Analysis of Flavonoids in Rhamnus davurica and Its Antiproliferative Activities
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of Chromatographic Conditions and HPLC Fingerprint Profile of R. davurica (Dongbei)
2.2. Structural Identifications of Flavonoids Using HPLC-ESI-MS/MS Analysis
2.2.1. Identification of Flavone Aglycones
2.2.2. Identification of Flavonoid Glycosides
2.2.3. Identification of Anthraquinones and Their O-glycosides
2.2.4. Others
2.3. Quantification of Flavonoids in R. davurica
2.4. Antiproliferation Assays on R. davurica
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Materials
3.3. Sample Preparation
3.4. Antiproliferation Assays in Vitro
3.4.1. Cell Culture
3.4.2. Sulforhodamine B Antiproliferation Assays
3.5. HPLC-ESI-MS/MS Analysis of R. davurica
3.5.1. HPLC Fingerprint Analysis
3.5.2. ESI-MS/MS Analysis
3.6. Quantitative Analysis of Flavonoids Compounds
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, S.S.; Wu, J.; Chen, L.G.; Du, H.; Xu, Y.J.; Wang, L.J.; Zhang, H.J.; Zheng, X.C.; Wang, L.S. Biogenesis of C-glycosyl flavones and profiling of flavonoid glycosides in lotus (Nelumbo nucifera). PLoS ONE 2014, 9, e108860. [Google Scholar] [CrossRef] [PubMed]
- Ablajan, K.; Abliz, Z.; Shang, X.Y.; He, J.M.; Zhang, R.P.; Shi, J.G. Structural characterization of flavonol 3,7-di-O-glycosides and determination of the glycosylation position by using negative ion electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 2006, 41, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kumar, S.; Bajpai, V.; Reddy, T.J.; Rameshkumar, K.B.; Kumar, B. Structural characterization of flavonoid C-and O-glycosides in an extract of Adhatoda vasica leaves by liquid chromatography with quadrupole time-of-flight mass spectrometry. Rapid Commun. Mass Spectrom. 2015, 29, 1095–1106. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Z.; Wu, W.; Jiao, L.L.; Yang, P.F.; Guo, M.Q. Analysis of flavonoids in lotus (Nelumbo nucifera) leaves and their antioxidant activity using macroporous resin chromatography coupled with LC-MS/MS and antioxidant biochemical assays. Molecules 2015, 20, 10553–10565. [Google Scholar] [CrossRef] [PubMed]
- Jin, Q.H.; Lee, C.; Lee, J.W.; Yeon, E.T.; Lee, D.H.; Han, S.B.; Hong, J.T.; Kim, Y.S.; Lee, M.K.; Hwang, B.Y. 2-phenoxychromones and prenylflavonoids from Epimedium koreanum and their inhibitory effects on LPS-induced nitric oxide and interleukin-1β production. J. Nat. Prod. 2014, 77, 1724–1728. [Google Scholar] [CrossRef] [PubMed]
- Bhouri, W.; Sghaier, M.B.; Kilani, S.; Bouhlel, I.; Dijoux-Franca, M.G.; Ghedira, K.; Ghedira, L.C. Evaluation of antioxidant and antigenotoxic activity of two flavonoids from Rhamnus alaternus L. (Rhamnaceae): Kaempferol 3-O-β-isorhamninoside and rhamnocitrin 3-O-β-isorhamninoside. Food Chem. Toxicol. 2011, 49, 1167–1173. [Google Scholar] [CrossRef] [PubMed]
- Malla, S.; Koffas, M.A.G.; Kazlauskas, R.J.; Kim, B.G. Production of 7-O-methyl aromadendrin, a medicinally valuable flavonoid, in Escherichia coli. Appl. Environ. Microbiol. 2012, 78, 684–694. [Google Scholar] [CrossRef] [PubMed]
- Marzouk, M.S.; EI-Toumy, S.A.A.; Merfort, I.; Nawwar, M.A.M. Polyphenolic metabolites of Rhamnus disperma. Phytochemistry 1999, 52, 943–946. [Google Scholar] [CrossRef]
- Payá, M.; Mánez, S.; Villar, A. Flavonoid constituents of Rhamnus lycioides L. Z. Naturforsch. 1986, 41, 976–978. [Google Scholar]
- Ammar, R.B.; Bhouri, W.; Sghaier, M.B.; Boubaker, J.; Skandrani, I.; Neffati, A.; Bouhlel, I.; Kilani, S.; Mariotte, A.M.; Chekir-Ghedira, L.; et al. Antioxidant and free radical-scavenging properties of three flavonoids isolated from the leaves of Rhamnus alaternus L. (Rhamnaceae): A structure-activity relationship study. Food Chem. 2009, 116, 258–264. [Google Scholar] [CrossRef]
- Cuoco, G.; Mathe, C.; Vieillescazes, C. Liquid chromatographic analysis of flavonol compounds in green fruits of three Rhamnus species used in Stil de grain. Microchem. J. 2014, 115, 130–137. [Google Scholar] [CrossRef]
- Sakushima, A.; Coskun, M.; Hisada, A.; Nishibe, S. Flavonoids from Rhamnus pallasii. Phytochemistry 1983, 22, 1677–1678. [Google Scholar] [CrossRef]
- Wang, J.; Kasai, R.; Sakimori, M.; Miyakoshi, M.; Tanaka, O.; Jia, M.R.; Ling, Y.K. Flavonol glycosides from the fruits of Rhamnus leptophylla. Phytochemistry 1988, 27, 3995–3996. [Google Scholar] [CrossRef]
- Mai, L.P.; Guéritte, F.; Dumontet, V.; Tri, M.V.; Hill, B.; Thoison, O.; Guénard, D.; Sévenet, T. Cytotoxicity of rhamnosylanthraquinones and rhamnosylanthrones from Rhamnus nepalensis. J. Nat. Prod. 2001, 64, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Sharp, H.; Latif, Z.; Bartholomew, B.; Thomas, D.; Thomas, B.; Sarker, S.D.; Nash, R.J. Emodin and syringaldehyde from Rhamnus pubescens (Rhamnaceae). Biochem. Syst. Ecol. 2001, 29, 113–115. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, A.R.; Kim, H.S.; Kim, H.W.; Park, Y.H.; You, J.S.; Park, Y.M.; Her, E.; Kim, H.S.; Kim, Y.M.; et al. Rhamnus davurica leaf extract inhibits fyn activation by antigen in mast cells for anti-allergic activity. BMC Complem. Altern. Med. 2015, 15, 80. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Ahn, H.; Kim, J.Y.; Kim, Y.K. Inhibitory activity of plant extracts on nitric oxide synthesis in LPS-activated macrophages. Phytother. Res. 2003, 17, 485–489. [Google Scholar] [CrossRef] [PubMed]
- Banzaraksheev, V.G.; Azhunova, T.A. The pharmacological properties of the complex plant remedy of traditional medicine. Int. J. Biomed. 2013, 3, 47–49. [Google Scholar]
- Lee, S.E.; Lee, J.H.; Lee, D.Y.; Kim, G.S.; Choi, J.H.; Ahn, Y.S. Effect of plant extracts on rat basophilic leukemia (RBL-2H3) cells sensitized with IgE. Planta Med. 2015, 81. [Google Scholar] [CrossRef]
- Wu, T.; Zang, X.X.; He, M.Y.; Pan, S.Y.; Xu, X.Y. Structure-activity relationship of flavonoids on their anti-Escherichia coli activity and inhibition of DNA gyrase. J. Agric. Food Chem. 2013, 61, 8185–8190. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, F.; Darbon, J.M. Effects of structurally related flavonoids on cell cycle progression of human melanoma cells: Regulation of cyclin-dependent kinases CDK2 and CDK1. Biochem. Pharmacol. 2001, 61, 1205–1215. [Google Scholar] [CrossRef]
- Truchado, P.; Vit, P.; Heard, T.A.; Tomas-Barberan, F.A.; Ferreres, F. Determination of interglycosidic linkages in O-glycosyl flavones by high-performance liquid chromatography/photodiode-array detection coupled to electrospray ionization ion trap mass spectrometry. Its application to Tetragonula carbonaria honey from australia. Rapid Commun. Mass Spectrom. 2015, 29, 948–954. [Google Scholar] [PubMed]
- Lai, J.P.; Lim, Y.H.; Su, J.; Shen, H.M.; Ong, C.N. Identification and characterization of major flavonoids and caffeoylquinic acids in three Compositae plants by LC/DAD-APCI/MS. J. Chromatogr. B 2007, 848, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Duan, K.F.; Yuan, Z.F.; Guo, W.; Meng, Y.; Cui, Y.; Kong, D.Z.; Zhang, L.T.; Wang, N. LC-MS/MS determination and pharmacokinetic study of five flavone components after solvent extraction/acid hydrolysis in rat plasma after oral administration of Verbena officinalis L. Extract. J. Ethnopharmacol. 2011, 135, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, H.K.; Willson, K.; Breidinger, S.; Rizk, M.L.; Wenning, L.; Woolf, E.J. Ultrasensitive liquid chromatography-tandem mass spectrometric methodologies for quantification of five HIV-1 integrase inhibitors in plasma for a microdose clinical trial. Anal. Chem. 2012, 84, 8614–8621. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.Z.; Dong, X.; Guo, M.Q. Phenolic profiling of Duchesnea indica combining macroporous resin chromatography (MRC) with HPLC-ESI-MS/MS and ESI-IT-MS. Molecules 2015, 20, 22463–22475. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.D.; Lu, C.J.; Zhao, R.Z. Qualitative and quantitative analysis of Rhizoma smilacis glabrae by ultra high performance liquid chromatography coupled with LTQ orbitrapXL hybrid mass spectrometry. Molecules 2014, 19, 10427–10439. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. Dietary assumption of plant polyphenols and prevention of allergy. Curr. Pharm. Des. 2014, 20, 811–839. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.S.; Galal, A.M.; Ross, S.A.; Ferreira, D.; ElSohly, M.A.; Ibrahime, A.R.S.; Mossa, J.S.; El-Feraly, F.S. A weakly antimalarial biflavanone from Rhus retinorrhoea. Phytochemistry 2001, 58, 599–602. [Google Scholar] [CrossRef]
- Chukwujekwu, J.C.; Kock, C.A.D.; Smith, P.J.; Heerden, F.R.V.; Staden, J.V. Antiplasmodial and antibacterial activity of compounds isolated from Ormocarpum trichocarpum. Planta Med. 2012, 78, 1857–1860. [Google Scholar] [CrossRef] [PubMed]
- Nindi, M.M.; Kgarebe, B.V.; Wolfender, L.J.; Abeaz, B.M. Electrospray liquid chromatography-mass spectrometry of the leaf extract of Rhamnus prinoides. Phytochem. Anal. 1999, 10, 69–75. [Google Scholar] [CrossRef]
- Abad-Garcia, B.; Berrueta, L.A.; Garmon-Lobato, S.; Gallo, B.; Vicente, F. A general analytical strategy for the characterization of phenolic compounds in fruit juices by high-performance liquid chromatography with diode array detection coupled to electrospray ionization and triple quadrupole mass spectrometry. J. Chromatogr. A 2009, 1216, 5398–5415. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Silva, B.M.; Andrade, P.B.; Seabra, R.M.; Ferreira, M.A. Approach to the study of C-glycosyl flavones by ion trap HPLC-PAD-ESI/MS/MS: Application to seeds of quince (Cydonia oblonga). Phytochem. Anal. 2003, 14, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.H.; Zhang, J.; Huang, Z.H.; Zhu, D.Y.; Xu, W. Profiling of phenolic constituents in Polygonum multiflorum thunb. By combination of ultra-high-pressure liquid chromatography with linear ion trap-orbitrap mass spectrometry. J. Chromatogr. A 2013, 1292, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, T.; Wang, X.D.; Dos Santos, J.S.; Fysikopoulos, A.; Tadrist, S.; Canlet, C.; Artigot, M.P.; Loiseau, N.; Oswald, I.P.; Puel, O. Trypacidin, a spore-borne toxin from Aspergillus fumigatus, is cytotoxic to lung cells. PLoS ONE 2012, 7, e29906. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.Y.; Chen, X.; Hu, S.; Tian, J.; Bai, X.H. Simultaneous preconcentration and analysis of anthraquinones based on ultrasound emulsification ionic liquid microextraction. J. Chromatogr. Sci. 2014, 52, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.Q.; Zhao, G.; Jiang, X.R.; Ding, Y. New ring construction strategy in taxane synthesis stereocontrolled synthesis of taxane CB-ring system. J. Chem. Soc. 1999, 1, 3531–3536. [Google Scholar] [CrossRef]
- Tao, J.H.; Zhao, M.; Wang, D.G.; Yang, C.; Chen, G.T.; Zhao, X.; Pu, X.L.; Jiang, S. UPLC-Q-TOF/MS-based screening and identification of two major bioactive components and their metabolites in normal and CKD rat plasma, urine and feces after oral administration of Rehmannia glutinosa libosch extract. J. Chromatogr. B 2015, 1001, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, A.R.; Sharma, S.; Mandal, S.; Goswami, A.; Mukhopadhyay, S.; Majumder, H.K. Luteolin, an emerging anti-cancer flavonoid, poisons eukaryotic DNA topoisomerase I. Biochem. J. 2002, 365, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, R.K.; Ahmad, S.; Abas, F.; Ismail, I.S.; Rukayadi, Y.; Akhtar, M.T.; Shaari, K. Bioactive constituents of Zanthoxylum rhetsa bark and its cytotoxic potential against B16-F10 melanoma cancer and normal human dermal fibroblast (HDF) cell lines. Molecules 2016, 21, 652–664. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.L.; Mu, R.H.; Li, L.; Liang, F.; Yao, L.; Su, L. Intracellular doxorubicin delivery of a core cross-linked, redox-responsive polymeric micelles. Int. J. Pharm. 2016, 498, 195–204. [Google Scholar]
- Rodriguez-Chavez, J.L.; Coballase-Urrutia, E.; Sicilia-Argumedo, G.; Ramirez-Apan, T.; Delgado, G. Toxicological evaluation of the natural products and some semisynthetic derivatives of Heterotheca inuloides Cass (Asteraceae). J. Ethnopharmacol. 2015, 175, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds are not available from the authors.
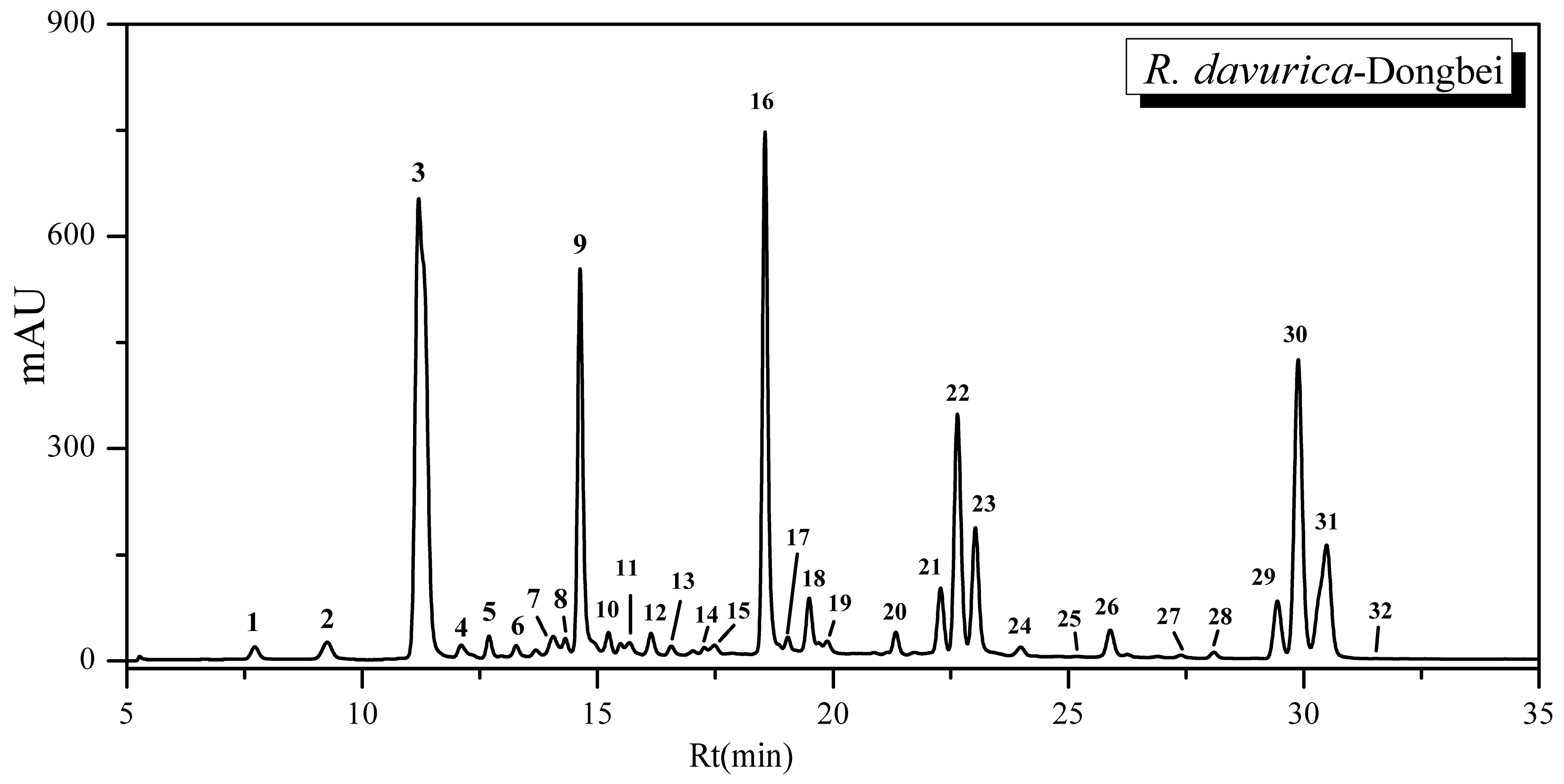
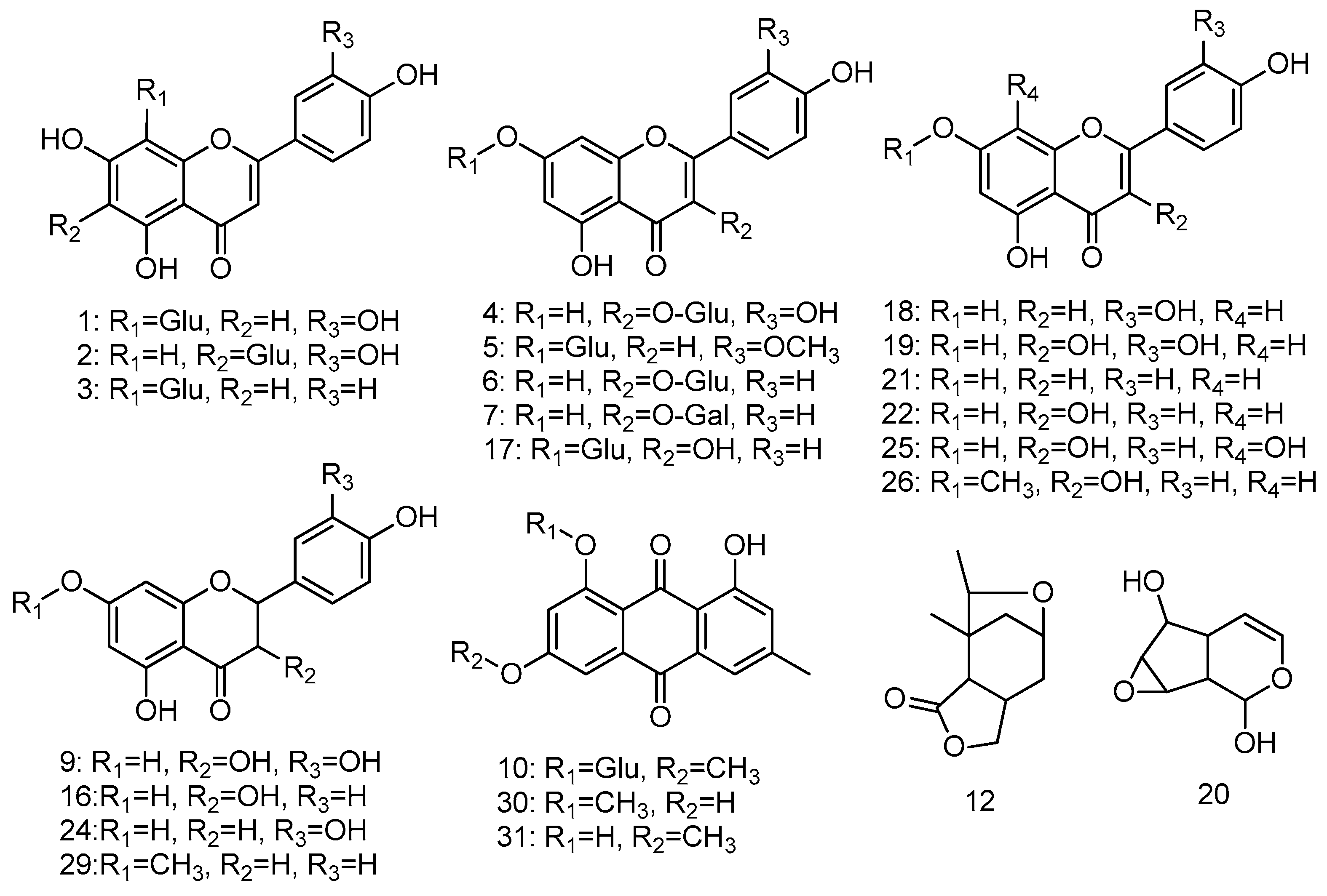
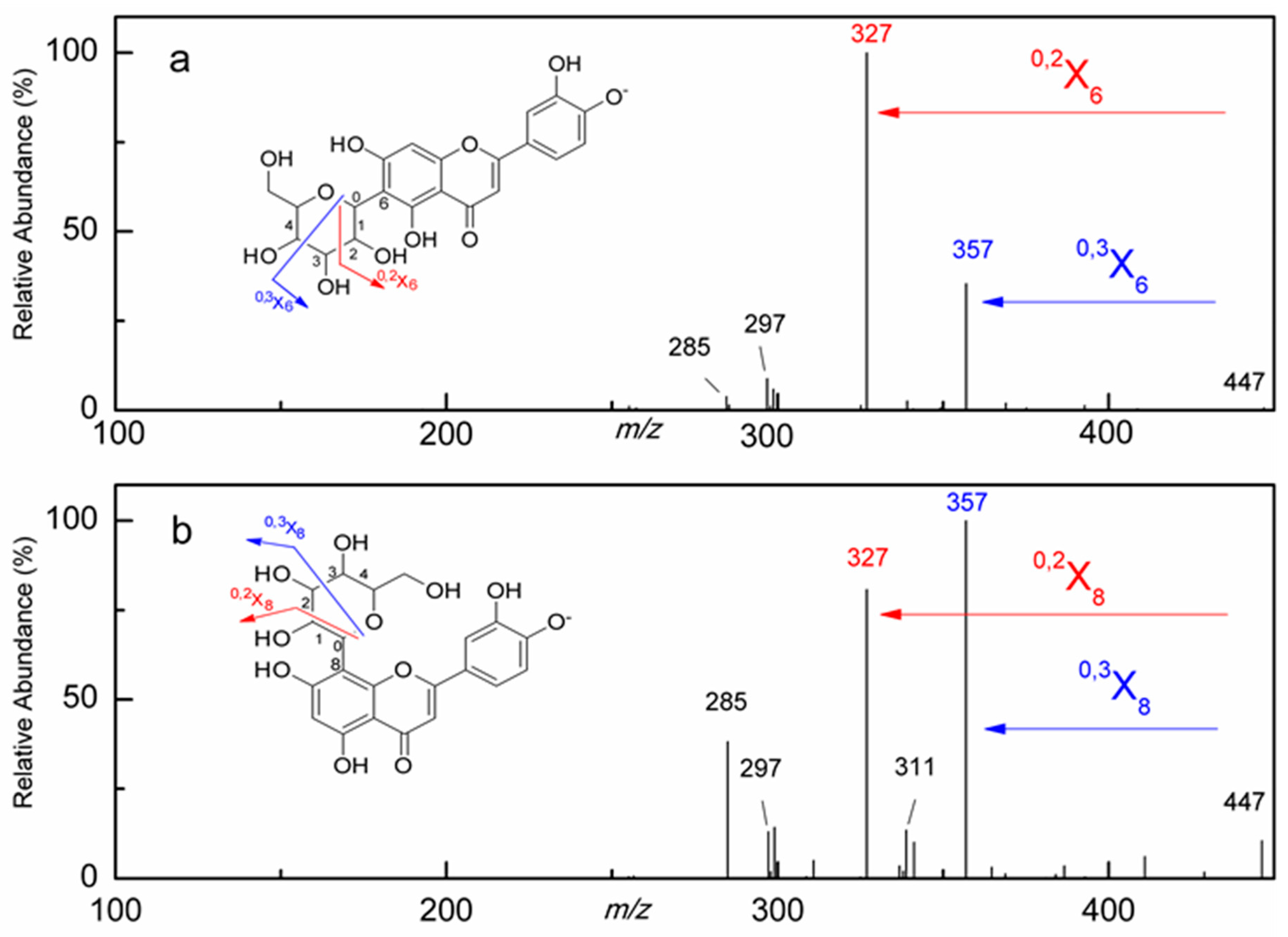

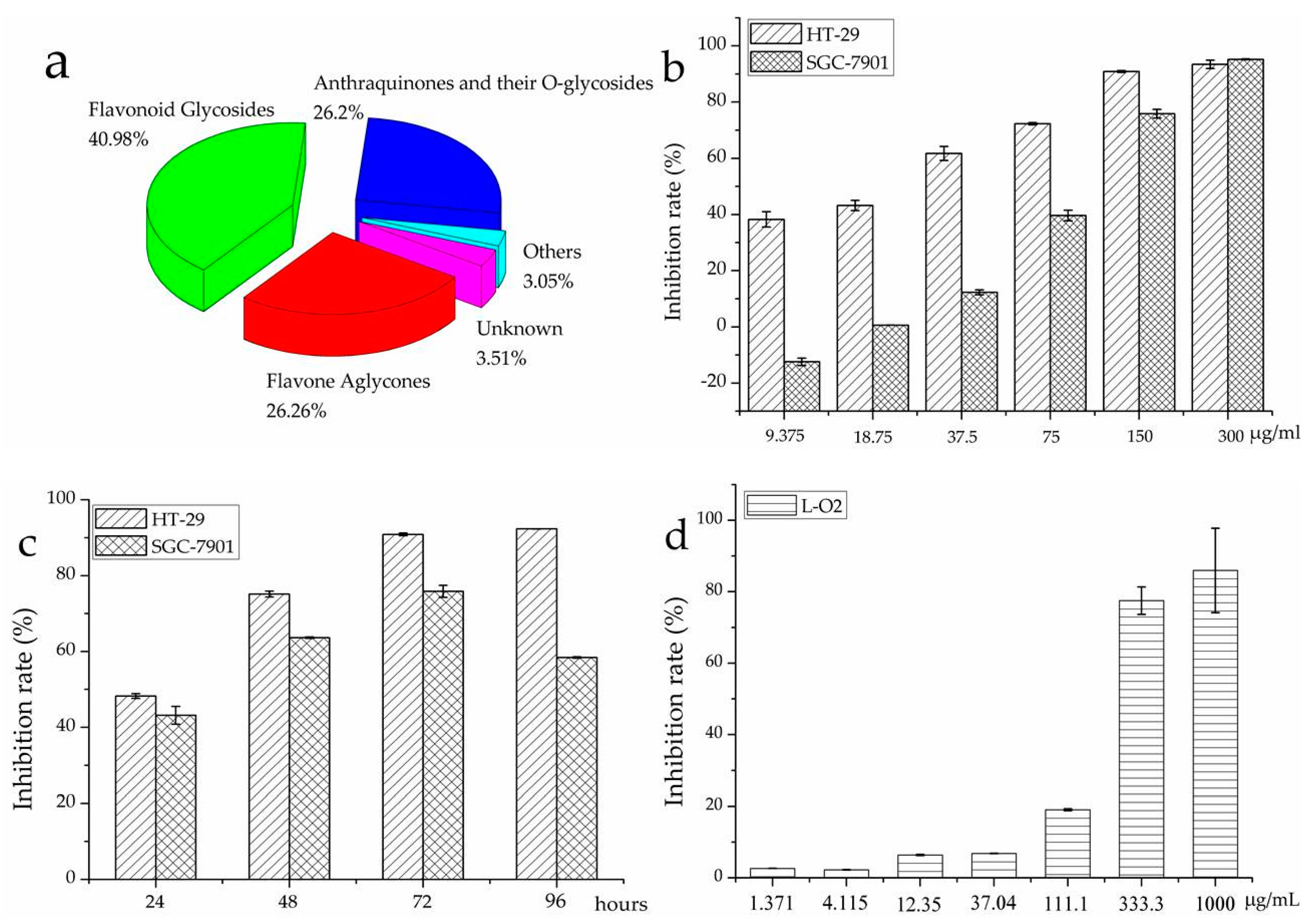
| Peak | Rt (min) | [M − H]− (m/z) | MS2 (m/z) | Identifications | Contents (mg/100 g) |
|---|---|---|---|---|---|
| 1 | 7.72 | 447 | 447, 357, 327, 311, 297, 285 | Orientin a | 1.43 |
| 2 | 9.26 | 447 | 447, 357, 327, 297, 285 | Isoorientin a | 2.87 |
| 3 | 11.20 | 431 | 431, 341, 311, 283, 269 | Vitexin a | 86.07 |
| 4 | 12.10 | 463 | 463, 301, 300 | Isoquercitrin a | 2.76 |
| 5 | 12.70 | 461 | 461, 299, 284 | Diosmetin 7-O-glucoside b | 2.66 |
| 6 | 13.27 | 447 | 447, 285, 284 | Astragaloside a | 2.19 |
| 7 | 14.06 | 447 | 447, 327, 301, 300, 285, 270 | Luteolin 5-O-glucoside b | 4.07 |
| 8 | 14.32 | 1017 | 1016, 903, 790, 677, 564, 451, 338, 225 | Unknown | 2.50 |
| 9 | 14.63 | 303 | 303, 285, 275, 241, 217, 199, 175, 151, 125 | Taxifolin b | 15.51 |
| 10 | 15.24 | 445 | 445, 283, 268 | Physcion 8-O-glucoside b | 3.40 |
| 11 | 15.68 | 463 | 463, 301, 300 | Quercetin 7-O-glucoside b | 3.08 |
| 12 | 16.14 | 195 | 195, 167, 152, 136, 108 | Iodolactone derivative b | 4.00 |
| 13 | 16.57 | 665 | 665, 470, 357, 338, 243 | Unknown | 2.49 |
| 14 | 17.27 | 553 | 553, 469, 425, 355, 243 | Unknown | 1.62 |
| 15 | 17.49 | 509 | 509, 449, 421, 359, 341, 315, 271, 239 | Unknown | 2.70 |
| 16 | 18.55 | 287 | 287, 269, 259, 243, 215, 201, 151, 125 | Aromadendrin b | 21.48 |
| 17 | 19.04 | 447 | 447, 285, 183, 165, 119, 93 | Kaempferol 7-O-glucoside b | 3.70 |
| 18 | 19.49 | 285 | 285, 267, 241, 217, 199, 175, 151, 133 | Luteolin a | 2.97 |
| 19 | 19.87 | 301 | 301, 273, 229, 179, 151, 121, 107 | Quercetin a | 1.17 |
| 20 | 21.33 | 169 | 169, 151, 125, 107, 83, 57 | Oxireno[4,5]cyclopenta[1,2-c]pyran b | 4.10 |
| 21 | 22.28 | 269 | 269, 241, 225, 201, 181, 159, 133 | Apigenin a | 3.47 |
| 22 | 22.64 | 285 | 285, 257, 241, 229, 213, 185, 151, 107, 93 | Kaempferol a | 11.77 |
| 23 | 23.02 | 301 | 301, 283, 245, 227, 151, 125 | Quercetin isomer | 7.29 |
| 24 | 23.99 | 271 | 271, 185, 151, 125, 119 | Naringenin b | 0.89 |
| 25 | 24.81 | 301 | 301, 283, 179, 165, 135, 109 | Quercetin isomer | 0.08 |
| 26 | 25.89 | 299 | 299, 284, 283, 256, 255, 240, 227 | Rhamnocitrin b | 1.62 |
| 27 | 26.90 | 325 | 325, 307, 289, 271, 263, 185, 169, 137, 125 | Naringenin derivative | 0.05 |
| 28 | 28.09 | 551 | 285, 179, 165, 119 | Sakuranetin dimer | 0.22 |
| 29 | 29.44 | 285 | 285, 270, 243, 165, 151, 119, 93 | Sakuranetin b | 2.95 |
| 30 | 29.88 | 283 | 285, 270, 243, 165, 151, 119, 93 | Questin b | 42.17 |
| 31 | 30.49 | 283 | 283, 268, 267, 239, 211 | Physcion b | 24.00 |
| 32 | 32.15 | 359 | 359, 285, 267, 241, 223 | Luteolin derivative | 0.27 |
| Compound | Liner Equation | R2 | LOD (μg/mL) | LOQ (μg/mL) | Linear Range (μg/mL) |
|---|---|---|---|---|---|
| Rutin | Y = 31.794x − 24.028 | 0.9992 | 0.30 | 1.0 | 1.0–333 |
| Vitexin | Y = 33.007x + 42.294 | 0.9991 | 0.30 | 1.0 | 1.0–333 |
| Kaempferol | Y = 78.286x + 79.191 | 0.9990 | 0.1 | 0.33 | 0.33–333 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.; Li, X.; Saleri, F.; Guo, M. Analysis of Flavonoids in Rhamnus davurica and Its Antiproliferative Activities. Molecules 2016, 21, 1275. https://doi.org/10.3390/molecules21101275
Chen G, Li X, Saleri F, Guo M. Analysis of Flavonoids in Rhamnus davurica and Its Antiproliferative Activities. Molecules. 2016; 21(10):1275. https://doi.org/10.3390/molecules21101275
Chicago/Turabian StyleChen, Guilin, Xun Li, Flora Saleri, and Mingquan Guo. 2016. "Analysis of Flavonoids in Rhamnus davurica and Its Antiproliferative Activities" Molecules 21, no. 10: 1275. https://doi.org/10.3390/molecules21101275
APA StyleChen, G., Li, X., Saleri, F., & Guo, M. (2016). Analysis of Flavonoids in Rhamnus davurica and Its Antiproliferative Activities. Molecules, 21(10), 1275. https://doi.org/10.3390/molecules21101275





