Antibacterial and Antitumor Activities of Biscoumarin and Dihydropyran Derivatives
Abstract
:1. Introduction
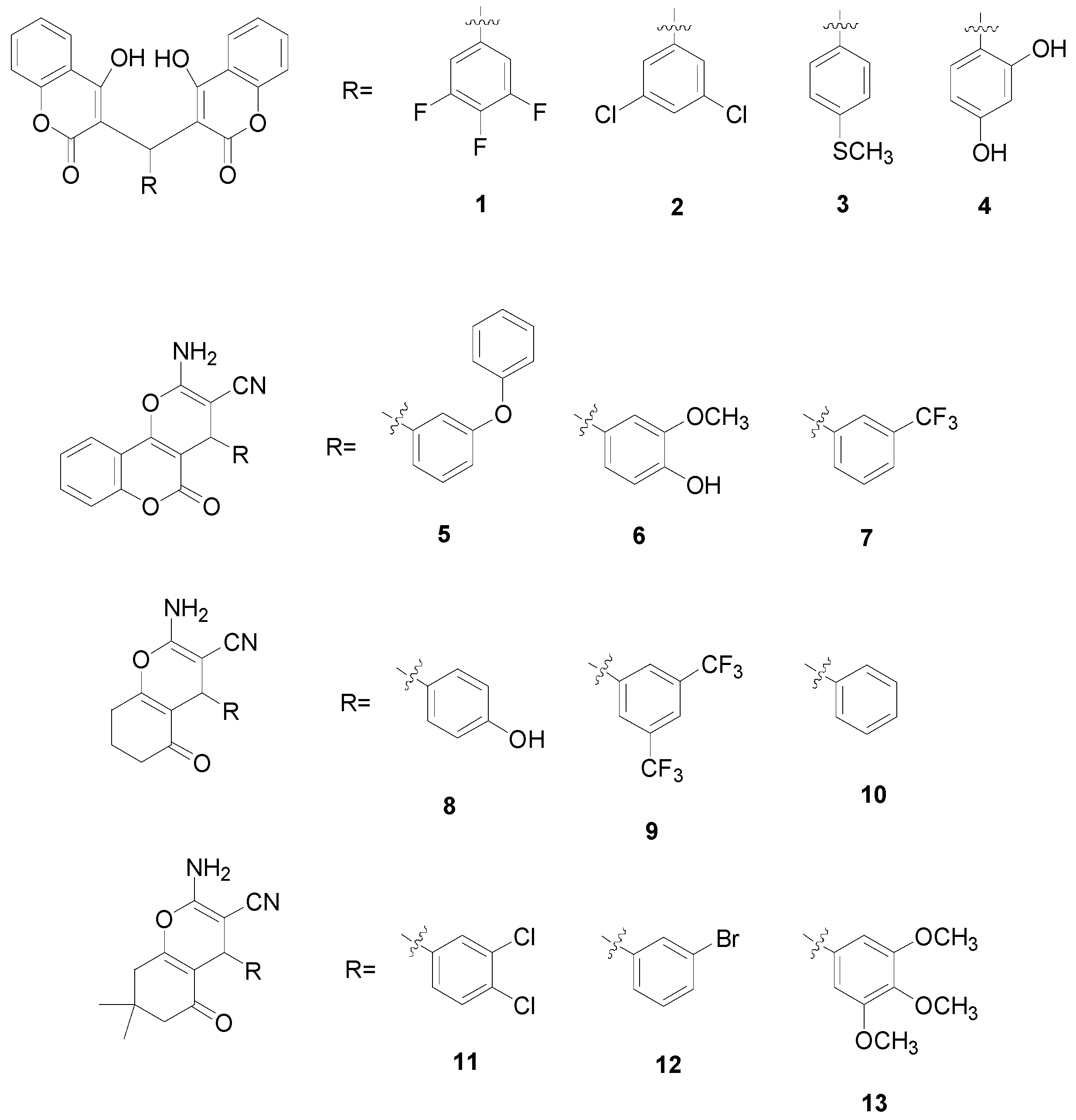
2. Results and Discussion
2.1. Molecular Structure
2.2. Hydrogen Bonds Energies in Biscoumarins 1–4
| System | Total Electronic Energies a,b | E(O6–H6···O1) | E(O3–H3···O4) | E(Total HB) c |
|---|---|---|---|---|
| 1ab | −1711.038294 | −116.20463 | ||
| 1a | −1711.018741 | −51.3364015 | ||
| 1b | −1711.013587 | −64.8682285 | ||
| 2ab | −2332.538245 | −115.7031595 | ||
| 2a | −2332.518779 | −51.107983 | ||
| 2b | −2332.513642 | −64.5951765 | ||
| 3ab | −2025.68685 | −118.0346035 | ||
| 3a | −2025.666896 | −52.389227 | ||
| 3b | −2025.661847 | −65.6453765 | ||
| 4ab | −1625.791083 | −121.970228 | ||
| 4a | −1625.770804 | −53.2425145 | ||
| 4b | −1625.764906 | −68.7277135 |
2.3. Minimal Inhibitory Concentration (MIC) Assay
| Drugs | MIC (µg/mL) | |||
|---|---|---|---|---|
| S. aureas (ATCC 29213) | MRSA (XJ 75302) | Mu50 (ATCC 700699) | LAC (USA 300) | |
| Compound 1 | 16 | 16 | 8 | 8 |
| Compound 2 | 4 | 4 | 2 | 2 |
| Compound 3 | 64 | 64 | 64 | 64 |
| Compound 4 | >256 | >256 | >256 | >256 |
| Compound 5 | >256 | >256 | >256 | >256 |
| Compound 6 | >256 | >256 | >256 | >256 |
| Compound 7 | >256 | >256 | >256 | >256 |
| Compound 8 | >256 | >256 | >256 | >256 |
| Compound 9 | >256 | >256 | >256 | >256 |
| Compound 10 | >256 | >256 | >256 | >256 |
| Compound 11 | >256 | >256 | >256 | >256 |
| Compound 12 | >256 | >256 | >256 | >256 |
| Compound 13 | >256 | >256 | >256 | >256 |
| Levofloxacin | <0.125 (S) | 4 (R) | 4 (R) | 8 (R) |
| Ceftazidime | 8 (S) | >256 (R) | 256 (R) | 64 (R) |
| Ceftriaxone | 2 (S) | >256 (R) | 256 (R) | 32 (R) |
| Gentamicin | 0.12 (S) | 64 (R) | 32 (R) | 0.25 (S) |
| Piperacillin | 2 (S) | >128 (R) | >128 (R) | 8 (R) |
2.4. In Vitro Antitumor Activity
| Drugs | HUTU 80 | 4T1 | PANC1 | |||
|---|---|---|---|---|---|---|
| IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | |
| Compound 1 | 32.63 | 64.55 | 22.09 | 39.58 | 27.52 | 51.33 |
| Compound 2 | 28.94 | 55.87 | 18.78 | 36.05 | 25.05 | 46.67 |
| Compound 3 | 28.42 | 55.31 | 20.08 | 38.21 | 28.01 | 51.75 |
| Compound 4 | 28.55 | 55.61 | 19.33 | 36.92 | 26.11 | 48.56 |
| Compound 5 | 116.00 | 216.00 | 138.00 | 248.07 | 79.23 | 154.62 |
| Compound 6 | 130.67 | 264.00 | 163.67 | 304.96 | 149.42 | 302.09 |
| Compound 7 | 209.00 | 409.00 | 91.44 | 186.16 | 174.71 | 339.00 |
| Compound 8 | 266.79 | 483.83 | 214.87 | 330.92 | 172.99 | 317.39 |
| Compound 9 | 582.88 | 1079.16 | 333.93 | 638.81 | 744.59 | 1340.72 |
| Compound 10 | 493.58 | 942.01 | 303.98 | 601.82 | 566.14 | 1055.13 |
| Compound 11 | 268.72 | 490.94 | 316.54 | 569.07 | 305.25 | 592.40 |
| Compound 12 | 509.78 | 985.97 | 621.34 | 1117.62 | 604.45 | 1143.73 |
| Compound 13 | 374.50 | 707.83 | 481.83 | 900.82 | 205.17 | 410.09 |
| Carboplatin | 65.62 | 126.24 | 45.85 | 102.13 | 52.94 | 109.94 |
3. Experimental Section
3.1. Apparatus and Materials
3.2. Synthesis and Characterization of Compounds 1–13
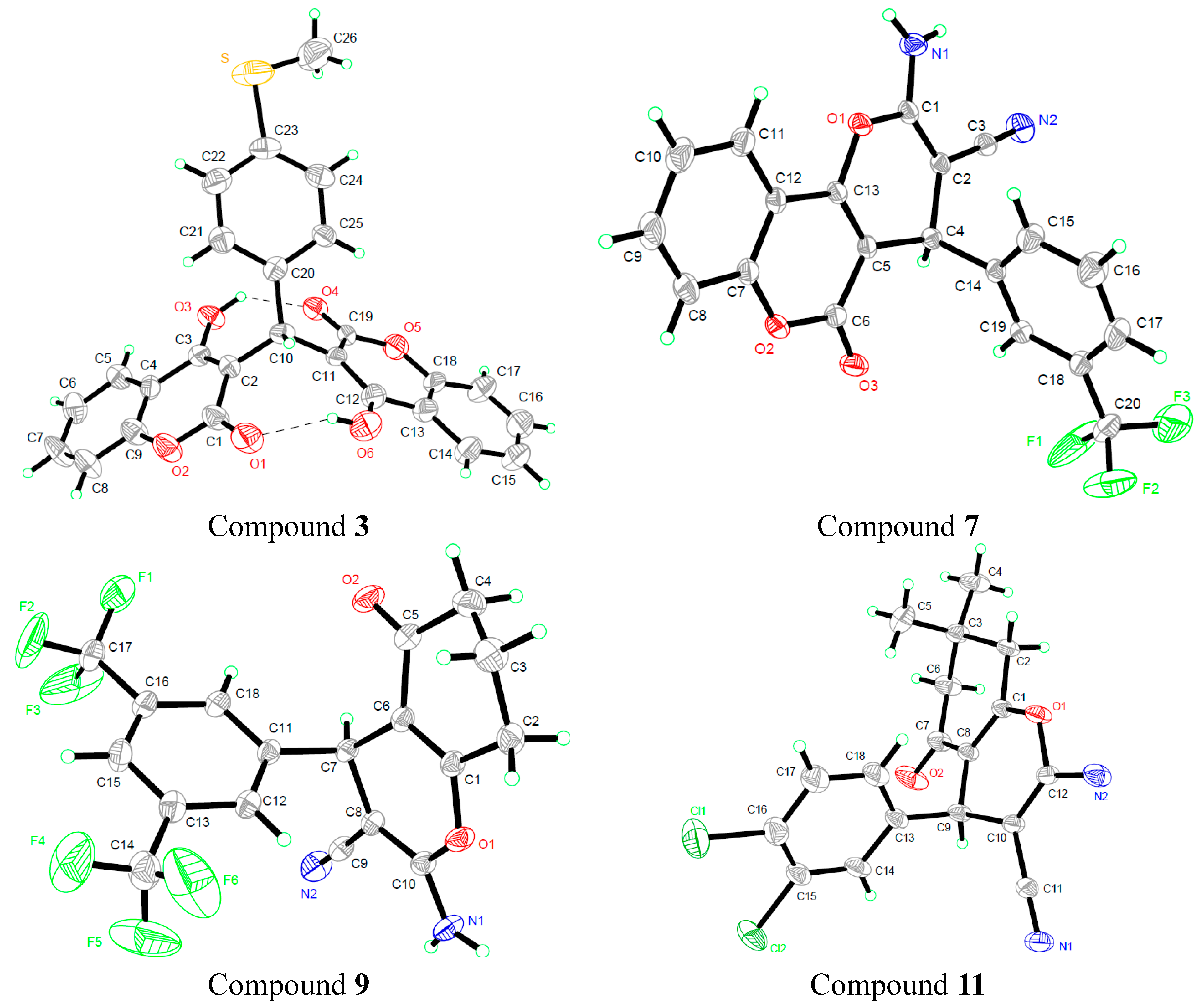
3.3. X-ray Crystallography
| Compound 3 | Compound 7 | Compound 9 | Compound 11 | |
|---|---|---|---|---|
| Formula | C26H18O6S | C20H11F3N2O3 | C18H12F6N2O2 | C18H16Cl2N2O2 |
| Mr | 458.08 | 384.31 | 402.3 | 362.06 |
| Crystal system | Triclinic | Triclinic | Triclinic | Triclinic |
| Space group | Pī | Pī | Pī | Pī |
| a/Å | 10.947 (3) | 7.9416 (6) | 8.5762 (6) | 8.2045 (5) |
| b/Å | 20.402 (7) | 10.9917 (9) | 8.8891 (8) | 14.1658 (8) |
| c/Å | 20.694 (7) | 11.4755 (6) | 12.1026 (8) | 23.3891 (15) |
| α/° | 109.44 (3) | 112.429 (7) | 109.583 (7) | 94.540 (5) |
| β/° | 90.09 (3) | 105.094 (6) | 92.163 (6) | 97.474 (5) |
| γ/° | 93.29 (3) | 97.364 (6) | 97.257 (7) | 98.928 (5) |
| V/Å3 | 4350 (3) | 864.45 (11) | 859.13 (11) | 2648.9 (3) |
| Z | 2 | 2 | 2 | 2 |
| Dcalc/g·cm−3 | 1.401 | 1.447 | 1.555 | 1.357 |
| μ(Mo Kα)/mm−1 | 0.191 | 0.122 | 0.146 | 0.38 |
| θ range/° | 2.43 to 25.00 | 2.74 to 27.68 | 2.43 to 27.35 | 2.76 to 27.51 |
| Reflections collected | 18355 | 5790 | 5819 | 18090 |
| No. unique data [R(int)] | 13442 [0.0377] | 3045 [0.0233] | 3030 [0.0213] | 9317 [0.0442] |
| No. data with I ≥ 2σ(I) | 5431 | 2170 | 2318 | 6552 |
| R1 | 0.0783 | 0.0595 | 0.0745 | 0.0824 |
| ωR2(all data) | 0.2469 | 0.1774 | 0.2276 | 0.261 |
3.4. Quantum Chemical Calculations
3.5. Minimal Inhibitory Concentration (MIC) Assay
3.6. Cell Viability Assay
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.B.; Tiwari, V.K. Natural products: An evolving role in future drug discovery. Eur. J. Med. Chem. 2011, 46, 4769–4807. [Google Scholar] [CrossRef] [PubMed]
- Fylaktakidou, K.C.; Hadjipavlou-Litina, D.J.; Litinas, K.E.; Nicolaides, D.N. Natural and synthetic coumarin derivatives with anti-inflammatory/antioxidant activities. Curr. Pharm. Des. 2004, 10, 3813–3833. [Google Scholar] [CrossRef] [PubMed]
- Ostrov, D.A.; Hernandez Prada, J.A.; Corsino, P.E.; Finton, K.A.; Le, N.; Rowe, T.C. Discovery of novel DNA gyrase inhibitors by high-throughput virtual screening. Antimicrob. Agents Chemother. 2007, 51, 3688–3698. [Google Scholar] [CrossRef] [PubMed]
- Elinos-Baez, C.M.; Leon, F.; Santos, E. Effects of coumarin and 7OH-coumarin on BCL-2 and BAX expression in two human lung cancer cell lines in vitro. Cell Biol. Int. 2005, 29, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Kawaii, S.; Tomono, Y.; Ogawa, K.; Sugiura, M.; Yano, M.; Yoshizawa, Y. The antiproliferative effect of coumarins on several cancer cell lines. Anticancer Res. 2001, 21, 917–923. [Google Scholar] [PubMed]
- Lopez-Gonzalez, J.S.; Prado-Garcia, H.; Aguilar-Cazares, D.; Molina-Guarneros, J.A.; Morales-Fuentes, J.; Mandoki, J.J. Apoptosis and cell cycle disturbances induced by coumarin and 7-hydroxycoumarin on human lung carcinoma cell lines. Lung Cancer 2004, 43, 275–283. [Google Scholar] [CrossRef] [PubMed]
- MacDonagh, L.; Gray, S.G.; Finn, S.P.; Cuffe, S.; O’Byrne, K.J.; Barr, M.P. The emerging role of microRNAs in resistance to lung cancer treatments. Cancer Treat. Rev. 2015, 41, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Kohli, S.; Sandhu, S.; Bansal, Y.; Bansal, G. Coumarin: A Promising Scaffold for Anticancer Agents. Anticancer Agents Med. Chem. 2015, 15, 1032–1048. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Li, J.; Yang, X.H.; Zhang, Z.D.; Luo, X.X.; Li, M.K.; Li, X. New Biscoumarin derivatives: synthesis, crystal Structure, theoretical study and antibacterial activity against Staphylococcus aureus. Molecules 2014, 19, 19868–19879. [Google Scholar] [CrossRef] [PubMed]
- Trendafilova, N.; Bauer, G.; Mihaylov, T. DFT and AIM studies of intramolecular hydrogen bonds in dicoumarols. Chem. Phys. 2004, 302, 95–104. [Google Scholar] [CrossRef]
- Mihaylov, T.; Georgieva, I.; Bauer, G.; Kostova, I.; Manolov, I.; Trendafilova, N. Theoretical study of the substituent effect on the intramolecular hydrogen bonds in di(4-hydroxycoumarin) derivatives. Int. J. Quantum Chem. 2006, 106, 1304–1315. [Google Scholar] [CrossRef]
- Li, J.; Hou, Z.; Chen, G.H.; Li, F.; Zhou, Y.; Xue, X.Y.; Li, Z.P.; Jia, M.; Zhang, Z.D.; Li, M.K.; et al. Synthesis, antibacterial activities, and theoretical studies of dicoumarols. Org. Biomol. Chem. 2014, 12, 5528–5535. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.T.; Lal, M.; Ali, S.; Khan, M.M. One-pot three-component reaction for the synthesis of pyran annulated heterocyclic compounds using DMAP as a catalyst. Tetrahedron Lett. 2011, 52, 5327–5332. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97, Program for Solution Crystal Structure and Refinement; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01, Gaussian, Inc.: Wallingford, CT, USA, 2009.
- Prüller, S.; Frömke, C.; Kaspar, H.; Klein, G.; Kreienbrock, L.; Kehrenberg, C. Recommendation for a standardized method of broth microdilution susceptibility testing for porcine bordetella bronchiseptica. PLoS ONE 2015, 10, e0123883. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–13 are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sui, Y.-P.; Huo, H.-R.; Xin, J.-J.; Li, J.; Li, X.-J.; Du, X.-L.; Ma, H.; Zhou, H.-Y.; Zhan, H.-D.; Wang, Z.-J.; et al. Antibacterial and Antitumor Activities of Biscoumarin and Dihydropyran Derivatives. Molecules 2015, 20, 17614-17626. https://doi.org/10.3390/molecules200917614
Sui Y-P, Huo H-R, Xin J-J, Li J, Li X-J, Du X-L, Ma H, Zhou H-Y, Zhan H-D, Wang Z-J, et al. Antibacterial and Antitumor Activities of Biscoumarin and Dihydropyran Derivatives. Molecules. 2015; 20(9):17614-17626. https://doi.org/10.3390/molecules200917614
Chicago/Turabian StyleSui, Yun-Peng, Hai-Ru Huo, Jia-Jia Xin, Jing Li, Xiao-Jun Li, Xin-Liang Du, Hai Ma, Hai-Yu Zhou, Hong-Dan Zhan, Zhu-Ju Wang, and et al. 2015. "Antibacterial and Antitumor Activities of Biscoumarin and Dihydropyran Derivatives" Molecules 20, no. 9: 17614-17626. https://doi.org/10.3390/molecules200917614
APA StyleSui, Y.-P., Huo, H.-R., Xin, J.-J., Li, J., Li, X.-J., Du, X.-L., Ma, H., Zhou, H.-Y., Zhan, H.-D., Wang, Z.-J., Li, C., Sui, F., & Li, M.-K. (2015). Antibacterial and Antitumor Activities of Biscoumarin and Dihydropyran Derivatives. Molecules, 20(9), 17614-17626. https://doi.org/10.3390/molecules200917614






