contacts. J. Am. Chem. Soc. 2005, 127, 7815–7824. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio calculation of vibrational absorption and circular dichroism spectra using density functional force fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-adjusted ab initio pseudopotentials for the second and third row transition elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Valiev, M.; Bylaska, E.J.; Govind, N.; Kowalski, K.; Straatsma, T.P.; van Dam, H.J.J.; Wang, D.; Nieplocha, J.; Apra, E.; Windus, T.L.; et al. NWChem: A comprehensive and scalable open-source solution for large scale molecular simulations. Comp. Phys. Commun. 2010, 181, 1477–1489. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are not available.
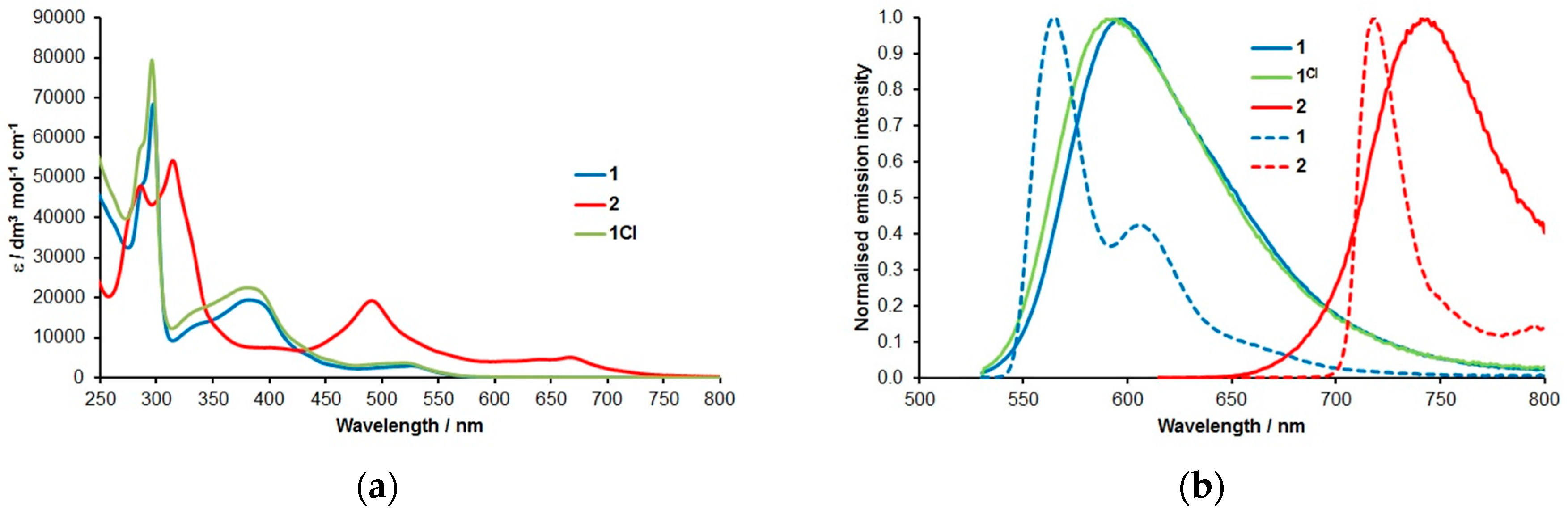
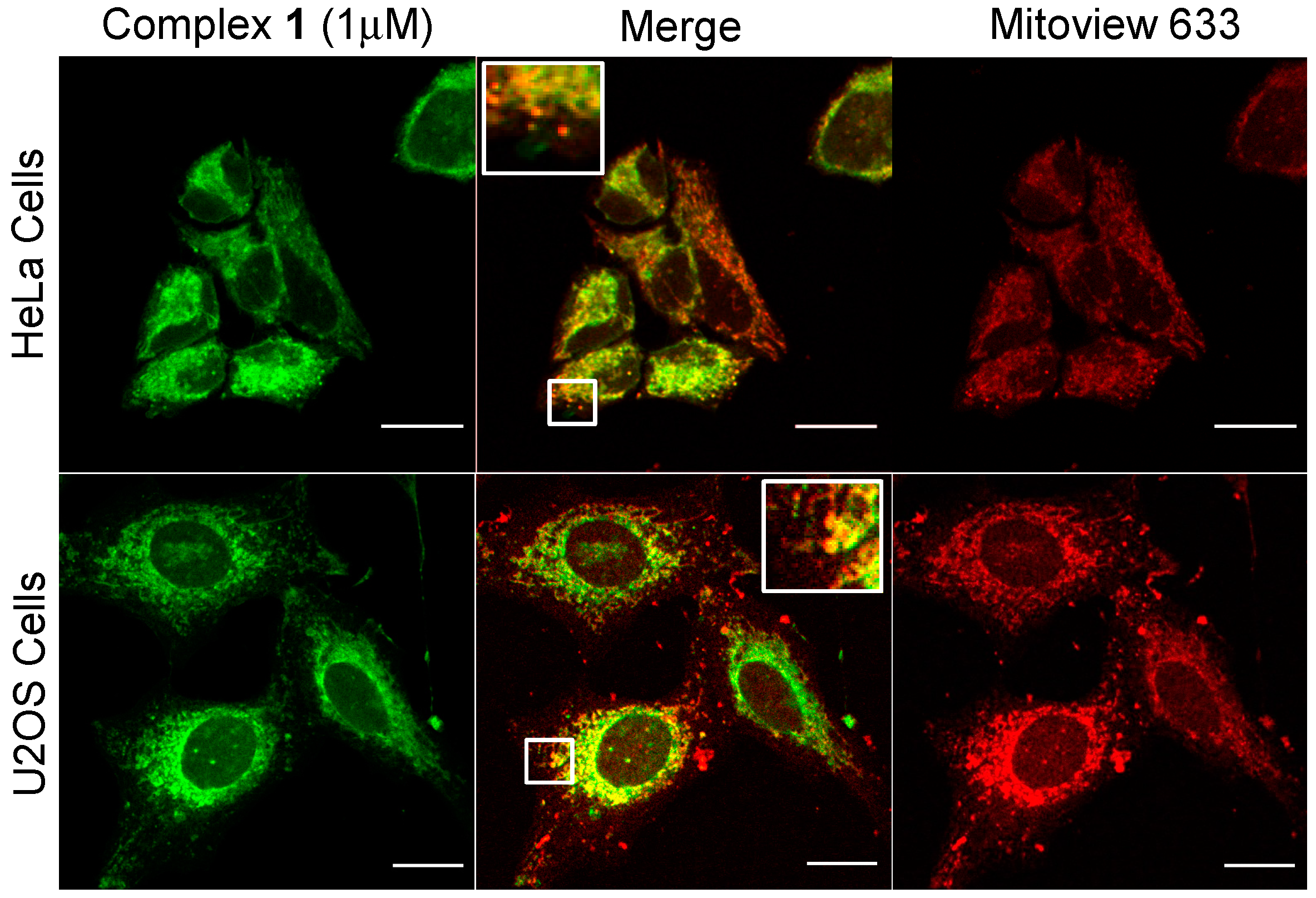
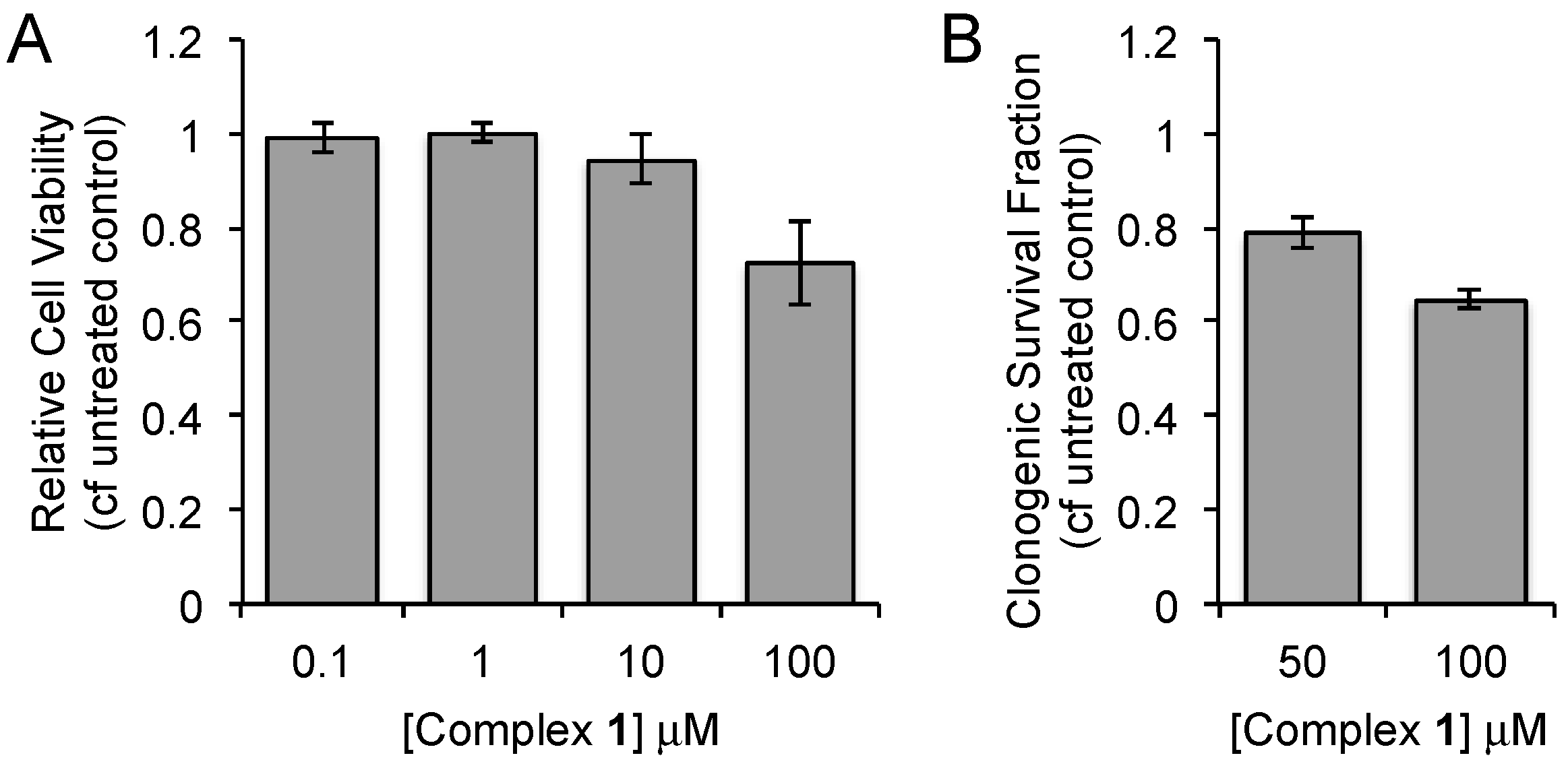
| Complex | λabs/nm 1 (ε/dm3·mol−1·cm−1) | λem/nm 1 | τ/ns 2,3 | φem/% 2,4 | λem/nm 5 |
|---|---|---|---|---|---|
| 1 | 526 (3025), 434 (5700), 382 (19,500), 337 (13,500), 297 (68,500), 288 (49,000) | 595 6 | 937 ± 12 | 9.3 | 564, 606 6 |
| 1Cl | 534 (3315), 438 (5800), 390 (24,750), 345 (17,350), 297 (90,800), 287 (62,500) | 599 6 (589) 6,8 | 884 ± 6 (273 ± 3) 8 | 9.7 (5.4) 8 | - |
| 2 | 669 (5070), 645 (4600), 491 (1930), 406 (7520), 314 (54,300), 286 (48,000) | 738 7 | 339 ± 4 | 3.2 | 718, 795 7 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omar, S.A.E.; Scattergood, P.A.; McKenzie, L.K.; Bryant, H.E.; Weinstein, J.A.; Elliott, P.I.P. Towards Water Soluble Mitochondria-Targeting Theranostic Osmium(II) Triazole-Based Complexes. Molecules 2016, 21, 1382. https://doi.org/10.3390/molecules21101382
Omar SAE, Scattergood PA, McKenzie LK, Bryant HE, Weinstein JA, Elliott PIP. Towards Water Soluble Mitochondria-Targeting Theranostic Osmium(II) Triazole-Based Complexes. Molecules. 2016; 21(10):1382. https://doi.org/10.3390/molecules21101382
Chicago/Turabian StyleOmar, Salem A. E., Paul A. Scattergood, Luke K. McKenzie, Helen E. Bryant, Julia A. Weinstein, and Paul I. P. Elliott. 2016. "Towards Water Soluble Mitochondria-Targeting Theranostic Osmium(II) Triazole-Based Complexes" Molecules 21, no. 10: 1382. https://doi.org/10.3390/molecules21101382
APA StyleOmar, S. A. E., Scattergood, P. A., McKenzie, L. K., Bryant, H. E., Weinstein, J. A., & Elliott, P. I. P. (2016). Towards Water Soluble Mitochondria-Targeting Theranostic Osmium(II) Triazole-Based Complexes. Molecules, 21(10), 1382. https://doi.org/10.3390/molecules21101382







