Synthesis of Some Novel 2-Amino-5-arylazothiazole Disperse Dyes for Dyeing Polyester Fabrics and Their Antimicrobial Activity
Abstract
:1. Introduction
2. Results and Discussion
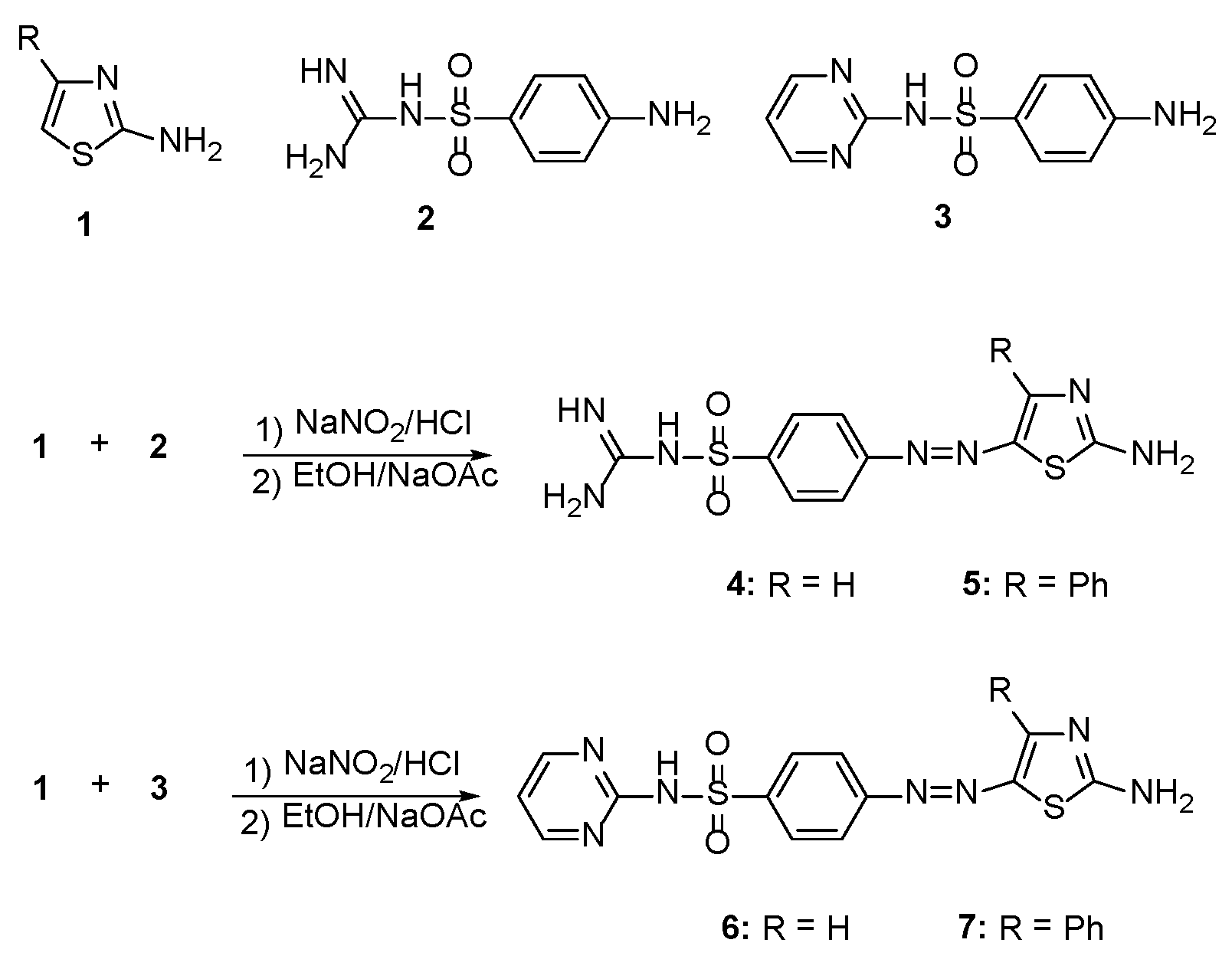
2.1. Synthesis
2.2. Dyeing and Fastness Properties
| Dye | Washing 1 | Perspiration 1 | Rubbing 1 | Sublimation 1 | Light 2 (60 h) | |||
|---|---|---|---|---|---|---|---|---|
| Acid | Alkali | Dry | Wet | Staining at 180 °C | Staining at 210 °C | |||
| 4 | 4–5 | 4–5 | 4–5 | 3–4 | 4–5 | 4 | 3 | 5 |
| 5 | 4–5 | 3–4 | 4–5 | 3 | 3 | 5 | 4 | 4–5 |
| 6 | 4–5 | 4–5 | 4–5 | 3–4 | 4–5 | 5 | 4 | 5 |
| 7 | 4–5 | 4 | 4 | 4 | 4 | 3 | 3 | 4–5 |
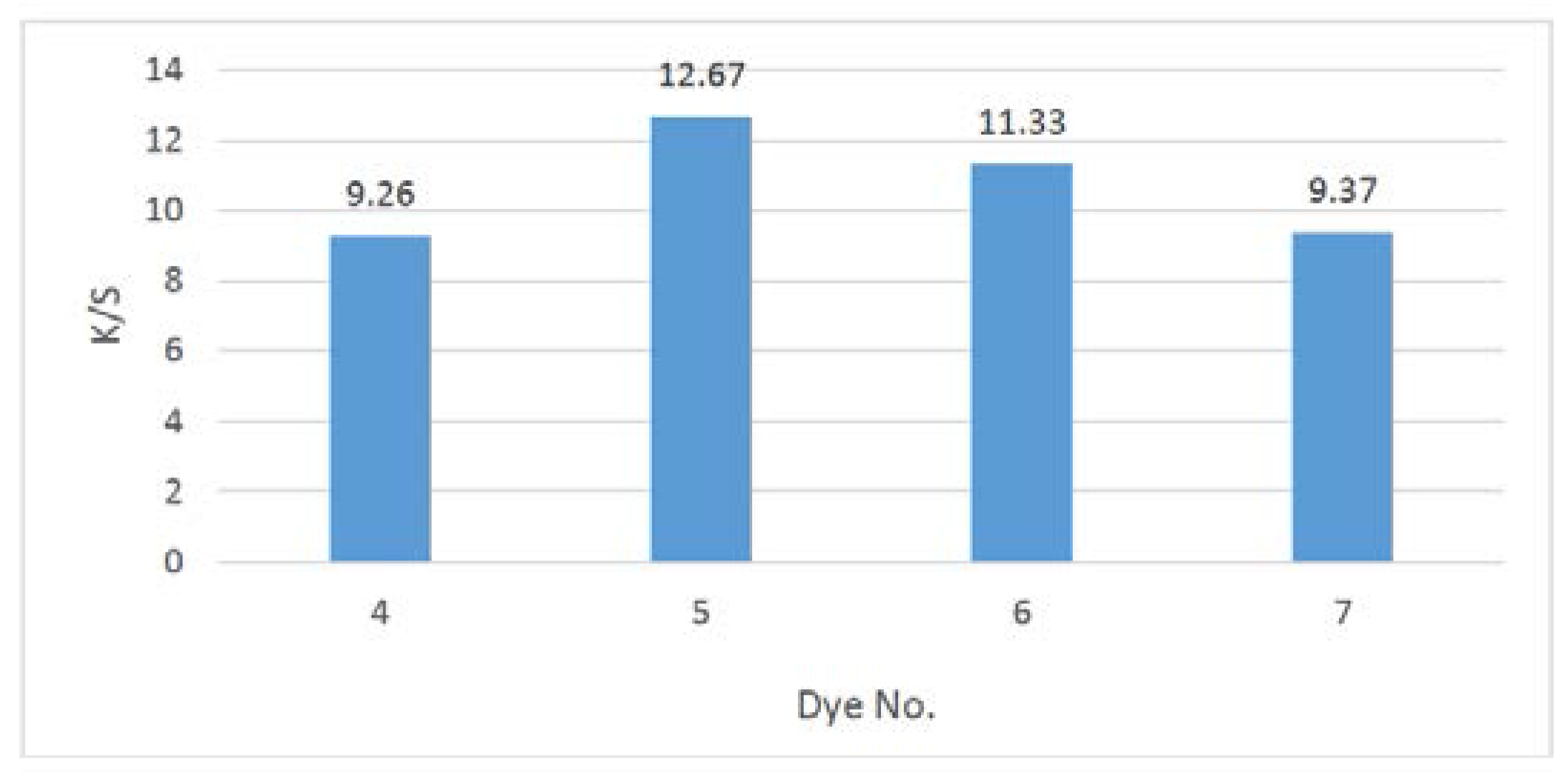
2.3. Color Assessment
| Dye | %Exhaustion | Absorption λmax/nm | K/S | L* | a* | b* | C* | H |
|---|---|---|---|---|---|---|---|---|
| 4 | 65 | 446 | 9.26 | 81.09 | 3.35 | 20.42 | 40.58 | 80.71 |
| 5 | 71 | 444 | 12.67 | 73.23 | −1.48 | 23.13 | 43.88 | 76.75 |
| 6 | 69 | 463 | 11.33 | 74.22 | 8.17 | 37.20 | 48.71 | 76.21 |
| 7 | 66 | 458 | 9.37 | 89.35 | −1.78 | 3.14 | 43.51 | 85.84 |
2.4. Antimicrobial Activity
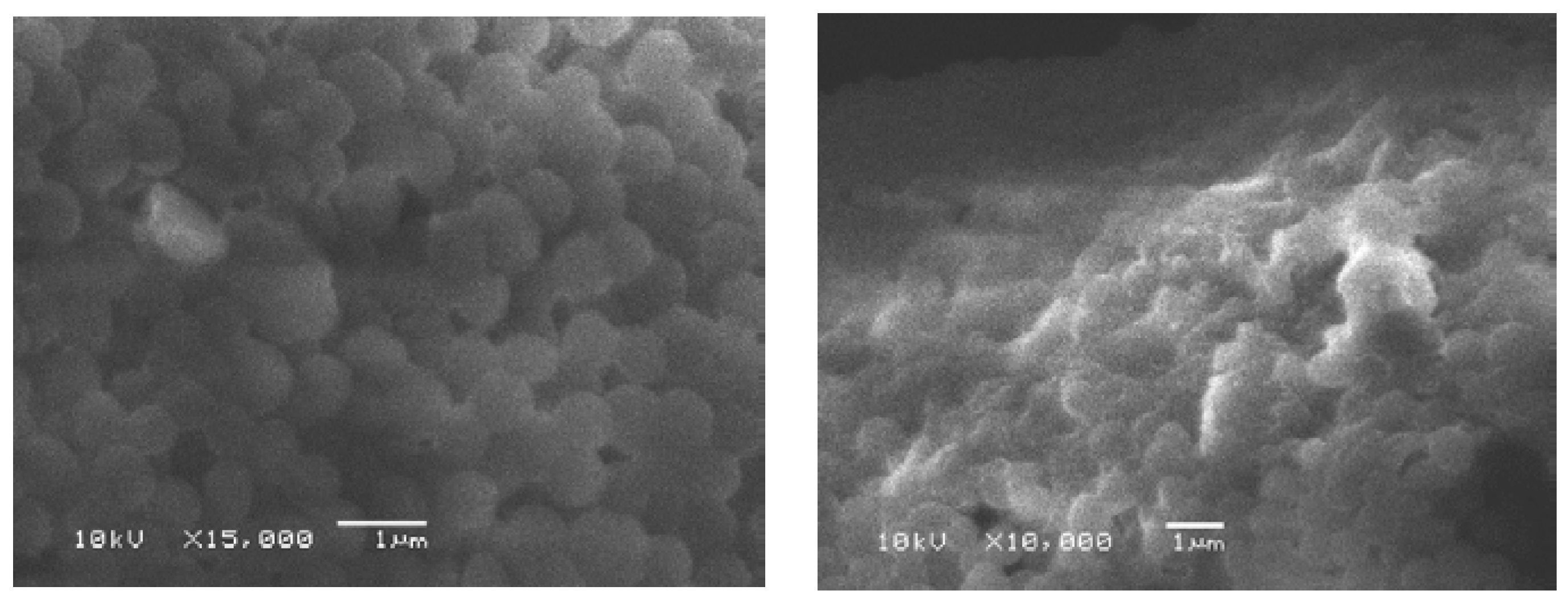
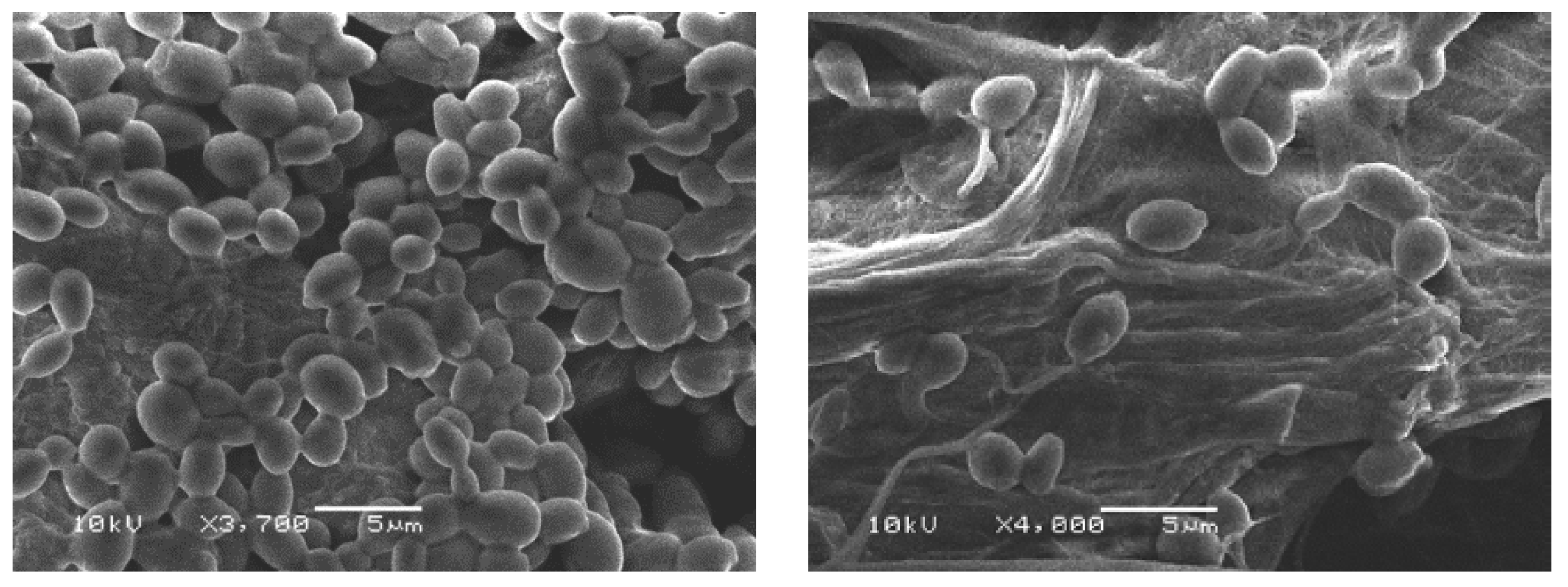
2.5. Scanning Electron Microscopy
2.6. Minimum Inhibitory Concentration (MIC)
| Compd. No. | Gram-Positive Bacteria | Gram-Negative Bacteria | Fungi | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | S. pyogenes | S. typhi | P. aeruginosa | K. pneumoniae | C. albicans | ||||||||||||
| 0.01 | 0.5 | 1.0 | 0.01 | 0.5 | 1 | 0.01 | 0.5 | 1 | 0.01 | 0.5 | 1 | 0.01 | 0.5 | 1.0 | 0.005 | 0.05 | |
| 4 | 0.6 | 0.7 | 1 | 0.4 | 0.6 | 0.65 | 0.6 | 0.9 | 1.2 | 0.3 | 0.6 | 0.8 | 0.2 | 0.6 | 0.7 | 0.7 | 1.1 |
| 5 | 0.6 | 0.8 | 1.2 | 0.1 | 0.2 | 0.6 | 0.6 | 0.8 | 1 | 0.2 | 0.4 | 0.6 | 0.2 | 0.6 | 0.7 | 0.9 | 1.3 |
| 6 | 0.6 | 1 | 1.7 | 0.3 | 0.6 | 1.6 | 0.6 | 1 | 1.2 | 0.7 | 1 | 1.4 | 0.3 | 0.5 | 1.2 | 0.7 | 1.4 |
| 7 | 0.6 | 1.2 | 1.3 | 0.6 | 1.1 | 1.9 | 0.7 | 1.2 | 2 | 0.1 | 0.3 | 0.7 | 0.4 | 1 | 2 | 0.8 | 1.3 |
| Ciprofloxacin | 0.9 | 1.2 | 1.4 | 0.8 | 1.0 | 1.3 | 0.8 | 0.9 | 1.5 | 0.8 | 1.0 | 1.4 | 0.5 | 0.8 | 1.2 | -- | -- |
| Ketoconazole | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | -- | 0.8 | 1.4 |
| Compd.# | Control Conc. (%) | S. aureus | Control Conc. (%) | S. typhi | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 60 | 150 | 5 | 10 | 60 | 150 | |||
| 4 | 49.32 × 103 (0 %) | 37.65 × 103 (23.7 %) | 31.44 × 103 (36.3 %) | 24.80 × 103 (49.7 %) | 19.39 × 103 (60.7 %) | 36.09 × 103 (0 %) | 34.22 × 103 (5.2 %) | 25.36 × 103 (29.7 %) | 22.71 × 103 (37.1 %) | 16.64 × 103 (53.9 %) |
| 5 | 49.32 × 103 (0 %) | 43.47 × 103 (11.9 %) | 26.16 × 103 (47.0 %) | 25.22 × 103 (48.9 %) | 17.32 × 103 (64.9 %) | 36.09 × 103 (0 %) | 36.56 × 103 (1.3 %) | 31.89 × 103 (11.6 %) | 22.24 × 103 (38.4 %) | 15.56 × 103 (56.9 %) |
| 6 | 49.32 × 103 (0 %) | 48.94 × 103 (0.8 %) | 48.19 × 103 (1.0 %) | 46.31 × 103 (6.1 %) | 28.24 × 103 (42.7 %) | 36.09 × 103 (0 %) | 32.67 × 103 (9.5 %) | 20.22 × 103 (44.0 %) | 19.44 × 103 (46.1 %) | 16.18 × 103 (55.2 %) |
| 7 | 49.32 × 103 (0 %) | 48.21 × 103 (21.2 %) | 37.65 × 103 (23.7 %) | 20.52 × 103 (58.4 %) | 19.20 × 103 (61.1 %) | 36.09 × 103 (0 %) | 31.89 × 103 (11.6 %) | 24.00 × 103 (33.5 %) | 21.78 × 103 (39.7 %) | 16.33 × 103 (54.8 %) |
3. Experimental Section
3.1. Materials and Methods
3.1.1. Materials, Chemicals and Reagents
3.1.2. Instrumentation
3.2. Synthesis
General Procedure for the Synthesis of 2-Amino-4-arylazo-thiazole Disperse Dyes 4–7
3.3. Disperse Dyeing Application
3.3.1. Dyebath Preparation Dyeing Procedure
3.3.2. Dyeing Characteristics on Polyester Fabrics
Color Fastness Tests
Color Properties of the Dyes on Polyester
3.4. Biological Assessment
3.4.1. Test-Pathogens
3.4.2. Antimicrobial and Antifungal Activity
3.4.3. Minimum Inhibitory Concentration (MIC)
3.4.4. Scanning Electron Microscopy
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dawson, J.F. Developments in disperse dyes. Color. Technol. 1978, 9, 25–35. [Google Scholar] [CrossRef]
- Malik, G.M.; Zadafiya, S.K. Thaizole based disperse dyes and their dyeing application on polyester fiber and their antimicrobial activity. Chem. Sin. 2010, 1, 15–21. [Google Scholar]
- Ibatullin, U.G.; Petrushina, T.F.; Leitis, L.Y.; Minibaev, I.Z.; Logvin, B.O. Synthesis and transformations of 4-substituted 2-aminothiazoles. Chem. Heterocycl. Compd. 1993, 29, 612–615. [Google Scholar] [CrossRef]
- Gaffer, H.E.; Abdel-Latif, E. Antimicrobial activity of some new 4-arylazo-3-methylthiophene disperse dyes on polyester fabrics. J. Appl. Polym. 2011, 122, 83–89. [Google Scholar] [CrossRef]
- Beuchet, P.; Varache-Lembège, M.; Neveu, A.; Léger, J.M.; Vercauteren, J.; Larrouture, S.; Deffieux, G.; Nuhrich, A. New 2-sulfonamidothiazoles substituted at C-4: Synthesis of polyoxygenated aryl derivatives and in vitro evaluation of antifungal activity. Eur. J. Med. Chem. 1999, 34, 773–779. [Google Scholar] [CrossRef]
- Geronikaki, A.; Vicini, P.; Dabarakis, N.; Lagunin, A.; Poroikov, V.; Dearden, J.; Modarresi, H.; Hewitt, M.; Theophilidis, G. Evaluation of the local anaesthetic activity of 3-aminobenzo[d]isothiazole derivatives using the rat sciatic nerve model. Eur. J. Med. Chem. 2009, 44, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, C.; Geronikaki, A.; Hadjipavlou-Litina, D. Synthesis and biological evaluation of new thiazolyl/benzothiazolyl-amides, derivatives of 4-phenyl-piperazine. Farmaco 2005, 60, 969–973. [Google Scholar] [CrossRef] [PubMed]
- Gouda, M.; Gaffer, H.E.; Gouda, M.A. Synthesis and anti-hypertensive activity of novel sulphadimidine derivatives. J. Med. Chem. Res. 2012, 21, 3902–3906. [Google Scholar] [CrossRef]
- Kreutzberger, A.; Schimmelpfennig, H. Antivirale Wirkstoffe, 18. Mitt. 2-Aminothiazole durch Spaltung der S-S-Bindung des Disulfidodicarbamidins. Arch. Pharm. 1981, 314, 385–391. [Google Scholar] [CrossRef]
- Keil, D.; Flaig, R.; Schroeder, A.; Hartmann, H. Synthesis and characterisation of methine dyes derived from N,N-disubstituted 2-aminoselenazoles and some of their heterocyclic sulfur analogues. Dyes Pigment. 2001, 50, 67–76. [Google Scholar] [CrossRef]
- Metwally, M.A.; Abdel-latif, E.; Khalil, A.M.; Amer, F.A.; Kaupp, G. New azodisperse dyes with thiazole ring for dyeing polyester fabrics. Dyes Pigment. 2004, 62, 181–195. [Google Scholar] [CrossRef]
- Singh, K.; Singh, S.; Taylor, J.A. Monoazo disperse dyes—Part 1: Synthesis, spectroscopic studies and technical evaluation of monoazo disperse dyes derived from 2-aminothiazoles. Dyes Pigment. 2002, 54, 189–200. [Google Scholar] [CrossRef]
- Yen, M.S.; Wang, I.J. Synthesis and solvent characteristics of bishetaryl monoazo dyes derived from polysubstituted-2-aminothiophene derivatives. Dyes Pigment. 2005, 67, 183–188. [Google Scholar] [CrossRef]
- Khalifa, M.E.; Abdel-Latif, E.; Gobouri, A.A. Disperse dyes based on 5-arylazothiazol-2-ylcarbamoyl-thiophenes: Synthesis, antimicrobial activity and their application on polyester. J. Heterocycl. Chem. 2015, 52, 674–680. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Gaffer, H.E.; Fahmy, H.M.; Fouda, M.E. Synthesis of some new 2-[(2,3-dihydroinden-1-ylidene) hydrazinyl]-4-methylthiazole derivatives for simultaneous dyeing and finishing for UV protective cotton fabrics. J. Appl. Polym. Sci. 2009, 112, 2221–2228. [Google Scholar] [CrossRef]
- Abdel-Latif, E.; Amer, F.A.; Metwally, M.A.; Khalifa, M.E. Synthesis of some 5-arylazo-2-(arylidenehydrazino)-thiazole disperse dyes for dyeing polyester fibers. Pigment Resin Technol. 2009, 38, 105–110. [Google Scholar] [CrossRef]
- Society of Dyes and Colorists. Standard Methods for the Determination of the Color Fastness of Textiles and Leather, 5th ed.; Society of Dyes and Colorists Publication: Bradford, UK, 1990. [Google Scholar]
- Al-Etaibi, A.M.; El-Apasery, M.A.; Ibrahim, M.R.; Al-Awadi, N.A. A facile synthesis of new monoazo disperse dyes derived from 4-hydroxyphenylazopyrazole-5-amines: Evaluation of microwave assisted dyeing behavior. Molecules 2012, 17, 13891–13909. [Google Scholar] [CrossRef] [PubMed]
- Chipalkatti, H.R.; Desai, N.F.; Giles, C.H.; Macaulay, N. The influence of the substrate upon the light fading of azo dyes. J. Soc. Dyers Color. 1954, 70, 487–501. [Google Scholar] [CrossRef]
- Műller, C. Recent developments in the chemistry of disperse dyes and their intermediate. Am. Dyest. Rep. 1970, 59, 37–44. [Google Scholar]
- Mcfarland, J. Nephelometer: An instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. JAMA 1907, 14, 1176–1178. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Venkatesalu, V. Antibacterial and antifungal activity of Syzygium jambolanum seeds. J. Ethnopharmacol. 2004, 91, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Adesokan, A.; Akanji, M.A.; Yakubu, M.T. Antibacterial potentials of aqueous extract of Enantia chlorantha stem bark. A. J. Biotechnol. 2007, 6, 2502–2505. [Google Scholar]
- Hayat, M.A. Principles and Techniques of Electron Microscopy: Biological Applications; University Park Press: Baltimore, MD, USA, 1981; Volume 1, p. 522. [Google Scholar]
- Sample Availability: Samples of the compounds 4–7 are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaffer, H.E.; Fouda, M.M.G.; Khalifa, M.E. Synthesis of Some Novel 2-Amino-5-arylazothiazole Disperse Dyes for Dyeing Polyester Fabrics and Their Antimicrobial Activity. Molecules 2016, 21, 122. https://doi.org/10.3390/molecules21010122
Gaffer HE, Fouda MMG, Khalifa ME. Synthesis of Some Novel 2-Amino-5-arylazothiazole Disperse Dyes for Dyeing Polyester Fabrics and Their Antimicrobial Activity. Molecules. 2016; 21(1):122. https://doi.org/10.3390/molecules21010122
Chicago/Turabian StyleGaffer, Hatem E., Moustafa M. G. Fouda, and Mohamed E. Khalifa. 2016. "Synthesis of Some Novel 2-Amino-5-arylazothiazole Disperse Dyes for Dyeing Polyester Fabrics and Their Antimicrobial Activity" Molecules 21, no. 1: 122. https://doi.org/10.3390/molecules21010122
APA StyleGaffer, H. E., Fouda, M. M. G., & Khalifa, M. E. (2016). Synthesis of Some Novel 2-Amino-5-arylazothiazole Disperse Dyes for Dyeing Polyester Fabrics and Their Antimicrobial Activity. Molecules, 21(1), 122. https://doi.org/10.3390/molecules21010122






