An Unusual Stress Metabolite from a Hydrothermal Vent Fungus Aspergillus sp. WU 243 Induced by Cobalt
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of Strain WU 243
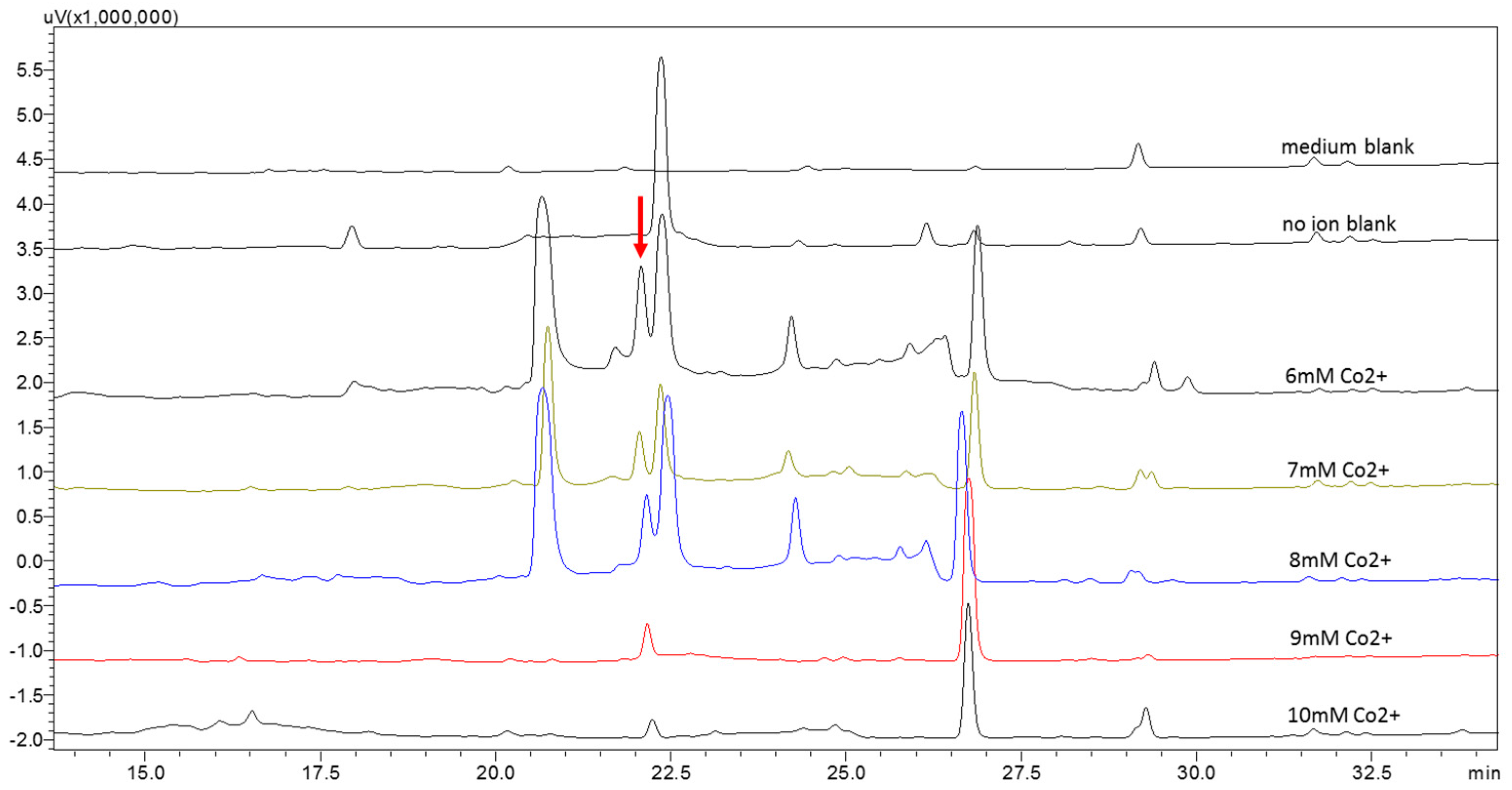
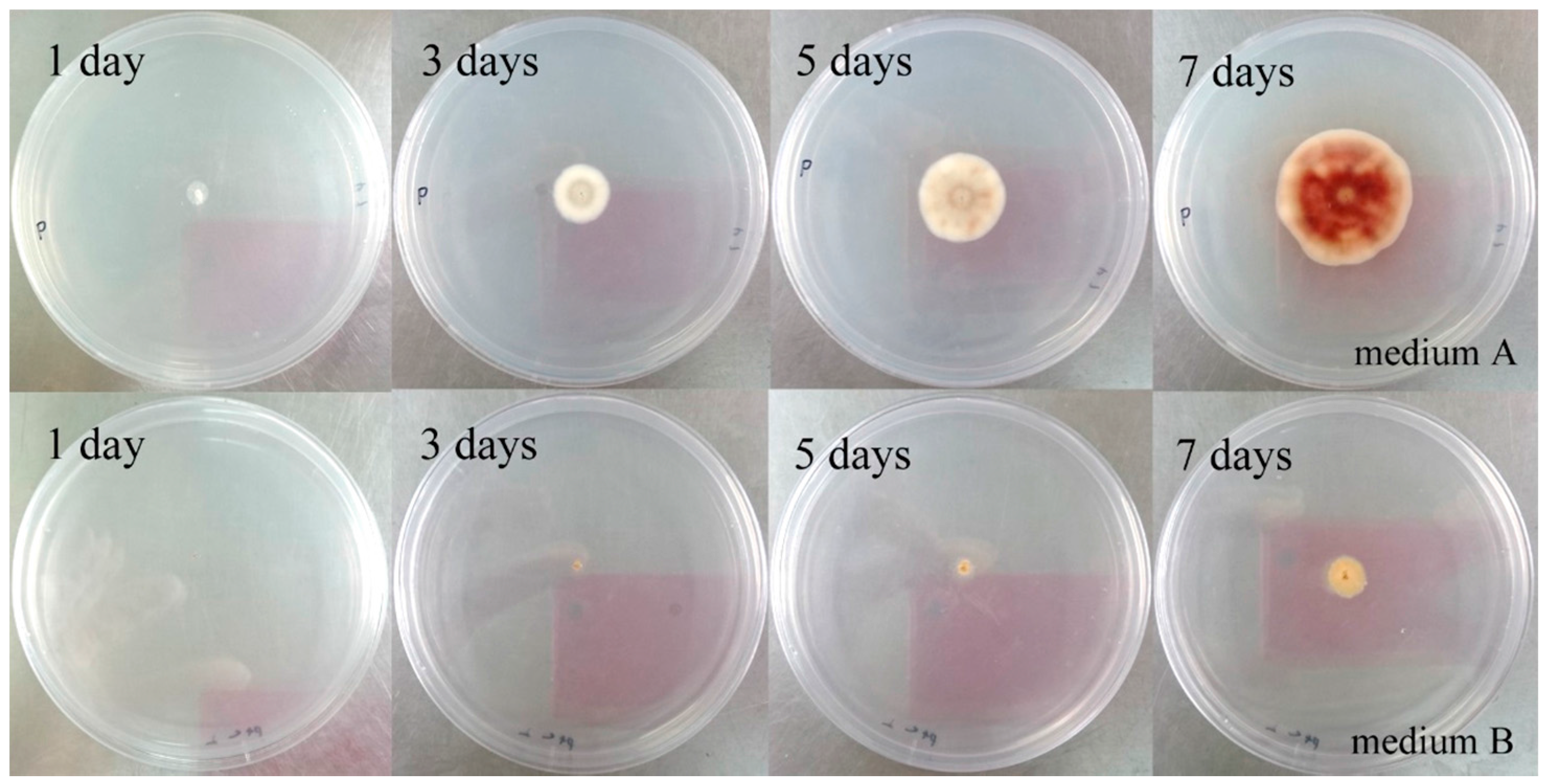
2.2. Metabolic Profiles of the Strain WU 243 after Metal-Stress Application and Optimization of Stress Fermentation

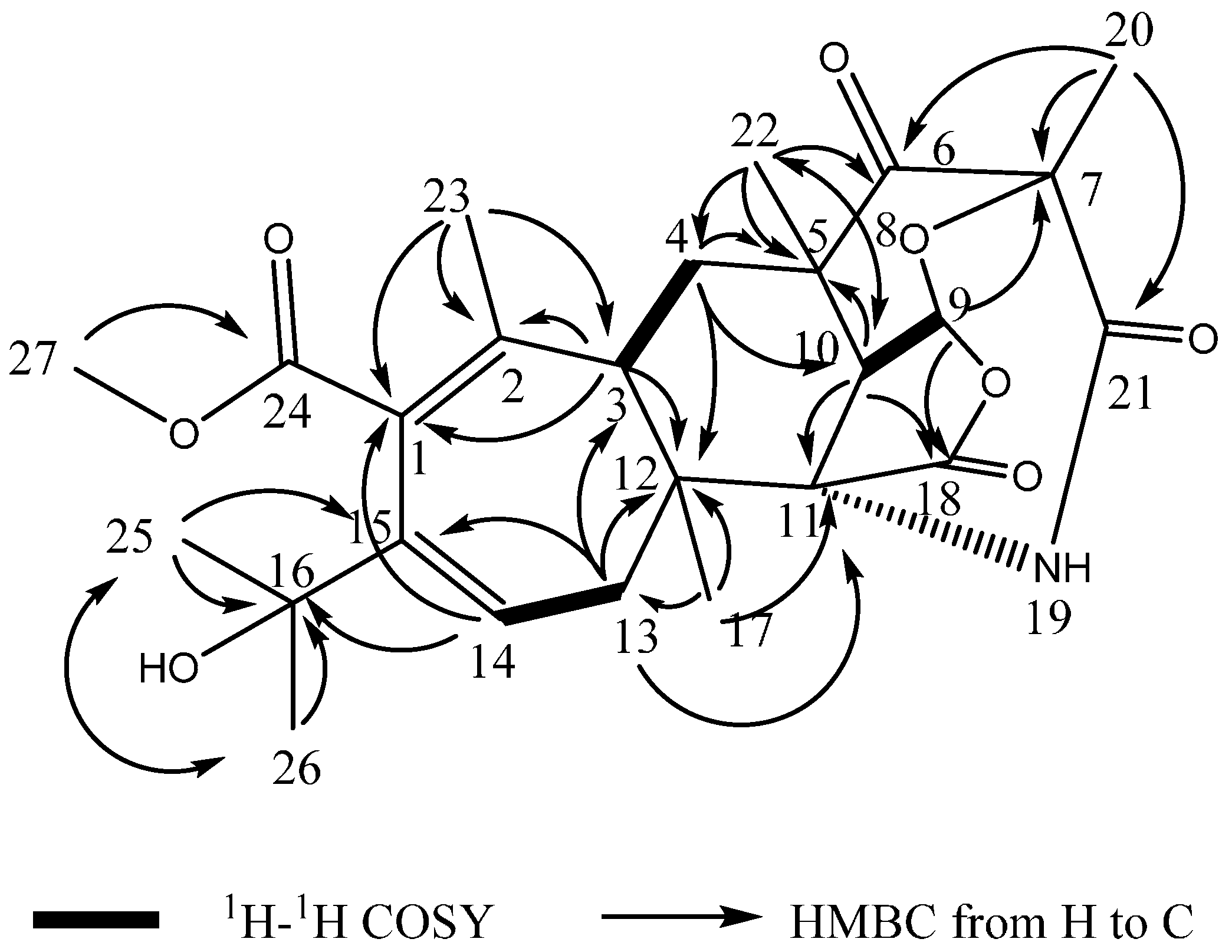
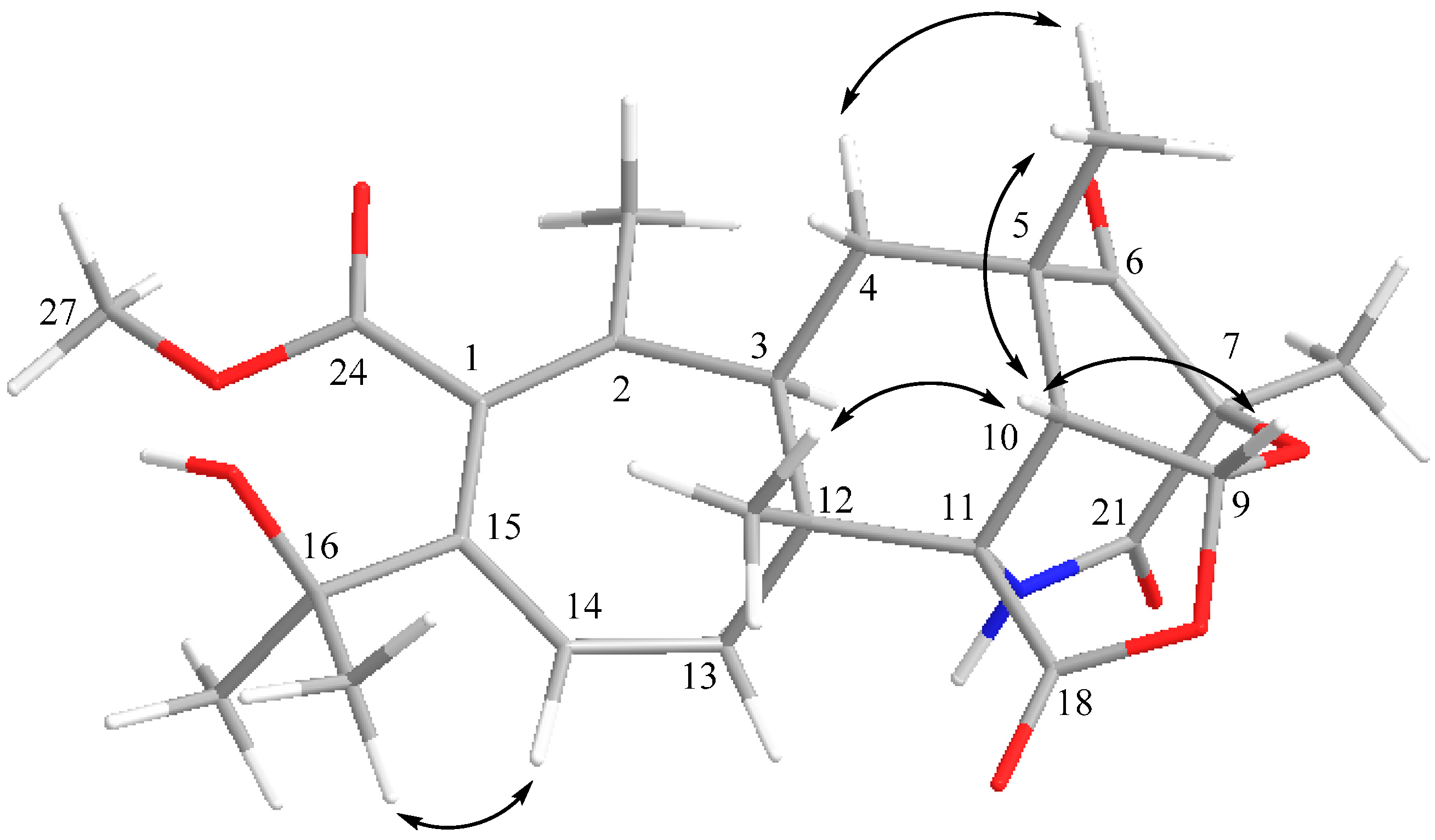
2.3. Structural Elucidation
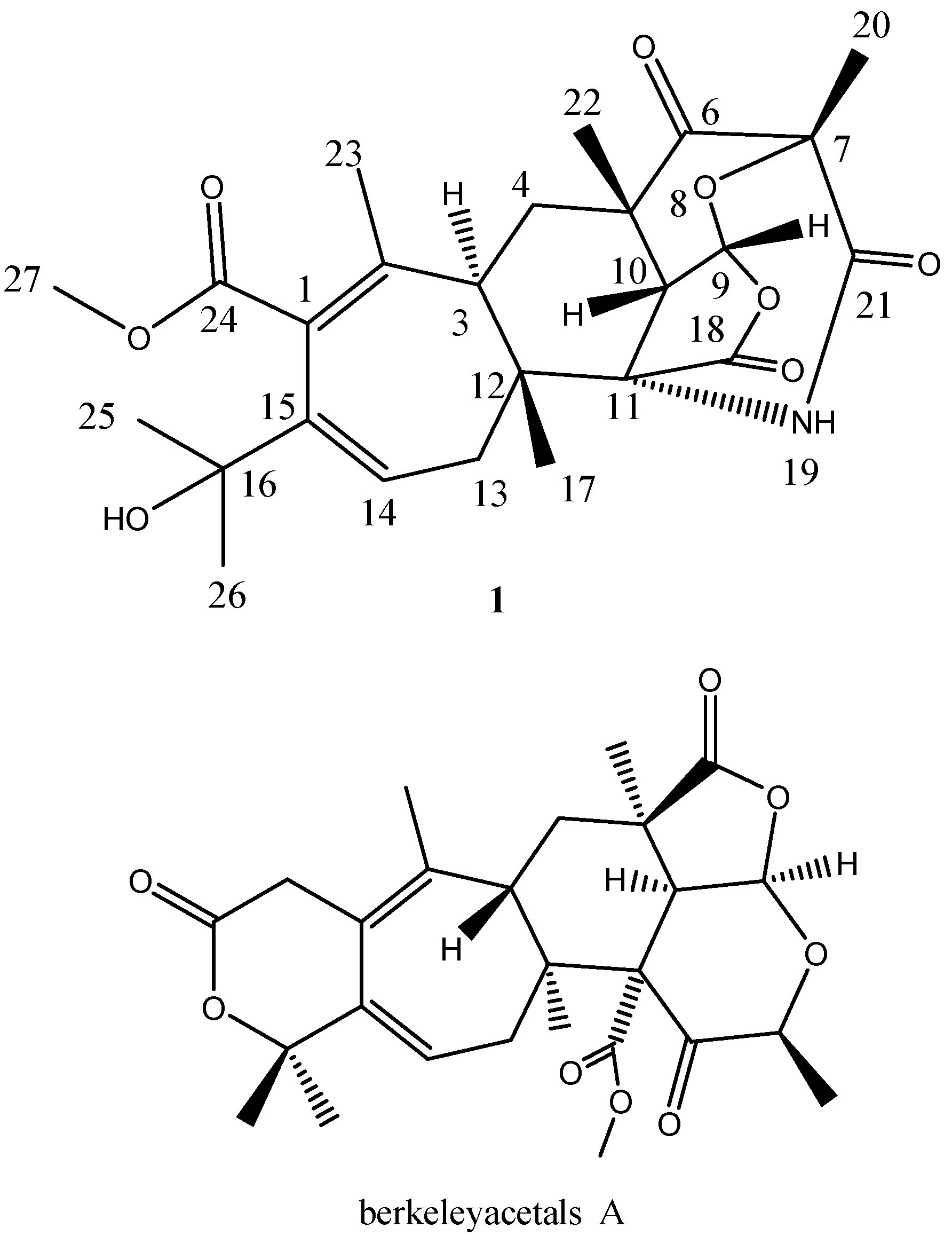
| Position | δC a,b, Mult. | δH c, Mult. (J in Hz) | HMBC | NOESY |
|---|---|---|---|---|
| 1 | 127.9 | |||
| 2 | 139.8 | |||
| 3 | 44.8, CH | 2.07, dd (13.5, 2.1) | C-1/C-2/C-12/C-17/C-23 | H-4β |
| 4β | 32.4, CH2 | 1.49, t (13.5) | C-3/C-5/C-6/C-12/C-22 | H-4β/H-23/H-17/H-22 |
| 4α | 2.39, dd (13.6, 2.6) | C-5/C-6/C-10/C-12 | H-4α/H-22/H-3/H-23 | |
| 5 | 48.0 | |||
| 6 | 211.2 | |||
| 7 | 81.8 | |||
| 8 | O | |||
| 9 | 100.0, CH | 6.10,d (7.1) | C-5/C-7/C-10/C-18 | H-10 |
| 10 | 45.2, CH | 3.27, d (7.1) | C-5/C-6/C-9/C-11/C-18/C-22 | H-9/H-17/H-22 |
| 11 | 51.7 | |||
| 12 | 56.6 | |||
| 13α | 40.3, CH2 | 2.51, dd (13.2, 8.0) | C-3/C-11/C-12/C-14/C-15 | H-13β/H-14 |
| 13β | 1.78, dd (13.2, 5.2) | C-11/C-12/C-14/C-15/C-17 | H-13α/H-17 | |
| 14 | 131.5, CH | 6.40,dd (8.0, 5.2) | C-1/C-12/C-13/C-16 | H-25/H-13α |
| 15 | 140.9 | |||
| 16 | 85.6 | |||
| 17 | 26.4, CH3 | 1.04, s | C-3/C-11/C-12/C-13/C-14/C-18 | H-10/H-13β/H-4α |
| 18 | 174.3 | |||
| 19 | N | |||
| 20 | 26.8, CH3 | 1.56, s | C-6/C-7/C-21 | H-22 |
| 21 | 173.2 | |||
| 22 | 32.3, CH3 | 1.40, s | C-4/C-5/C-6/C-10 | H-10/H-20/H-4α/H-4β |
| 23 | 16.5, CH3 | 1.83, s | C-1/C-2/C-3 | H-4α/H-4β |
| 24 | 173.2 | |||
| 25 | 27.8, CH3 | 1.56,s | C-15/C-16/C-26 | H-14/H-26 |
| 26 | 30.7, CH3 | 1.39, s | C-15/C-16/C-25 | H-25 |
| 27 | 54.7, CH3 | 3.67, s | C-24 |
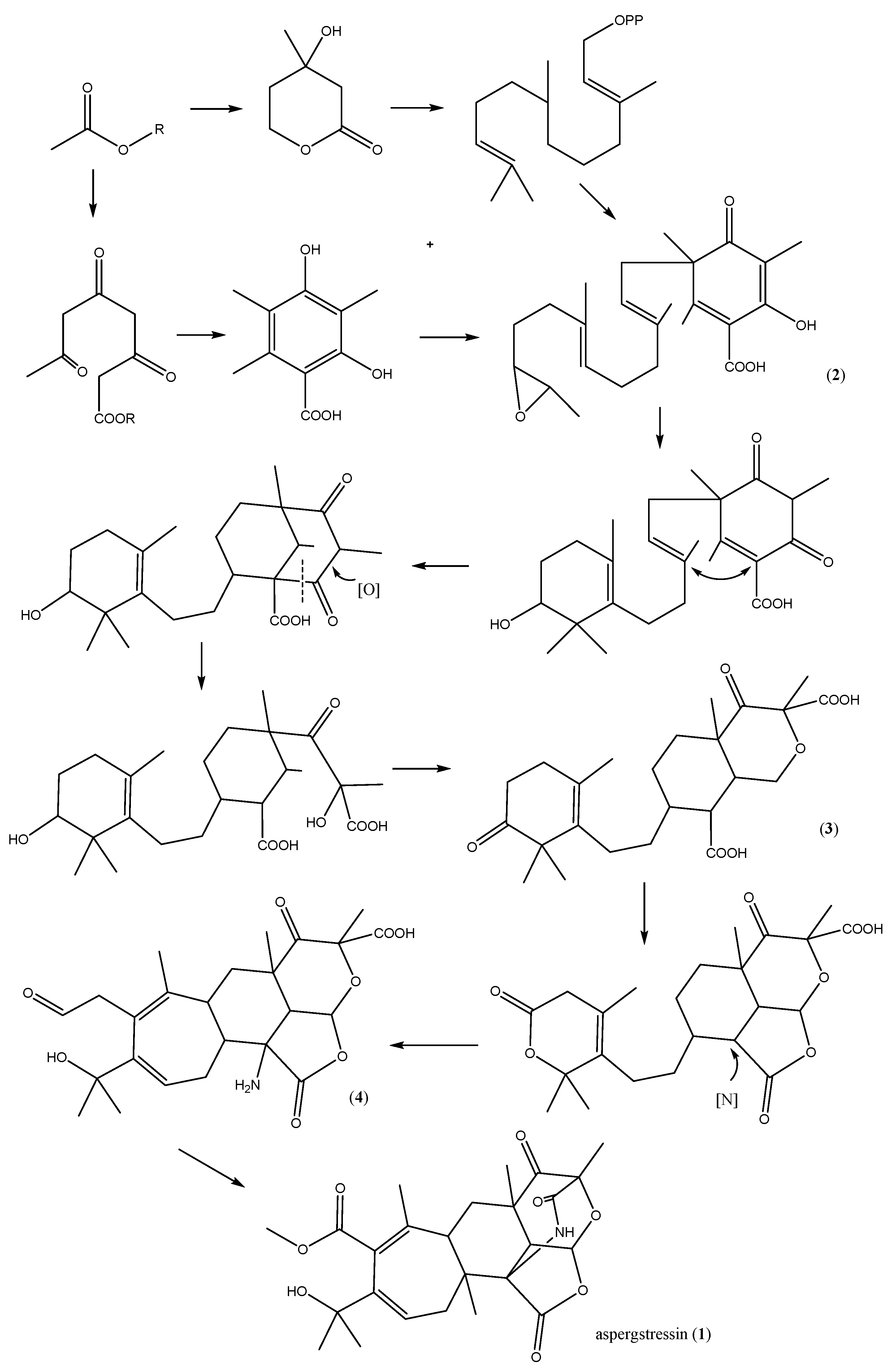
2.4. Proposed Biosynthetic Pathway
3. Materials and Methods
3.1. General Procedures
3.2. Isolation, Cultivation, and Storage of the Strain WU 243
3.3. Normal Culture and Metal-Stress Cultivation
3.4. HPLC Analysis and Identification of Stress Metabolites
3.5. Confirmation and Fermentation in Large Scale
3.6. Extraction and Isolation of Compound 1
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Holden, J.F.; Adams, M.W.W. Microbe-metal interactions in marine hydrothermal environments. Curr. Opin. Chem. Biol. 2003, 7, 160–165. [Google Scholar] [CrossRef]
- Thornburg, C.C.; Zabriskie, T.M.; McPhail, K.L. Deep-sea hydrothermal vents: Potential hot spots for natural products discovery? J. Nat. Prod. 2010, 73, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Debbab, A.; Aly, A.H.; Lin, W.H.; Proksch, P. Bioactive compounds from marine bacteria and fungi. Microb. Biotechnol. 2010, 3, 544–563. [Google Scholar] [CrossRef] [PubMed]
- Saleema, M.; Ali, M.S.; Hussain, S.; Jabbar, A.; Ashraf, M.; Lee, Y.S. Marine natural products of fungal origin. Nat. Prod. Rep. 2007, 24, 1142–1152. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Wu, X.; Sun, M.; Li, M. Two novel tyrosinase inhibitory sesquiterpenes induced by CuCl2 from a marine-derived fungus Pestalotiopsis sp. Z233. Mar. Drugs 2013, 11, 2713–2721. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Zhong, Y.; Shen, L.; Wu, X.; Ye, Y.; Chen, C.T.A.; Wu, B. Stress-driven Discovery of Natural Products from Extreme Marine Environment—Kueishantao Hydrothermal Vent, a Case Study of Metal Switch Valve. Curr. Org. Chem. 2014, 18, 925–934. [Google Scholar] [CrossRef]
- Haferburg, G.; Groth, I.; Möllmann, U.; Kothe, E.; Sattler, I. Arousing sleeping genes: Shifts in secondary metabolism of metal tolerant actinobacteria under conditions of heavy metal stress. Biometals 2009, 22, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Van den Brenk, A.L.; Fairlie, D.P.; Hanson, G.R.; Gahan, L.R.; Hawkins, C.J.; Jones, A. Binding of copper(II) to the cyclic octapeptide patellamide D. Inorg. Chem. 1994, 33, 2280–2289. [Google Scholar] [CrossRef]
- Yang, J.X.; Qiu, S.X.; She, Z.G. Metabolites of Mangrove Endophytic Fungus Gx-3a from South China Sea. Technol. Dev. Chem. Ind. 2013, 2, 168–170. [Google Scholar]
- Kimura, Y.; Tani, K.; Kojima, A. Cyclo-(l-tryptophyl-l-phenylalanyl), a plant growth regulator produced by the fungus Penicillium sp. Phytochemistry 1996, 41, 665–669. [Google Scholar] [CrossRef]
- Taridaporn, B.; Seangaroon, Y.; Kamolphan, I. New Diphenyl Ethers from the Insect Pathogenic Fungus Cordyceps sp. BCC 1861. Chem. Pharm. Bull. 2007, 55, 304–307. [Google Scholar]
- Zheng, C.J.; Shao, C.L.; Wang, K.L. Secondary metabolites and their bioactivities of a soft coral-derived fungus Aspergillus versicolor(ZJ-2008015). Chin. J. Mar. Drugs 2012, 31, 7–13. [Google Scholar]
- Stierle, D.B.; Stierle, A.A.; Patacini, B. The Berkeleyacetals, three meroterpenes from a deep water Acid mine waste Penicillium. J. Nat. Prod. 2007, 70, 1820–1823. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, E.; Yamazaki, M.; Kobayashi, K.; Sakurai, T. Paraherquonin, a new meroterpenoid from penicillium paraherquei. Tetraheadron Lett. 1983, 24, 3113–3114. [Google Scholar] [CrossRef]
- Ebel, R.; Rusman, Y.; Brauers, G.; Proksch, P.; Frank, W.; Wray, V. Abstracts of the 54th Annual Congress on Medicinal Plant Research, 29 August–2 September 2006, Helsinki, Finland. Planta Med. 2006, 72, 961–1083. [Google Scholar]
- Simpson, T.J.; Ahmed, S.A.; Mcintyre, C.R.; Scott, F.E.; Sadler, I.H. Biosynthesis of polyketide-terpenoid (meroterpenoid) metabolites andibenin b and andilesin a in aspergillus variecolor. Tetrahedron 1997, 53, 4013–4034. [Google Scholar] [CrossRef]
- Mcintyre, C.R.; Simpson, T.J. Biosynthesis of terretonin, a polyketide-terpenoid metabolite of aspergillus terreus. J. Chem. Soci. Chem. Commun. 1981, 20, 1043–1044. [Google Scholar] [CrossRef]
- Aleem, A.A. Distribution and ecology of marine fungi in Sierra Leone (tropical West Africa). Bota Mari 1980, 23, 679–688. [Google Scholar]
- Fang, F.; Ui, H.; Shiomi, K.; Masuma, R.; Yamaguchi, Y.; Zhang, C.G. Two new components of the aspochalasins produced by Aspergillus sp. J. Antibiot. 1995, 11, 919–925. [Google Scholar] [CrossRef]
- Hanson, F.R.; Eble, T.E. An antiphage agent isolated from aspergillus sp. J. Bacteriol. 1949, 4, 527–529. [Google Scholar]
- Iqbal, A.; Mohd, A.; Farrukh, A. Biosorption of Ni, Cr and Cd by metal tolerant Aspergillus niger and penicillium sp. using single and multi-metal solution. India J. Exp. Biol. 2006, 44, 73–76. [Google Scholar]
- Shi, Y.; Wei, J.; Auckloo, B.N.; Wu, B. Wu, B. Several Classes of Natural Products with Metal ion Chelating Ability. Curr. Org. Chem. 2015, 19, 1935–1953. [Google Scholar] [CrossRef]
- Sample Availability: Sample of the compound 1 is available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, C.; Wu, X.; Auckloo, B.N.; Chen, C.-T.A.; Ye, Y.; Wang, K.; Wu, B. An Unusual Stress Metabolite from a Hydrothermal Vent Fungus Aspergillus sp. WU 243 Induced by Cobalt. Molecules 2016, 21, 105. https://doi.org/10.3390/molecules21010105
Ding C, Wu X, Auckloo BN, Chen C-TA, Ye Y, Wang K, Wu B. An Unusual Stress Metabolite from a Hydrothermal Vent Fungus Aspergillus sp. WU 243 Induced by Cobalt. Molecules. 2016; 21(1):105. https://doi.org/10.3390/molecules21010105
Chicago/Turabian StyleDing, Chihong, Xiaodan Wu, Bibi Nazia Auckloo, Chen-Tung Arthur Chen, Ying Ye, Kuiwu Wang, and Bin Wu. 2016. "An Unusual Stress Metabolite from a Hydrothermal Vent Fungus Aspergillus sp. WU 243 Induced by Cobalt" Molecules 21, no. 1: 105. https://doi.org/10.3390/molecules21010105
APA StyleDing, C., Wu, X., Auckloo, B. N., Chen, C.-T. A., Ye, Y., Wang, K., & Wu, B. (2016). An Unusual Stress Metabolite from a Hydrothermal Vent Fungus Aspergillus sp. WU 243 Induced by Cobalt. Molecules, 21(1), 105. https://doi.org/10.3390/molecules21010105






