Evidence for the Formation of Benzacridine Derivatives in Alkaline-Treated Sunflower Meal and Model Solutions
Abstract
:1. Introduction
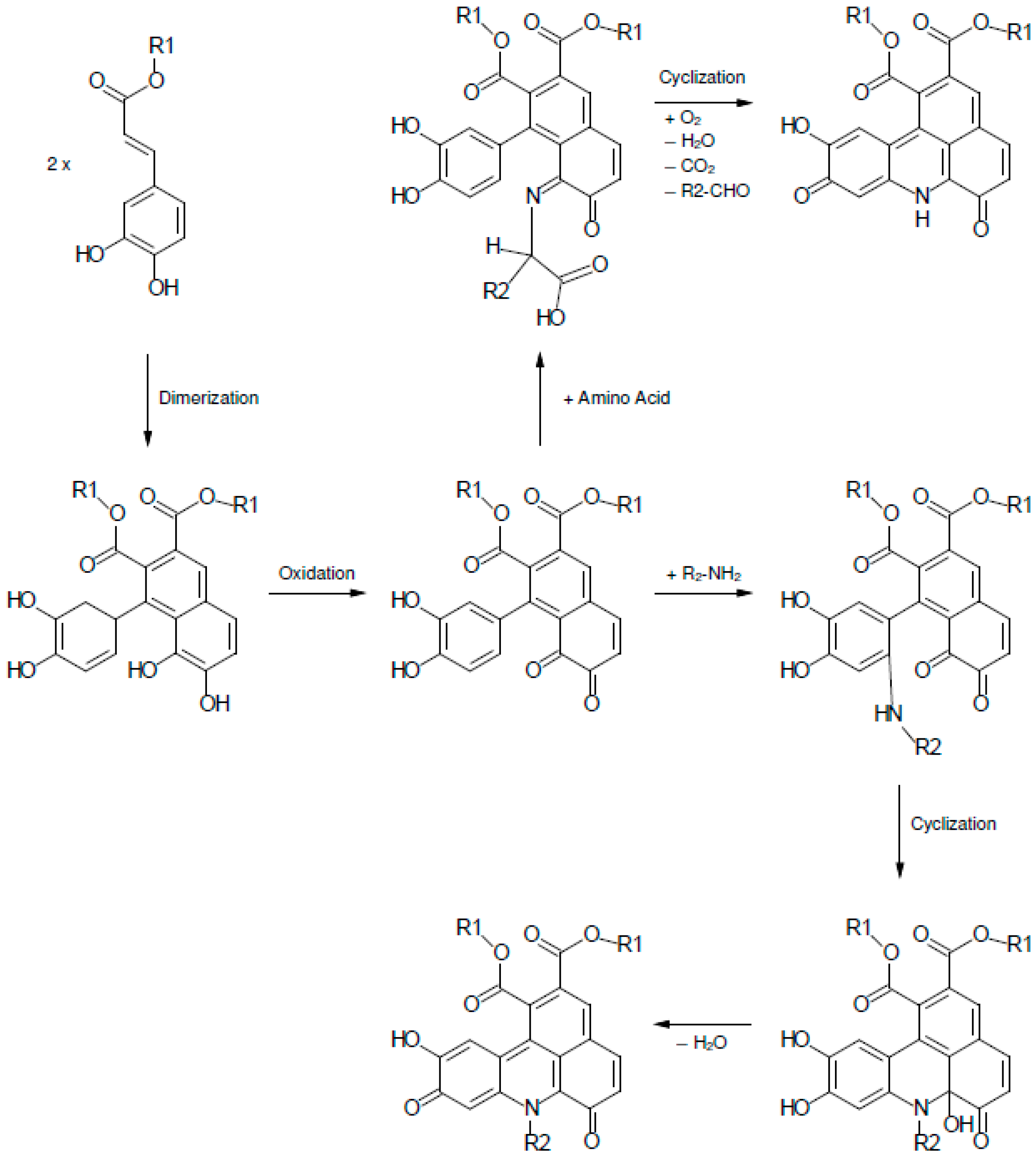
2. Results and Discussion
2.1. Color Development in SEM Extract and Model Solutions
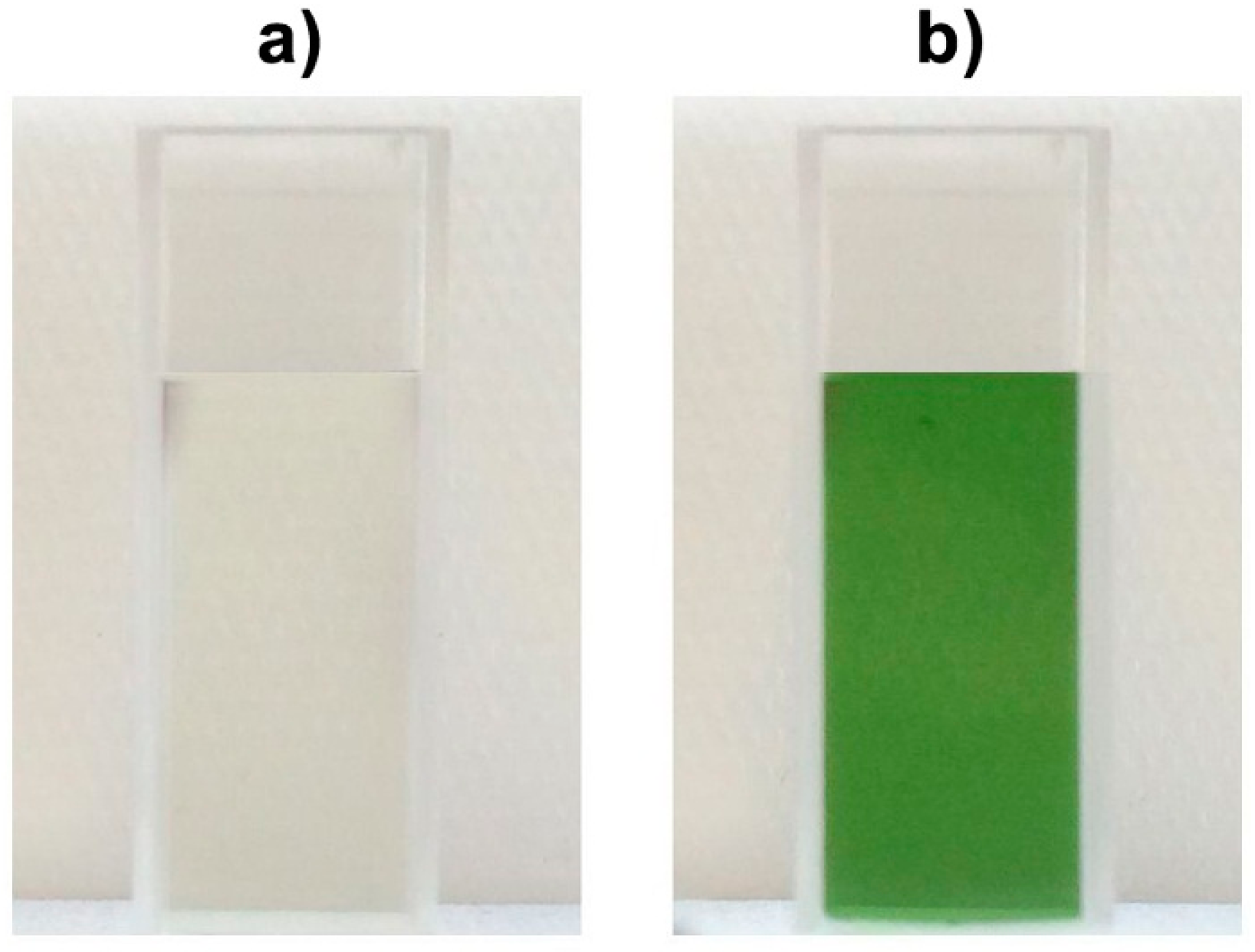
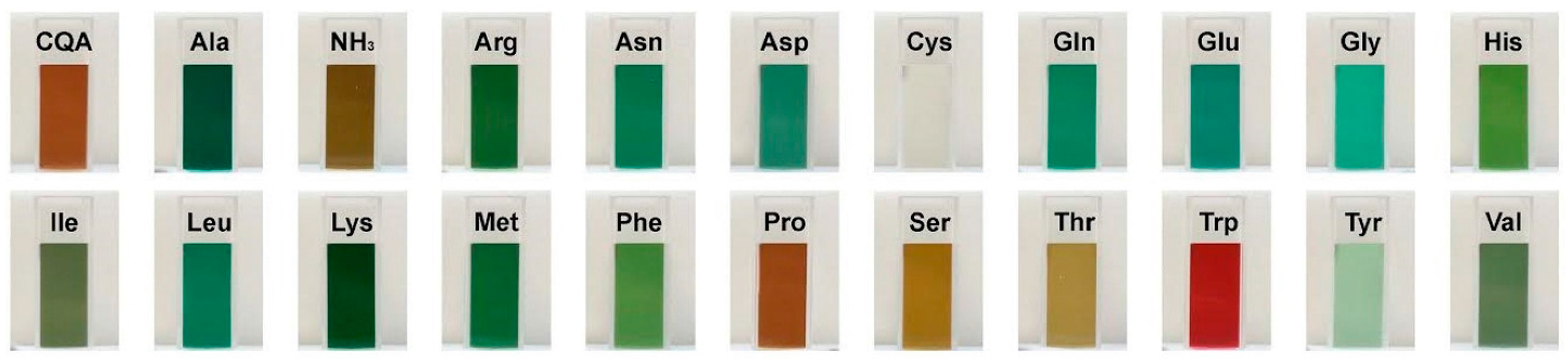
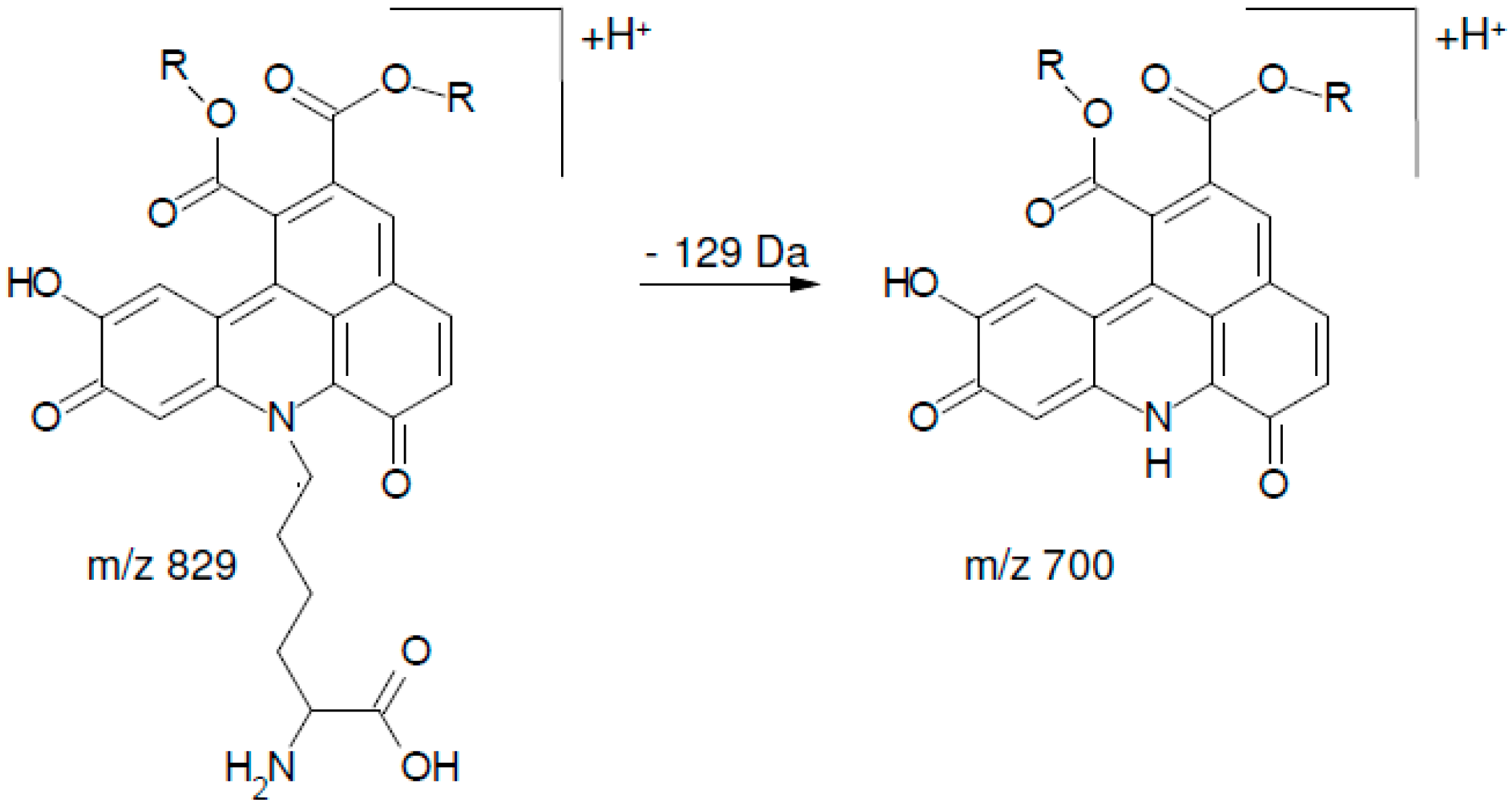
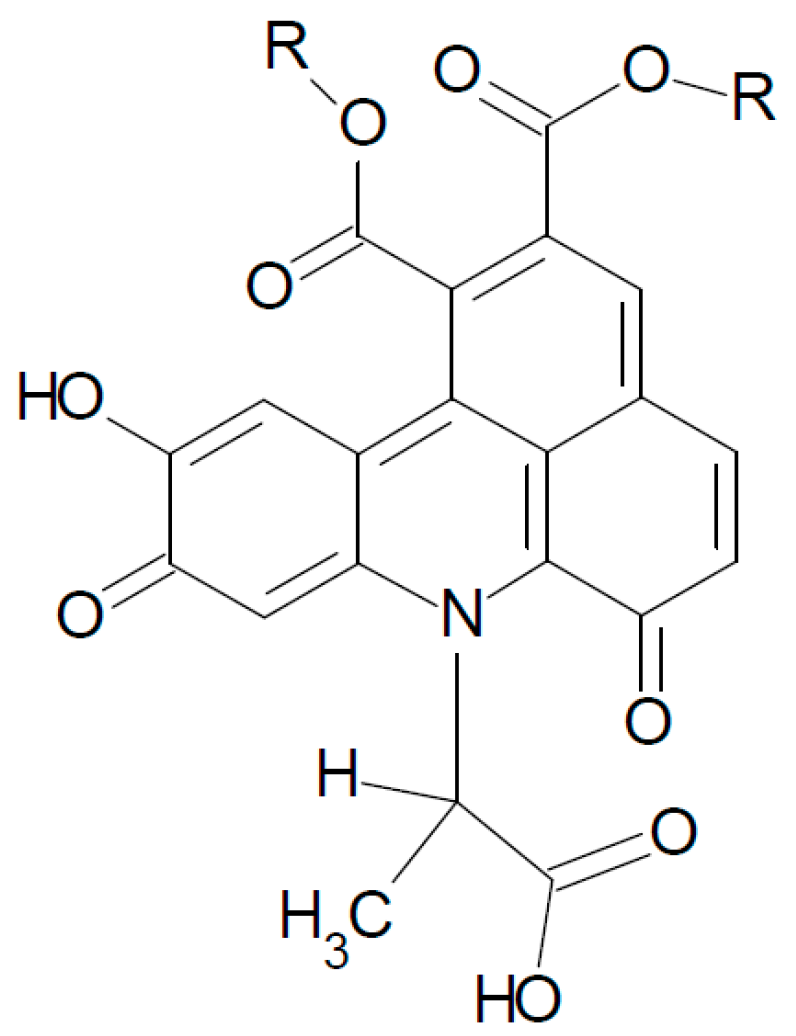
2.2. UHPLC-DAD-MS/MS Analysis
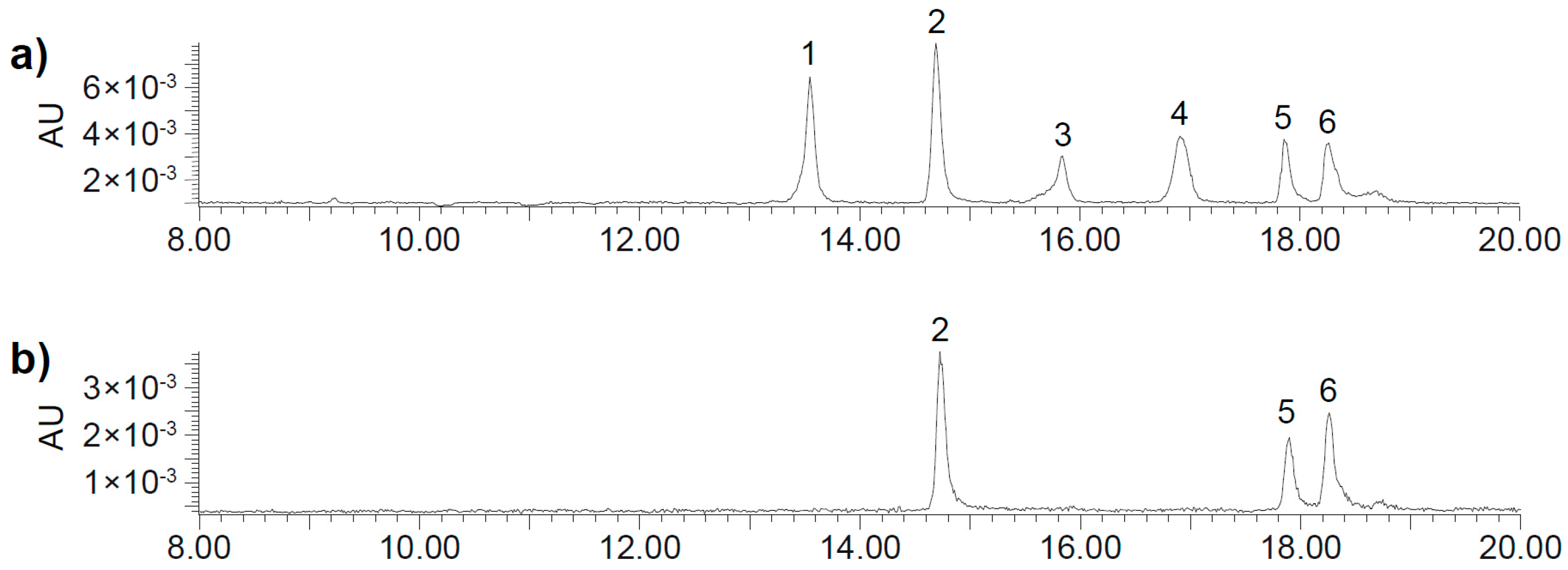
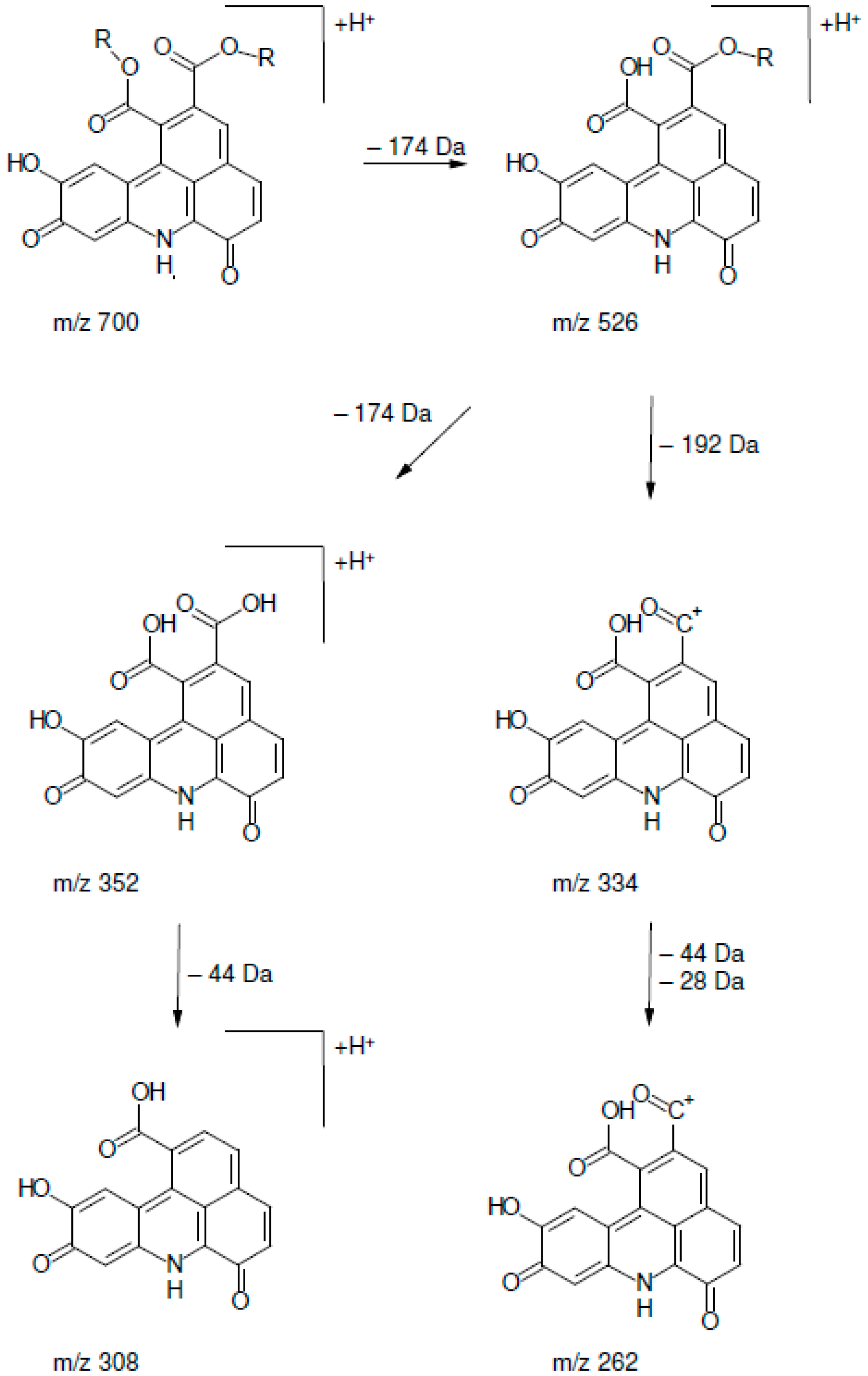
| Peak | Rt (min) | [M + H]+ | MS2 Fragments |
|---|---|---|---|
| 1 | 13.51 | 700.3 | 334 (100), 352 (54), 526 (11), 262 (7), 308 (7), 654 (5) 700 (3) |
| 2 | 14.65 | 700.3 | 334 (100), 352 (83), 526 (18), 262 (5), 308 (9), 654 (4), 700 (6) |
| 3 | 15.80 | 700.3 | 334 (100), 352 (50), 526 (16), 262 (6), 308 (4), 654 (1), 700 (4) |
| 4 | 16.87 | 700.3 | 334 (100), 352 (47), 526 (9), 262 (8), 308 (7), 654 (1), 700 (5) |
| 5 | 17.79 | 700.3 | 334 (100), 352 (48), 526 (7), 262 (5), 308 (5), 654 (4) 700 (1) |
| 6 | 18.20 | 700.3 | 334 (100), 352 (60), 526 (14), 262 (9), 308 (6), 654 (3), 700 (2) |
| Peak | Rt (min) | [M + H]+ | MS2 Fragments |
|---|---|---|---|
| 1 | 13.48 | 829 | 334 (100), 128 (83), 352 (20), 526 (29), 336 (27), 654 (7) 700 (5), 130 (19), 318 (4), 480 (6) |
| 2 | 14.59 | 829 | 334 (100), 128 (76), 352 (25), 526 (23), 336 (19), 654 (2) 700 (3), 130 (26) 318 (6), 480 (7) |
| 3 | 15.73 | 829 | 334 (100), 128 (96), 352 (27), 526 (40), 336 (52), 654 (3), 700 (22), 130 (9), 318 (11), 480 (5) |
| 4 | 16.74 | 829 | 334 (100), 128 (84), 352 (51), 526 (36), 336 (40), 654 (3), 700 (17), 130 (16), 318 (7), 480 (5) |
| 5 | 17.73 | 829 | 334 (100), 128 (80), 352 (48), 526 (30), 336 (18), 654 (8), 700 (21), 130 (14), 318 (10), 480 (3) |
| 6 | 18.11 | 829 | 334 (100), 128 (78), 352 (54), 526 (36), 336 (16), 654 (4), 700 (16), 130 (15), 318 (10), 480 (5) |
3. Experimental Section
3.1. Plant Material
3.2. Chemicals and Reagents
3.3. Sample Preparation—SEM Samples
3.3.1. Alkaline Treatment of SEM
3.3.2. Pressurized Liquid Extraction of SEM
3.4. Sample Preparation—Model Systems with 5-CQA and Amino Acids or Ammonia
3.5. UHPLC-DAD-MS/MS
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Weisz, G.M.; Schneider, L.; Schweiggert, U.; Kammerer, D.R.; Carle, R. Sustainable sunflower processing—I. Development of a process for the adsorptive decolorization of sunflower [Helianthus annuus L.] protein extracts. Innov. Food Sci. Emerg. Technol. 2010, 11, 733–741. [Google Scholar] [CrossRef]
- González-Pérez, S.; Merck, K.B.; Vereijken, J.M.; van Koningsfeld, G.A.; Gruppen, H.; Voragen, A.G.J. Isolation and characterization of undenatured chlorogenic acid free sunflower (Helianthus annuus) proteins. J. Agric. Food Chem. 2002, 50, 1713–1719. [Google Scholar] [CrossRef] [PubMed]
- Kammerer, D.R.; Saleh, Z.S.; Carle, R.; Stanley, R.A. Adsorptive recovery of phenolic compounds from apple juice. Eur. Food Res. Technol. 2007, 224, 605–613. [Google Scholar] [CrossRef]
- Pierpoint, W. o-Quinones formed in plant extracts: Their reactions with amino acids and peptides. Biochem. J. 1969, 112, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Rawel, H.M.; Meidtner, K.; Kroll, J. Binding of selected phenolic compounds to proteins. J. Agric. Food Chem. 2005, 53, 4228–4235. [Google Scholar] [CrossRef] [PubMed]
- Le Bourvellec, C.; Renard, C. Interactions between polyphenols and macromolecules: Quantification methods and mechanisms. Crit. Rev. Food Sci. Nutr. 2012, 52, 213–248. [Google Scholar] [CrossRef] [PubMed]
- Namiki, M.; Yabuta, G.; Koizumi, Y.; Yano, M. Development of free radical products during the greening reaction of caffeic acid esters (or chlorogenic acid) and a primary amino compound. Biosci. Biotechnol. Biochem. 2001, 65, 2131–2136. [Google Scholar] [CrossRef] [PubMed]
- Pedrosa, M.M.; Muzquiz, M.; Garcia-Vallejo, C.; Burbano, C.; Cuadrado, C.; Ayet, G.; Robredo, L.M. Determination of caffeic and chlorogenic acids and their derivatives in different sunflower seeds. J. Sci. Food Agric. 2000, 80, 459–464. [Google Scholar] [CrossRef]
- Yabuta, G.; Koizumi, Y.; Namiki, K.; Hida, M.; Namiki, M. Structure of green pigment formed by the reaction of caffeic acid esters (or chlorogenic acid) with a primary amino compound. Biosci. Biotechnol. Biochem. 2001, 65, 2121–2130. [Google Scholar] [CrossRef] [PubMed]
- Kroll, J.; Rawel, H.M.; Rohn, S. Reactions of plant phenolics with food proteins and enzymes under special consideration of covalent bonds. Food Sci. Technol. Res. 2003, 9, 205–218. [Google Scholar] [CrossRef]
- Prigent, S.V.E. Interactions of Phenolic Compounds with Globular Proteins and Their Effects on Food-Related Functional Properties. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2005. [Google Scholar]
- Prigent, S.V.E.; Voragen, A.G.J.; Li, F.; Visser, A.J.W.G.; van Koningsfeld, G.A.; Gruppen, H. Covalent interactions between amino acid side chains oxidation products of caffeoylquinic acid (chlorogenic acid). J. Sci. Food Agric. 2008, 88, 1748–1754. [Google Scholar] [CrossRef]
- García Cárnovas, F.G.; Sánchez, J.V.; Iborra Pastor, J.L.; Lozano Teruel, J.A. The role of pH in the melanin biosynthesis pathway. J. Biol. Chem. 1982, 15, 8738–8744. [Google Scholar]
- Clifford, M.N.; Johnston, K.L.; Knight, S.; Kuhnert, N. Hierarchical scheme for LC-MSn identification of chlorogenic acids. J. Agric. Food Chem. 2003, 51, 2900–2911. [Google Scholar] [CrossRef] [PubMed]
- Schilling, S.; Sigolotto, C.-I.; Carle, R.; Schieber, A. Characterization of covalent addition products of chlorogenic acid quinone with amino acid derivatives in model systems and apple juice by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2008, 22, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Homann, T.; Kreisel, J.; Khalil, M.; Puhlmann, R.; Kruse, H.-P.; Rawel, H. Characterization and modeling of the interactions between coffee storage proteins and phenolic Compounds. J. Agric. Food Chem. 2012, 60, 11601–11608. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Homann, T.; Khalil, M.; Kruse, H.-P.; Rawel, H. Milk whey protein modification by coffee-specific phenolics: Effect on structural and functional properties. J. Agric. Food Chem. 2013, 61, 6911–6920. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Limited sample amounts of the compounds in Section 3.1, Section 3.2, Section 3.3 and Section 3.4 are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bongartz, V.; Brandt, L.; Gehrmann, M.L.; Zimmermann, B.F.; Schulze-Kaysers, N.; Schieber, A. Evidence for the Formation of Benzacridine Derivatives in Alkaline-Treated Sunflower Meal and Model Solutions. Molecules 2016, 21, 91. https://doi.org/10.3390/molecules21010091
Bongartz V, Brandt L, Gehrmann ML, Zimmermann BF, Schulze-Kaysers N, Schieber A. Evidence for the Formation of Benzacridine Derivatives in Alkaline-Treated Sunflower Meal and Model Solutions. Molecules. 2016; 21(1):91. https://doi.org/10.3390/molecules21010091
Chicago/Turabian StyleBongartz, Verena, Lisa Brandt, Mai Linh Gehrmann, Benno F. Zimmermann, Nadine Schulze-Kaysers, and Andreas Schieber. 2016. "Evidence for the Formation of Benzacridine Derivatives in Alkaline-Treated Sunflower Meal and Model Solutions" Molecules 21, no. 1: 91. https://doi.org/10.3390/molecules21010091
APA StyleBongartz, V., Brandt, L., Gehrmann, M. L., Zimmermann, B. F., Schulze-Kaysers, N., & Schieber, A. (2016). Evidence for the Formation of Benzacridine Derivatives in Alkaline-Treated Sunflower Meal and Model Solutions. Molecules, 21(1), 91. https://doi.org/10.3390/molecules21010091






