Synthesis and Herbicidal Activity of 5-Heterocycloxy-3-methyl-1-substituted-1H-pyrazoles
Abstract
:1. Introduction
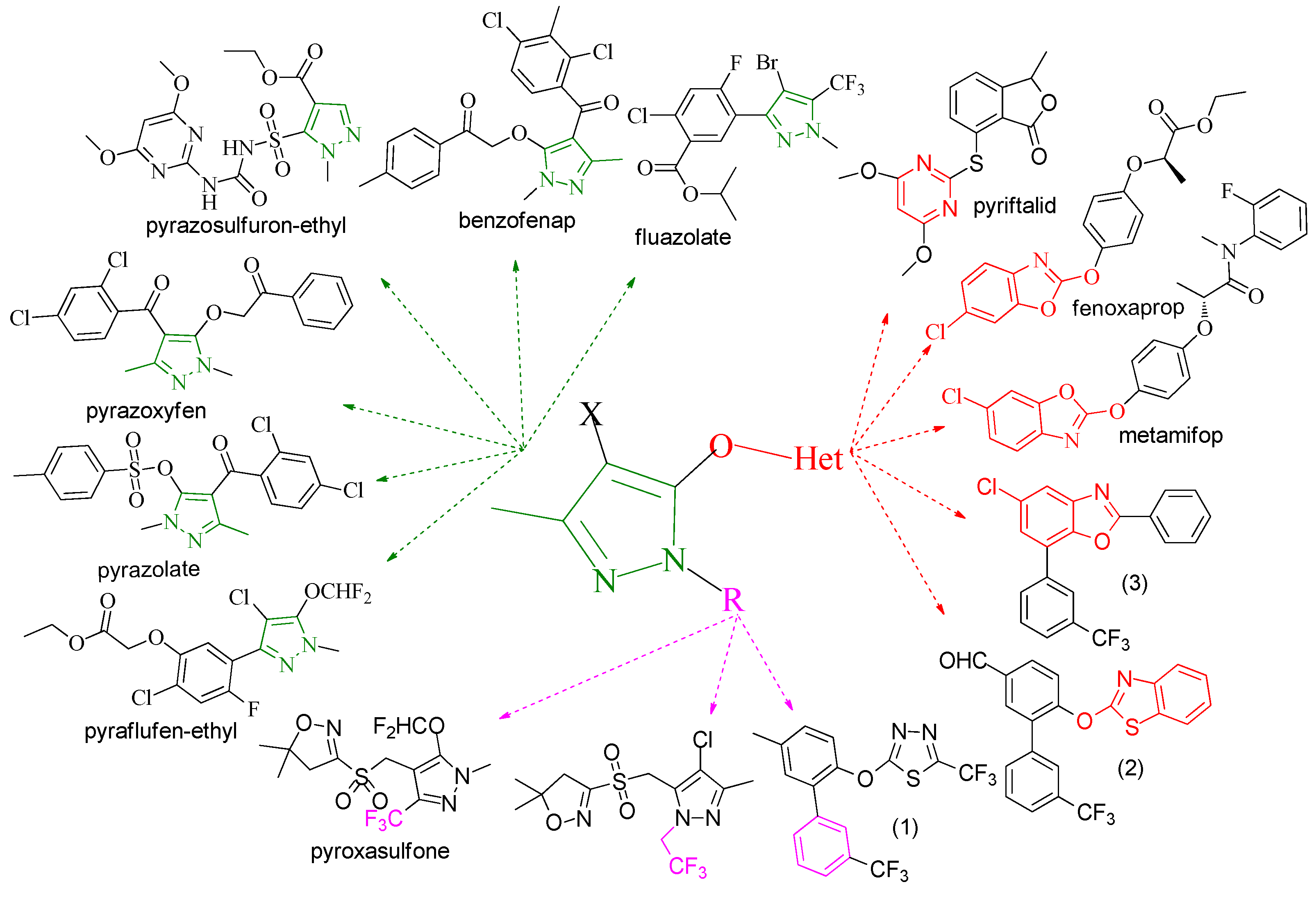
2. Results and Discussion
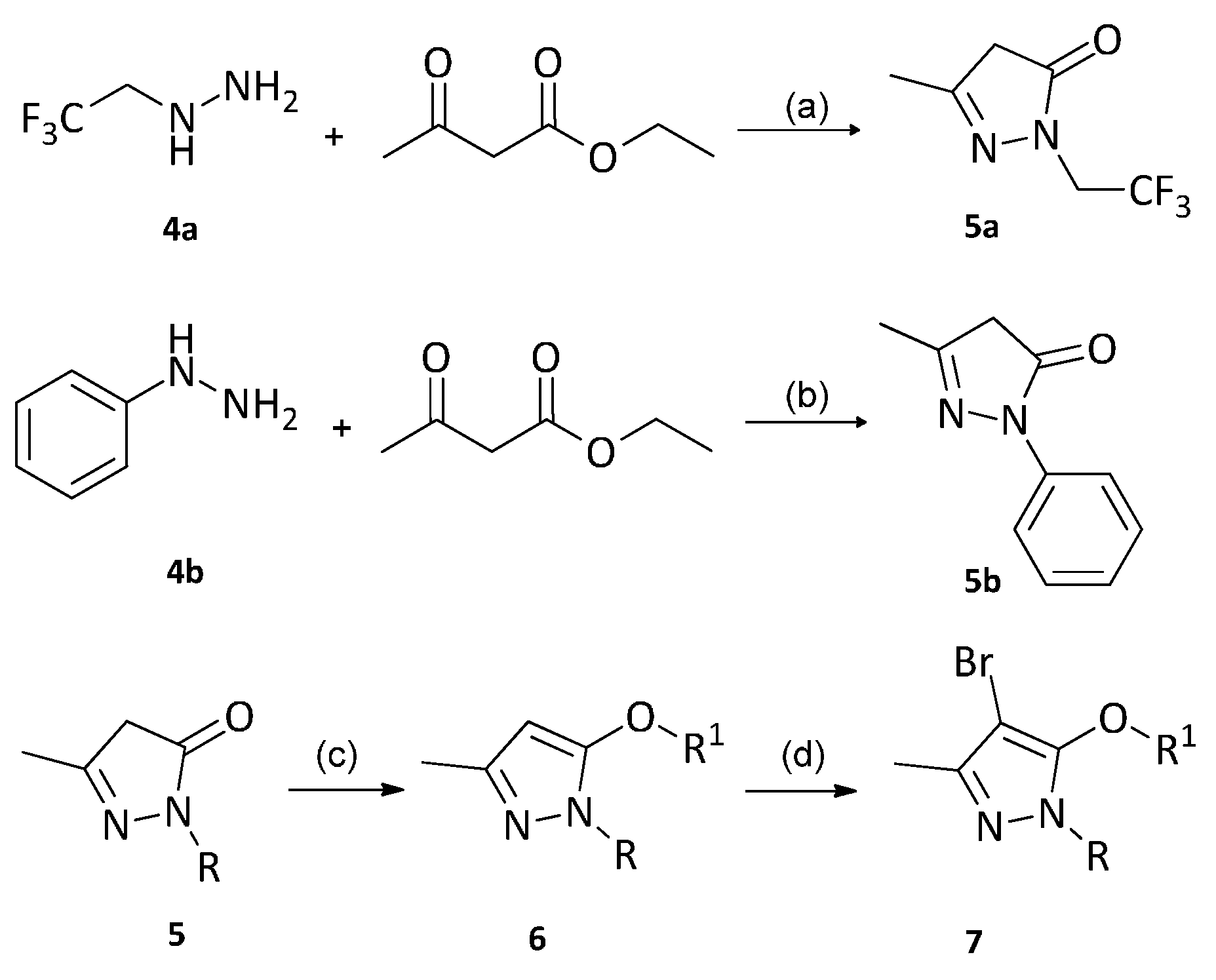
2.1. Synthesis
| Compd. | R | R1 | Compd. | R | R1 |
|---|---|---|---|---|---|
| 6a | CH2CF3 |  | 6h | C6H5 | 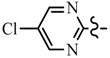 |
| 6b | CH2CF3 | 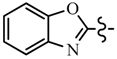 | 6i | C6H5 | 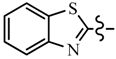 |
| 6c | CH2CF3 |  | 7a | CH2CF3 | 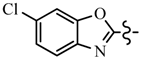 |
| 6d | CH2CF3 | 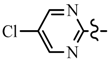 | 7b | CH2CF3 |  |
| 6e | C6H5 | 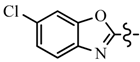 | 7c | CH2CF3 |  |
| 6f | C6H5 | 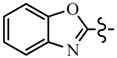 | 7d | CH2CF3 | 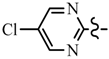 |
| 6g | C6H5 | 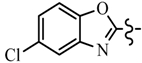 | 7e | C6H5 | 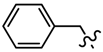 |
2.2. Growth Inhibition of Weed Roots and Shoots
| Compd. | Relative Inhibition (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B. campestris | A. retroflexus | P. oleracea | P. alopecuroides | E. crus-galli | D. sanguinalis | |||||||
| Root | Root | Root | Shoot | Shoot | Root | |||||||
| 10 mg/L | 100 mg/L | 10 mg/L | 100 mg/L | 10 mg/L | 100 mg/L | 10 mg/L | 100 mg/L | 10 mg/L | 100 mg/L | 10 mg/L | 100 mg/L | |
| 6a | 69 ± 2.1 | 78 ± 1.1 | 39 ± 1.1 | 58 ± 2.1 | 3 ± 1.3 | 48 ± 1.6 | 47 ± 1.0 | 59 ± 0.5 | 47 ± 1.1 | 57 ± 2.3 | 27 ± 1.5 | 58 ± 1.1 |
| 6b | 80 ± 1.2 | 81 ± 1.1 | 53 ± 1.7 | 60 ± 2.4 | 29 ± 1.2 | 50 ± 0.8 | 56 ± 2.2 | 59 ± 1.6 | 60 ± 0.6 | 70 ± 1.3 | 31 ± 2.9 | 75 ± 0.7 |
| 6c | 37 ± 1.5 | 46 ± 2.0 | 26 ± 3.3 | 58 ± 1.5 | 30 ± 1.7 | 74 ± 2.1 | 38 ± 2.7 | 55 ± 1.0 | 26 ± 1.4 | 61 ± 0.6 | 9 ± 2.4 | 21 ± 0.8 |
| 6d | 49 ± 2.4 | W | 41 ± 0.3 | 100 | 42 ± 1.8 | 100 | 34 ± 1.6 | W | 30 ± 2.0 | W | W | W |
| 6e | 21 ± 0.5 | 22 ± 1.6 | 0 ± 1.2 | 27 ± 2.5 | 4 ± 2.2 | 32 ± 1.9 | 22 ± 1.2 | 32 ± 1.2 | 33 ± 2.4 | 36 ± 2.7 | 2 ± 1.4 | 28 ± 0.7 |
| 6f | 42 ± 1.3 | 54 ± 2.1 | 0 ± 1.3 | 7 ± 1.7 | 3 ± 2.0 | 23 ± 2.0 | 30 ± 1.9 | 58 ± 1.9 | 30 ± 2.4 | 52 ± 0.5 | 36 ± 1.3 | 46 ± 1.3 |
| 6g | 16 ± 1.0 | 36 ± 1.0 | 3 ± 1.8 | 13 ± 0.3 | 11 ± 0.5 | 53 ± 0.7 | 21 ± 1.4 | 34 ± 1.1 | 12 ± 1.6 | 24 ± 1.5 | 2 ± 4.4 | 7 ± 3.5 |
| 6h | 17 ± 1.6 | 62 ± 3.0 | 9 ± 0.2 | 35 ± 1.5 | 41 ± 1.0 | 66 ± 0.7 | 40 ± 1.9 | 63 ± 1.6 | 29 ± 1.5 | 53 ± 0.8 | 1 ± 1.5 | W |
| 6i | 61 ± 1.4 | 64 ± 0.5 | 6 ± 1.1 | 29 ± 1.2 | 3 ± 2.6 | 32 ± 0.9 | 48 ± 1.3 | 54 ± 1.7 | 31 ± 1.4 | 37 ± 2.1 | 9 ± 2.8 | 22 ± 2.2 |
| 7a | 27 ± 1.4 | 59 ± 2.4 | 25 ± 2.2 | 53 ± 0.6 | 11 ± 2.2 | 55 ± 0.5 | 24 ± 2.8 | 41 ± 0.8 | 27 ± 2.3 | 54 ± 2.3 | 32 ± 2.4 | 74 ± 0.3 |
| 7b | 36 ± 1.7 | 53 ± 0.9 | 39 ± 1.0 | 58 ± 1.0 | 34 ± 0.7 | 61 ± 0.4 | 48 ± 1.7 | 78 ± 1.7 | 53 ± 1.7 | 78 ± 1.7 | 44 ± 1.9 | 67 ± 1.9 |
| 7c | 22 ± 1.2 | 72 ± 0.5 | 8 ± 3.5 | 20 ± 2.1 | 25 ± 1.8 | 49 ± 2.0 | 37 ± 2.1 | 56 ± 1.4 | 18 ± 0.1 | 68 ± 0.2 | 28 ± 2.1 | 63 ± 0.2 |
| 7d | 46 ± 1.6 | 79± 0.5 | 46 ± 1.8 | 79 ± 1.0 | 48 ± 2.1 | 72 ± 0.8 | 49 ± 2.2 | 77 ± 0.6 | 22 ± 0.6 | 83 ± 1.1 | 45 ± 1.5 | 77 ± 0.7 |
| 7e | 32 ± 3.5 | 64 ± 1.7 | 4 ± 0.7 | 15 ± 0.7 | 3 ± 1.3 | 23 ± 1.4 | 32 ± 1.5 | 61 ± 0.4 | 21 ± 1.7 | 43 ± 2.5 | 13 ± 1.4 | 22 ± 2.5 |
2.3. Screening in Greenhouse Conditions
| Compd. | Relative Inhibition (%) | |||||
|---|---|---|---|---|---|---|
| A. theophrasti | A. retroflexus | P. oleracea | P. alopecuroides | E. crus-galli | D. sanguinalis | |
| 6a | 22 ± 1.3 | 27 ± 1.4 | 30 ± 1.2 | 42 ± 1.4 | 34 ± 2.6 | 21 ± 2.7 |
| 6b | 35 ± 2.0 | 29 ± 0.7 | 40 ± 1.1 | 25 ± 0.8 | 26 ± 2.2 | 17 ± 2.0 |
| 6c | 40 ± 1.0 | 44 ± 0.3 | 38 ± 1.7 | 32 ± 1.6 | 30 ± 1.6 | 33 ± 2.6 |
| 6d | 42 ± 2.0 | 50 ± 0.9 | 30 ± 0.7 | 6 ± 2.7 | 41 ± 2.6 | 82 ± 0.9 |
| 7a | 21 ± 0.6 | 46 ± 1.6 | 34 ± 2.6 | 12 ± 3.1 | 60 ± 0.5 | 56 ± 0.6 |
| 7b | 9 ± 0.6 | 56 ± 0.9 | 34 ± 1.5 | 5 ± 2.1 | 50 ± 2.3 | 46 ± 1.7 |
| 7d | 11 ± 2.2 | 37 ± 1.8 | 28 ± 1.3 | 17 ± 2.7 | 48 ± 0.4 | 52 ± 0.3 |
2.4. Inhibitory Effect of the Compound 6d on Chlorophyll of Weed
| Species | 6d | Diflufenican | ||
|---|---|---|---|---|
| IC50 (95% Confidence Intervals) (mg·L−1) | Slope (±SE) | IC50 (95% Confidence Intervals) (mg·L−1) | Slope (±SE) | |
| B. campestris | 20.01 (16.48–24.28) | 3.54 ± 0.32 | 19.79 (11.23–34.90) | 1.15 ± 0.39 |
| P. alopecuroides | 11.71 (10.57–12.98) | 1.53 ± 0.08 | 5.97 (4.04–8.82) | 0.45 ± 0.06 |
| E. crus-galli | 6.14 (5.83–6.46) | 2.09 ± 0.04 | 0.88 (0.07–9.81) | 2.24 ± 0.28 |
| D. sanguinalis | 8.09 (6.08–10.76) | 4.17 ± 0.34 | 1.09 (0.36–3.24) | 3.12 ± 0.16 |
3. Experimental Section
3.1. Analysis and Instruments
3.2. Synthesis and Characterization of Target Compounds
3.2.1. Synthesis of Compounds 5a and 5b
3.2.2. Synthesis of Compounds 6a–6i
3.2.3. Synthesis of Compounds 7a–7e
3.3. Biological Evaluation
3.3.1. Inhibitory Effect of the Target Compounds on the Growth of Weed Roots and Shoots
3.3.2. Treatment in Greenhouse Conditions
3.3.3. Inhibitory Effect of the Compound 6d on Chlorophyll of Weeds
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghorab, M.M.; Ragab, F.A.; Alqasoumi, S.I.; Alafeefy, A.M.; Aboulmagd, S.A. Synthesis of some new pyrazolo[3,4-d]pyrimidine derivatives of expected anticancer and radio-protective activity. Eur. J. Med. Chem. 2010, 45, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Rostom, S.A.; Shalaby, M.A.; El-Demellawy, M.A. Polysubstituted pyrazoles, part 5. Synthesis of new 1-(4-chlorophenyl)-4-hydroxy-1H-pyrazole-3-carboxylic acid hydrazide analogs and some derived ring systems. A novel class of potential antitumor and anti-HCV agents. Eur. J. Med. Chem. 2003, 38, 959–974. [Google Scholar] [CrossRef] [PubMed]
- Amnerkar, N.D.; Bhusari, K.P. Synthesis, anticonvulsant activity and 3D-QSAR study of some prop-2-eneamido and 1-acetyl-pyrazolin derivatives of aminobenzothiazole. Eur. J. Med. Chem. 2010, 45, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Yeu, J.P.; Yeh, J.T.; Chen, T.Y.; Uang, B.J. An expedient synthesis of 1-[3-(dimethylamino)propyl]-5-methyl-3-phenyl-1H-indazole(FS-32)-An antidepressant. Synthesis 2001, 12, 1775–1777. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kumar, S.; Kumar, P.; Kaushik, P.; Kaushik, D.; Dhingra, Y.; Aneja, K.R. Synthesis and biological evaluation of some pyrazolylpyrazolines as anti-inflammatory-antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 2650–2655. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, P.N.; Satasia, S.P.; Raval, D.K. Synthesis, identification and in vitro biological evaluation of some novel 5-imidazopyrazole incorporated pyrazoline and isoxazoline derivatives. New J. Chem. 2014, 38, 2902–2910. [Google Scholar] [CrossRef]
- Shiga, Y.; Okada, I.; Fukuchi, T. Synthesis and acaricidal activity of N-(1,3,4-thiadiazol-2-yl)pyrazole-5-carboxamides and N-(1,3,4-thiadiazol-2-yl) thiazole-5-carboxamides. J. Pestic. Sci. 2003, 28, 310–312. [Google Scholar] [CrossRef] [Green Version]
- Shiga, Y.; Okada, I.; Ikeda, Y.; Takizawa, E.; Fukuchi, T. Insecticidal activity of N-acy-N-(4-aryloxybenzyl)pyrazole-5-carboxamides. J. Pestic. Sci. 2003, 28, 313–314. [Google Scholar] [CrossRef]
- Ohno, R.; Watanabe, A.; Matsukawa, T.; Ueda, T.; Sakurai, H.; Hori, M.; Hirai, K. Synthesis and herbicidal activity of new pyrazole-4-carboxamide derivatives. J. Pestic. Sci. 2004, 29, 15–26. [Google Scholar] [CrossRef]
- Vicentini, C.B.; Mares, D.; Tartari, A.; Manfrini, M.; Forlani, G. Synthesis of pyrazole derivatives and their evaluation as photosynthetic electron transport inhibitors. J. Agric. Food Chem. 2004, 52, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Vicentini, C.B.; Romagnoli, C.; Andreotti, E.; Mares, D. Synthetic pyrazole derivatives as growth inhibitors of some phytopathogenic fungi. J. Agric. Food Chem. 2007, 55, 10331–10338. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Zhu, Y.Q.; Song, X.W.; Hu, F.Z.; Liu, B.; Li, Y.H.; Niu, Z.X.; Liu, P.; Wang, Z.H.; Song, H.B.; et al. Novel protoporphyrinogen oxidase inhibitors: 3H-pyrazolo[3,4-d]-[1,2,3]triazin-4-one derivatives. J. Agric. Food Chem. 2008, 56, 9535–9542. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.P.; Cai, X.J.; Chen, Z.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y.; Zeng, S. Synthesis and antiviral activities of pyrazole derivatives containing an oxime moiety. J. Agric. Food Chem. 2008, 56, 10160–10167. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, G.P.; Chen, Z.; Cai, X.J.; Song, B.A.; Bhadury, P.S.; Yang, S.; Jin, L.H.; Xue, W.; Hu, D.Y.; Zeng, S. Synthesis and antiviral activity of novel pyrazole derivatives containing oxime esters group. Bioorg. Med. Chem. 2008, 16, 9699–9707. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Uchida, A.; Ohno, R. Herbicide Classes in Development; Boger, P., Hirai, K., Wakabyashi, K., Eds.; Springer-Verlag: Heidelberg, Germany, 2002; pp. 179–289. [Google Scholar]
- Wang, H.X.; Wu, L.L.; Wang, Y.M.; Zhou, Z.H. Organocatalyzed asymmetric tandem Michaelcyclization reaction of 4-benzylidene-3-methylpyrazol-5-ones and malononitrile: Stereocontrolled construction of pyrano[2,3-c]pyrazole scaffold. RSC Adv. 2015, 5, 42836–42842. [Google Scholar] [CrossRef]
- Moon, J.K.; Kim, J.H.; Shibamoto, T. Photodegradation Pathways and mechanisms of the herbicide metamifop in a water/acetonitrile solution. J. Agric. Food Chem. 2010, 58, 12357–12365. [Google Scholar] [CrossRef] [PubMed]
- Kalaria, P.N.; Satasia, S.P.; Raval, D. K. l-Proline promoted green and regioselective synthesis of a novel pyrazole based trifluoromethylated fused thiazolopyran scaffold and its biological evaluation. RSC Adv. 2014, 4, 32353–32362. [Google Scholar] [CrossRef]
- Roe, R.M.; Burton, J.D.; Kuhr, R.J. Herbicide Activity: Toxicology, Biochemistry and Molecular Biology; Sandmann, G., Böger, P., Eds.; IOS Press: Amsterdam, The Netherlands, 1997; pp. 111–141. [Google Scholar]
- Sandmann, G. Bleaching activities of substituted pyrimidines and structure-activity comparison to related heterocyclic derivatives. Pestic. Biochem. Physiol. 2001, 70, 86–91. [Google Scholar] [CrossRef]
- Ogawa, H.; Yamada, I.; Arai, K.; Hirase, K.; Moriyasu, K.; Schneider, C.; Sandmann, G.; Boger, P.; Wakabayashi, K. Mode of Bleaching Phytotoxicity of Herbicidal Diphenylpyrrolidinones. Pest Manag. Sci. 2001, 57, 33–40. [Google Scholar] [CrossRef]
- Ohki, S.; Miller-Sulger, R.; Wakabayashi, K.; Pfleiderer, W.; Böger, P. Phytoene desaturase inhibition by O-(2-phenoxy)ethyl-N-aralkylcarbamates. J. Agric. Food Chem. 2003, 51, 3049–3055. [Google Scholar] [CrossRef] [PubMed]
- Laber, B.; Usunow, G.; Wiecko, E.; Franke, W.; Frank, H.; Köhn, A. Inhibition of narcissus pseudonarcissus phytoene desaturase by herbicidal 3-trifluoromethyl-1,1-biphenyl derivatives. Pestic. Biochem. Physiol. 1999, 63, 173–184. [Google Scholar] [CrossRef]
- Krämer, W.; Schirmer, U.; Jeschke, P.; Witschel, M. Herbicides with bleaching properties. In Modern Crop Protection Compounds; John Wiley & Sons: Malden, MA, USA, 2012; pp. 197–276. [Google Scholar]
- Xu, H.; Zou, X.M.; Zhu, Y.Q. Synthesis and herbicidal activity of novel α,α,α-trifluoro-m-tolyl pyridazinone derivatives. Pest. Manag. Sci. 2006, 62, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Hu, X.H.; Zou, X.M.; Zhu, Y.Q.; Liu, B.; Hu, F.Z.; Yang, H.Z. Synthesis and herbicidal activity of 5-heterocycloxy-3-substituted-1-(3-trifluoromethyl)phenyl-1H-pyrazole. Chem. Res. Chin. Univ. 2012, 28, 824–827. [Google Scholar]
- Kohn, A.; Franke, H.; Franke, W.; Bohner, J.; Rees, R. Substituted Biphenyl Derivatives and Their Use as Herbicides. EP Patent WO1996006089 A1, 29 February 1996. [Google Scholar]
- Welch, J.T. Tetrahedron report number 221: Advances in the preparation of biologically active organofluorine compounds. Tetrahedron 1987, 43, 3123–3197. [Google Scholar] [CrossRef]
- Ma, H.J.; Li, Y.H.; Zhao, Q.F.; Zhang, T.; Xie, R.L. Synthesis and herbicidal activity of novel N-(2,2,2)-trifluoroethylpyrazole derivatives. J. Agric. Food Chem. 2010, 58, 4356–4360. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.J.; Zhang, J.H.; Xia, X.D.; Xu, M.H.; Nin, J. Design, synthesis and herbicidal activities of novel 4-(1H-pyrazol-1-yl)-6-(alkynyloxy)-pyrimidine derivatives as potential pigment biosynthesis inhibitors. Pest Manag. Sci. 2014, 70, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.J.; Zhang, J.H.; Xia, X.D.; Kang, J.; Li, J.H. Design, synthesis and herbicidal evaluation of novel 4-(1H-pyrazol-1-yl)pyrimidine derivatives. Pest Manag. Sci. 2015, 71, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Masoud, M.S.; Mostafa, M.A.; Ahmed, R.H.; Moneim, N.H.A. Structures and chemical equilibria of some N-Heterocycles, containing amide linkages. Molecules 2003, 8, 430–438. [Google Scholar] [CrossRef]
- Shimomura, O.; Lee, B.S.; Meth, S.; Suzuki, H.; Mahajan, S.; Nomura, R.; Janda, K.D. Synthesis and application of polytetrahydrofuran-grafted polystyrene (PS–PTHF) resin supports for organic synthesis. Tetrahedron 2005, 61, 12160–12167. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.; Wellburn, A.R. Determinations of total carotenoids and chlorophylls a and b of leaf extracts in different solvents. Biochem. Soc. Trans. 1983, 11, 591–592. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 6a–6i are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Yue, X.L.; Chen, C.S.; Li, J.H.; Ma, H.J. Synthesis and Herbicidal Activity of 5-Heterocycloxy-3-methyl-1-substituted-1H-pyrazoles. Molecules 2016, 21, 39. https://doi.org/10.3390/molecules21010039
Kang J, Yue XL, Chen CS, Li JH, Ma HJ. Synthesis and Herbicidal Activity of 5-Heterocycloxy-3-methyl-1-substituted-1H-pyrazoles. Molecules. 2016; 21(1):39. https://doi.org/10.3390/molecules21010039
Chicago/Turabian StyleKang, Jing, Xia Li Yue, Chang Shui Chen, Jian Hong Li, and Hong Ju Ma. 2016. "Synthesis and Herbicidal Activity of 5-Heterocycloxy-3-methyl-1-substituted-1H-pyrazoles" Molecules 21, no. 1: 39. https://doi.org/10.3390/molecules21010039
APA StyleKang, J., Yue, X. L., Chen, C. S., Li, J. H., & Ma, H. J. (2016). Synthesis and Herbicidal Activity of 5-Heterocycloxy-3-methyl-1-substituted-1H-pyrazoles. Molecules, 21(1), 39. https://doi.org/10.3390/molecules21010039






