Abstract
In this paper, two tetrapyrrolic complexes, Zn(II)-5-(3-hydroxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl)porphyrin and Cu(II)-5-(3-hydroxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl)porphyrin were synthesized, and characterized from a spectral and biological point of view. The study provided data concerning the behavior of identical external substituents vs. two different core insertions. Some of the properties of the proposed tetrapyrrolic structures were highlighted, having photodynamic therapy of cancer as a targeted biomedical application. Elemental analysis, NMR, FTIR and UV-Vis data in various solvents were provided. A preliminary in vitro study on normal and cancer cultured cells was carried out for biocompatibility assessment in dark conditions. The preliminary in vitro study performed on human peripheral mononuclear cells exposed to tetrapyrrolic compounds (2 µM) showed that the proposed compounds had a convenient cytotoxic profile on human normal peripheral blood mononuclear cells under dark conditions. Meanwhile, the investigated compounds reduced the number of metabolically active breast tumor MCF-7 cells, with the exception of Zn(II) complex-containing a symmetrical ligand. Accordingly, preliminary in vitro data suggest that the proposed tetrapyrrolic compounds are good candidates for PDT, as they limit tumor expansion even under dark conditions, whilst sparing normal cells.
1. Introduction
Tetrapyrrolic compounds, especially porphyrins and metalloporphyrins, are still a spearhead in the studies related to diagnosis and photodynamic therapy (PDT) of malignant tumours [1,2,3,4,5,6,7,8,9]. The therapeutic effect of tetrapyrrolic compounds results from their ability to accumulate preferentially in the malignant tissue and to generate, following light-mediated activation in the presence of molecular oxygen, reactive oxygen species responsible for cell death [10,11,12]. The use of porphyrinic compounds as fluorescent markers in the detection of various cancer types is based on their photophysical characteristics, such as emissions in the spectral range 600–800 nm, large Stokes shift and high fluorescence lifetime [2,3].
In diagnostic and therapeutic applications the efficiency of tetrapyrrolic compounds depends on their structural and physical-chemical characteristics [13]. High absorption coefficients in the 600–680 nm spectral range, high singlet oxygen generation quantum yield, acceptable solubility in biologic fluids, selectivity for diseased tissues and superior uptake values, photostability and lack of toxicity in the absence of light excitation are the most important parameters that dictate the efficiency of porphyrins as photosensitizers [1,2,3].
Current mainstream research approaches include more and more complex long-chain studies of both porphyrins and metalloporphyrins configured as third generation photosensitizers [14,15]. The quest for optimal or at least most improved structures, remains active, despite the already clinical approved porphyrin-base drugs [16]. Among metalloporphyrins, some forms with Zn(II) have been recently studied as starting points for future efficient photodynamic agents, mostly with a focus on asymmetrically substituted bases; meanwhile, copper porphyrins are less present in this type of studies, for instance as compounds involved in the side-effects of phototherapy [17,18,19,20].
Taking into account that localization at a subcellular level of the tetrapyrrolic structure is directly influenced by the polarity of the molecule and by the type of metallic ion [21,22], our research has been focused on obtaining porphyrins with various degrees of hydrophobic/hydrophilic substitutions that favor their uptake by cellular targets [23,24,25,26,27,28,29,30]. As a continuation of our research, in this study we describe the synthesis of some new asymmetrical tetrapyrrolic complexes, Zn(II)-5-(3-hydroxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl)porphyrin (P1) and Cu(II)-5-(3-hydroxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl)porphyrin (P2) (Figure 1), their spectral properties and a preliminary in vitro evaluation focused on their dark toxicity in the context of their potential use in the diagnosis and PDT of cancer.
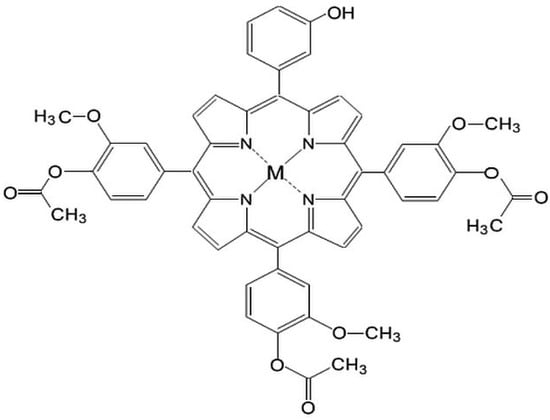
Figure 1.
General structures of M(II)-5-(3-hydroxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl)porphyrin, M = Cu, Zn.
2. Results and Discussion
2.1. Chemistry
The syntheses presented in this study have been successfully repeated several times with identical results. The dry media synthesis procedure was performed in three stages: irradiation followed by cooling and with UV-Vis analytical monitoring of samples. After synthesis and purification, the porphyrinic complexes were characterized by elemental analysis, FTIR, 1H- and 13C-NMR and UV-Vis spectrometry.
The infrared spectral data obtained for the synthesized metalloporphyrins revealed the presence of typical vibration modes of both the tetrapyrrolic macrocycles and phenyl substituents. This is generally in agreement with results previously reported for similar porphyrinic structures [25,26,27,28,29,30]. The large band registered at about 3410 cm−1 can be assigned to the O–H stretching vibration of the –OH functional group, near the typical 3423 cm−1 of the N–H vibration. In the higher wavenumber region at ~2920 cm−1 a medium band was registered, corresponding to C–H vibrations of the phenyl groups. Around 2850 cm−1 the band corresponding to C–H vibration frequencies of the –O–CH3 group was detected. For both synthesized metalloporphyrins the IR signals at 1709 cm−1 can be assigned to the C=O stretching vibration, while the absorption band evidenced at 1104 cm−1 was attributed to the C–O bond vibrations. In the infrared spectra of P1 and P2, the bands corresponding the C=N and C–N stretching vibrations were highlighted in the spectral range of 1490–1510 cm−1 and 1600 cm−1, respectively. Several signals belonging to the pyrrole ring are also present at 975 cm−1 for δC-H and 798 cm−1 for γC-N. The general shape of the spectra remains similar for both complexes even below 1600 cm−1; in this region several medium intensity signals attributed to C–C vibrations as 1397, 1349, 1326 cm−1 or γC-C at 880 and 846 cm−1 are present.
Absorption and Emission Spectra
The complexes were studied by UV-Vis spectroscopy to confirm their structure and to establish their profiles in environments with different polarities. Due to solvent dependence of the position of the Soret band and all the other bands, measurements were concentrated around organic solvents with impact in biomedical studies. Due to the presence of –OH groups at the periphery of the tetrapyrrolic complex, the solubility was increased, but remained an issue for systems containing water.
Parameters of the solvents from the polarity to the donor-acceptor process often cause significant changes in the band energy values leading to shifts in the spectral profiles. As expected, in our particular case the Soret band shifted in a narrow range of 4 nm for P1 but doubled in the case of P2. The relative intensities were sensitive to the nature of the solvent, but within narrow limits as can be seen in Table 1 and Figure 2.

Table 1.
Absorption and emission data of the porphyrinic complexes in various solvents (c = 2.5 × 10−6 M, λex = 420 nm).
| Solvent | Absorption λmax (nm) [lgε (L·mol−1·cm−1)] | Emission λmax (nm) |
|---|---|---|
| Soret Band B Qy (0,0) Qx(1,0) | Q(0,0) Q(0,1) | |
| Zn(II)-5-(3-hydroxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl)porphyrin | ||
| EtOH | 426 [5.63] 558 [4.30] 598 [3.92] | 605 653 |
| Iso-PrOH | 426 [5.61] 559 [4.21] 599 [3.90] | 605 652 |
| DCM | 423 [5.60] 549 [4.30] 588 [3.71] | 603 650 |
| DMF | 428 [5.58] 561 [4.24] 600 [3.94] | 608 656 |
| DMSO | 430 [5.61] 560 [4.26] 602 [4.00] | 609 656 |
| Cu(II)-5-(3-hydroxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl)porphyrin | ||
| EtOH | 413 [5.52] 537 [4.28] | - - |
| Iso-PrOH | 414 [5.58] 538 [4.36] | - - |
| DCM | 416 [5.50] 540 [4.14] | - - |
| DMF | 420 [5.52] 542 [4.20] | - - |
| DMSO | 421 [5.51] 544 [4.30] | - - |
EtOH = ethanol, Iso-PrOH = isopropyl alcohol, DCM = dichloromethane, DMF = dimethylformamide, DMSO = dimethyl sulfoxide.
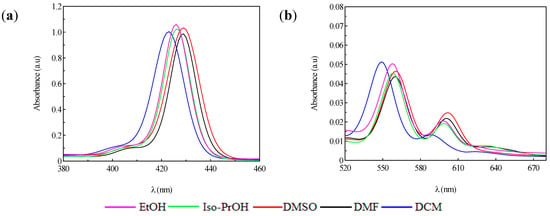
Figure 2.
Absorption spectra of P1 in different solvents; (a) Soret bands; (b) Q bands.
The insertion of an ionic metal in the porphyrinic core led to a decrease of the vibrational modes evidenced by the reduced number of Q bands. In this context, P1 displayed two Q bands, Qy(0,0) and Qx(1,0), respectively. Meanwhile, in the case of P2, the spectrum contained only one Q band.
Figure 3 and Figure 4 show the quenching influence of the more polar solvents both on Soret band and on the Q bands. The general tendencies are the same for P1 and P2, despite the small differences between these compounds, given in part by the different complexation power of the two metals. The absence of P2 emission is in agreement with data reported in the literature [31,32,33]. P1 on the other hand had two distinctive signals. The differences in terms of peak maximum values remained constant and independent of the solvent. Dichloromethane proved to have the most significant hypsochromic influence, suggesting that less polar solvents seem to be more suitable for further biological tests.
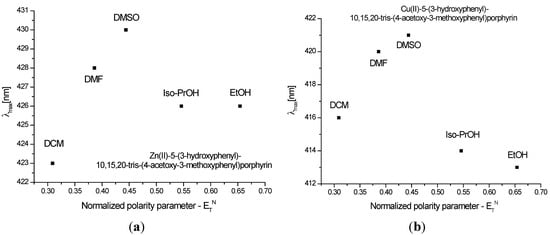
Figure 3.
Reichardt’s normalized polarity parameter vs. wavelengths maxima of the Soret bands for (a) P1 and (b) P2.
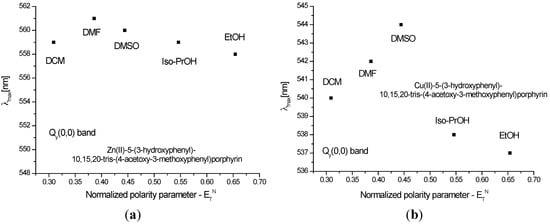
Figure 4.
Reichardt’s normalized polarity parameter vs. wavelength maxima of the Qy(0,0) bands for (a) P1 and (b) P2.
2.2. In Vitro Dark Cytotoxicity Study
Tetrapyrroles for biomedical applications with suitable physico-chemical and photophysical properties, are generally selected in a first stage of development based on their low dark toxicity. This can be assessed by in vitro studies on normal and diseased cells. To this end we studied the effects of the proposed porphyrinic compounds on human cancer cells (the breast human carcinoma MCF-7 cell line) and on human normal peripheral blood mononuclear cells (PBMC). The in vitro study focused on the viability and/or proliferation of the mentioned cells, assessed by the MTS reduction test that provides information regarding the number of metabolically active cells in a culture. We investigated the ligand 5-(3-hydroxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl)-21,23-H-porphyrin (P) [28] and its complexes with Zn(II) and Cu(II) Zn(II)-5-(3-hydroxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl)porphyrin (P1) and Cu(II)-5-(3-hydroxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxy-phenyl)porphyrin (P2). The role of the asymmetry element that was introduced in the structure of the porphyrinic compound was studied in relation to the cytotoxicity profile. Accordingly, the series of symmetric porphyrinic compounds was tested in parallel with the asymmetric ones: 5,10,15,20-meso-tetrakis-(4-acetoxy-3-methoxyphenyl)-21,23-H-porphyrin (P3), Zn(II)-5,10,15,20-meso-tetrakis-(4-acetoxy-3-methoxyphenyl)porphyrin (P4), Cu(II)-5,10,15,20-meso-tetrakis-(4-acetoxy-3-methoxy-phenyl)porphyrin (P5) [29]. The compounds dissolved in DMSO were tested in vitro at the concentration of 2 μM, corresponding to a 0.12% final concentration of DMSO in the cell cultures.
The Effect of Porphyrinic Compounds on Human Tumor MCF-7 Cells
Results presented in Figure 5 show that all the tested compounds at 2 µM concentration did not affect the viability of normal PBMC (P, P2, P3, P4) or even tended increase the MTS reduction (P1, P5).
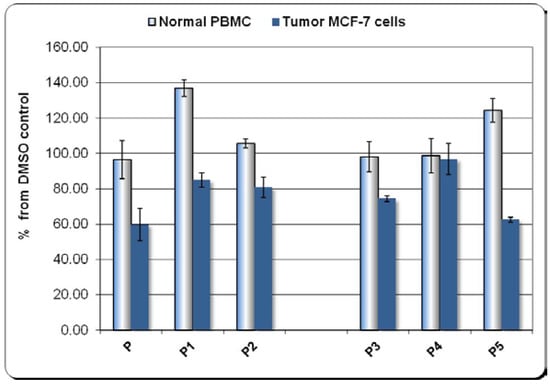
Figure 5.
The effect exerted in vitro by the groups of compounds P, P1, P2 and P3, P4, P5 on the number of metabolically active cells in culture, either on normal human peripheral mononuclear cells (PBMC), or on human breast carcinoma MCF-7 cells. Evaluation by the MTS reduction test. Results were presented as percentage of MTS reduction in presence of compounds from the corresponding DMSO-induced MTS reduction (mean ± SEM for three independent experiments).
Meanwhile, most of the investigated compounds reduced the number of metabolically active tumor cells (P, P1, P2, and P3, P5). This cytotoxic effect was more pronounced in the case of compounds P and P5. In turn, the Zn(II)-containing complex (P4) exerted no major effect on tumor cells. Compounds P and P5 proved to be promising candidates for cancer therapy by drastically inhibiting the growth of tumor cells, whilst having no cytotoxic action on normal PBMC. We emphasize that the Zn(II)-containing compound (P4) had almost no impact on normal and cancer cells
3. Experimental Section
3.1. General Information
Commercially available chemicals and solvents from Sigma-Aldrich (St. Louis, MO, USA) and Merck (Whitehouse Station, NJ, USA) were used for chemical synthesis. For the microwave-assisted synthesis a MWG775H type temperature-controlled microwave oven (Clatronic, Kempen, Germany) was used. The elemental analysis of C, H and N was performed with an automatic 1108 analyzer (Carlo Erba, Milan, Italy). IR spectra were recorded with a FT-IR Tensor 27 spectrophotometer (Bruker, Fremont, CA, USA). The UV-Vis spectra of the porphyrinic complexes were recorded with a Lambda 35 spectrophotometer (Perkin-Elmer, Waltham, MA, USA) and fluorescence spectra were recorded with a FP 6500 spectrofluorimeter (Jasco, Tokyo, Japan). The metalloporphyrin solutions in different organic solvents (ethanol, isopropyl alcohol, dimethylformamide, dichloromethane, dimethyl sulfoxide) were freshly prepared in the spectrally pure solvents at the concentration 2.5 × 10−6 M and kept in dark until the measurement to prevent photodegradation. The NMR spectra of the porphyrinic complex were recorded with a 400 MHz NMR spectrometer (Bruker).
3.2. Synthesis of Tetrapyrrolic Complexes
3.2.1. Synthesis of Zn(II)-5-(3-hydroxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl)porphyrin (P1)
A mixture of 3-hydroxybenzaldehyde (1.22 g, 0.01 mol), methyl 4-formyl benzoate (5.82 g, 0.03 mol), freshly distilled pyrrole (2.76 mL, 0.04 mol), anhydrous zinc chloride (1.36 g, 0.01 mol) and 2.5–3 g of silica gel 60 (200–500 μm, 35–70 mesh) in the presence of 2,6-dimethylpyridine (1 mL) was irradiated in a Pyrex bottle in a microwave oven set at 180 °C, 500 W for 10 min (Scheme 1). The complexation reaction was monitored using UV-Vis spectroscopy and TLC. Extraction of samples was performed after every 2 min of irradiation. TLC test of the final product of reaction revealed the presence of a six metalloporphyrin isomers (A4, A3B, A2B2 (cis and trans), AB3 and B4-type) with high content of A3B isomer. The crude product was first dissolved in dichloromethane/diethyl ether 25:1 (v/v), filtered and purified on a chromatography column by repeated elution, using silica gel (100–200 mesh size) as stationary phase and dichloromethane/diethyl ether 25:1 (v/v) as eluent. The solution of the Zn porphyrinic compound was concentrated by simple distillation, the obtained violet crystals were dried at 100 °C for 12 h. The yield was about 28%. Elemental analysis for C53H40N4O10Zn: calculated (found): C 66.45(66.35), H 4.18(4.10), N 5.85 (5.69). 1H-NMR (CDCl3), δH ppm: 3.90 (s, 9H), 4.0 (s, 9H), 5.98 (s, 1H), 7.15 (d, J = 8.5 Hz, 3H), 7.25 (s, 3H), 7.45 (d, J = 8.5 Hz, 3H), 7.70 (m, 1H), 7.75 (t, 1H), 7.80 (d, J = 8.0 Hz, 1H), 7.0 (s, 1H), 8.80 (d, J = 4.70 Hz, 6H), 8.92 (d, J = 4.72 Hz, 2H). 13C-NMR (CDCl3), δC ppm: 53.6, 76.7, 81.0, 115.0, 118.0, 120.0, 121.8, 122.0, 127.7, 128.0, 130.0, 132.3, 134.8, 139.0, 146.8, 150.3. IR (cm−1): 3408, 2922, 2851, 1709, 1601, 1496, 1434, 1267, 1104, 995, 791, 759. UV-Vis (CH2Cl2) λmax (nm): 423, 550, 588.
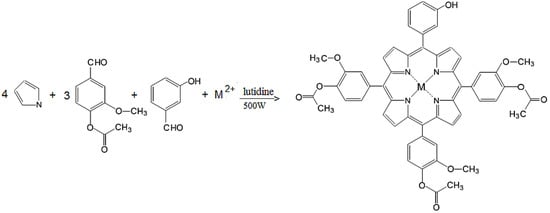
Scheme 1.
General scheme for synthesis of P1 and P2 complexes.
3.2.2. Synthesis of Cu(II)-5-(3-hydroxyphenyl)-10,15,20-tris-(4-acetoxy-3-methoxyphenyl)porphyrin (P2)
The synthesis of P2 was performed by a similar method to that of P1 (Scheme 1), by microwave irradiation at 500 W in the presence of 2,6-dimethylpyridine, of a mixture of 3-hydroxybenzaldehyde (1.22 g, 0.01 mol), methyl 4-formyl benzoate (5.82 g, 0.03 mol), pyrrole (2.76 mL, 0.04 mol) and anhydrous CuCl2 (1.34 g, 0.01 mol). The presence of the porphyrinic complex in the reaction mixture was confirmed by monitoring the Q bands in the visible spectra recorded for extracted samples after every 2 min of irradiation. After cooling, the reaction product was dissolved in dichloromethane/diethyl ether 25:1 (v/v) and then filtered. The solvent was removed under vacuum and the solid product was chromatographically separated on silica gel (100–200 mesh size) with dichloromethane/diethyl ether as eluent. The solution of the Cu porphyrinic complex was concentrated by simple distillation and the obtained dark red crystals were dried at 100 °C for 12 h. The obtained yield was 33%. Elemental analysis for C53H40N4O10Cu: calculated (found) C 66.56 (66.42), H 4.18 (4.10), N 5.86 (5.72). 1H-NMR (CDCl3), δH ppm: 3.90 (s, 9H), 4.15 (s, 9H), 6.10 (s, 1H), 7.18 (d, J = 8.5 Hz, 3H), 7.25 (s, 3H), 7.48 (d, J = 8.5 Hz, 3H), 7.72 (m, 1H), 7.77 (t, 1H), 7.81 (d, J = 8.0 Hz, 1H), 7.0 (s, 1H), 8.78 (d, J = 4.70 Hz, 6H), 8.90 (d, J = 4.72 Hz, 2H).13C-NMR (CDCl3), δC ppm: 53.3, 77.0, 81.0, 115.0, 119.0, 120.0, 121.8, 122.3, 128.0, 128.2, 130.0, 132.3, 134.5, 139.0, 146.8, 152.2. IR (cm−1): 3413, 2919, 2850, 1709, 1603, 1507, 1437, 1277, 1104, 995, 798, 722. UV-Vis (in CH2Cl2) λmax (nm): 416, 540.
3.3. In Vitro Dark Cytotoxicity Tests
3.3.1. Preparation of Tetrapyrrolic Compounds for the in Vitro Study
Tetrapyrrolic compounds were dissolved initially in DMSO and were further diluted in culture medium before the experiments to obtain working concentrations of 2 μM. The final DMSO concentration in cell cultures was 0.12%.
3.3.2. Cells
The human breast carcinoma MCF-7 cell line was purchased from ATCC (ATCC® HTB-22™) and was cultivated in DMEM (Biochrome, Berlin, Germany) culture medium supplemented with 10% fetal bovine serum (FBS, Biochrome) and antibiotic-antimycotic solution (Sigma-Aldrich). Passage of cells was performed at 48 h by trypsinization of adherent MCF-7 cells using 0.25%/0.02% Trypsin/EDTA (Gibco, Life Technologies, Carlsbad, CA, USA). For experiments, MCF-7 cells were plated in 96 well culture plates, at a density of 40,000 cells/cm2. Human peripheral mononuclear cells (PBMC) were isolated from blood samples of 6 healthy volunteers, based on their informed consent. Briefly, 3 mL blood collected in Li-heparin coated vacutainers was layered on Biocoll solution (Biochrome), and was centrifuged for 20 min at 2000 rpm, at room temperature. Mononuclear cells were collected and were washed twice in RPMI 1640 culture medium (Biochrome) by centrifugation for 10 min at 1500 rpm, 4 °C. Cells were finally suspended in RPMI 1640 medium supplemented with 10% FBS (Biochrome) and antibiotic-antimycotic solution (Sigma-Aldrich). Cells were counted in a Burker-Turk hemocytometer. Cellular viability was assessed by the trypan blue exclusion test. Only cell suspensions with viability >95% were used for experiments.
3.3.3. Cell Cultures
For experiments, MCF-7 cells were plated in 96 well culture plates, 7000 cells/well (21,000 cell/cm2), and were cultivated for 24 h to reach adherence. PBMC isolated from 6 healthy volunteers were mixed for obtaining activated PBMC by mixed lymphocyte reaction. PBMC were thereafter plated in 96 well culture plates, 105 cells/well. Cells were treated with the test substances (2 μM concentration). Non-treated cells and cells treated with the solvent (DMSO) were used as controls. Samples containing culture medium and dilutions of the test substances were used for background assessment. Final sample volume in experiments was 100 μL. All samples were plated in triplicate. Cell cultivation was performed at 37 °C, in 5% CO2 atmosphere.
3.3.4. The MTS Reduction Test
The MTS reduction test was used for assessing the number of metabolically active cells in culture, using the CellTiter 96® AQueous One Solution Cell Proliferation Assay (Promega, Madison, WI, USA). Cells were cultivated as described above, and treated with test substances for 48 h. At the end of the treatment time, 20 μL of the kit reagent were added to each well of the culture plate. Cells were further incubated for 3 h at 37 °C, in 5% CO2 atmosphere. The optical density (OD) of samples was measured at 490 nm against 620 nm reference, using a Sunrise ELISA reader (Tecan, Männedorf, Schweiz). Final OD in cellular samples was calculated by subtracting the OD of corresponding background samples. Data were processed as percentage of test sample OD relative to DMSO-corresponding OD, and were presented as mean ± standard error of the mean (SEM) for independent experiments.
4. Conclusions
The spectral data confirmed the expected general profiles of the studied tetrapyrrolic complexes, and placed them in suitable position for the next step in our quest to identify convenient photosensitizers. The preliminary in vitro study indicated that the new porphyrinic complexes did not display dark condition cytotoxic effects on normal PBMC, and generally reduced the number of metabolically active tumor MCF-7 cells. Taking into account that an appropriate PS candidate for efficient and safe PDT in tumors should minimally affect normal and diseased cells under dark conditions, the Zn(II)-containing compounds proved to be more convenient for potential biomedical use than Cu(II)-containing ones.
Author Contributions
All authors conceived and designed the structure of the paper and contributed to write the manuscript. R. Socoteanu, R. Boscencu, G. Vasiliu and A.S. Oliveira performed the synthesis and spectral characterized porphyrinic complexes. G. Manda designed the preliminary in vitro study, performed cellular experiments on the proposed tetrapyrrolic complexes, analyzed the ensuing experimental data, and contributed to the elaboration of the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ethirajan, M.; Chen, Y.; Josi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.P.; Spring, B.Q.; Rizvi, I.; Evans, C.L.; Samkoe, K.S.; Verma, S.; Pogue, B.W.; Hasan, T. Imaging and Photodynamic Therapy: Mechanisms, Monitoring, and Optimization. Chem. Rev. 2010, 110, 2795–2838. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.F.; Liu, T.W.B.; Chen, J.; Zheng, G. Activatable Photosensitizers for Imaging and Therapy. Chem. Rev. 2010, 110, 2839–2857. [Google Scholar] [CrossRef] [PubMed]
- Detty, M.R.; Gibson, S.L.; Wagner, S.J. Current Clinical and Preclinical Photosensitizers for Use in Photodynamic Therapy. J. Med. Chem. 2004, 47, 3897–3915. [Google Scholar] [CrossRef] [PubMed]
- Hilderbrand, S.; Weissleder, R. Near-infrared fluorescence: Application to in vivo molecular imaging. Curr. Opin. Chem. Biol. 2010, 14, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, D.K.; Fong, L.S.; Zhang, Y. Nanoparticles in photodynamic therapy: An emerging paradigm. Adv. Drug Deliv. Rev. 2008, 60, 1627–1637. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, S.; Bizet, C.V.; Mojzisova, H.; Brault, D. Tetrapyrrole-photosensitizers vectorization and plasma LDL: A physic-chemical approach. Int. J. Pharm. 2007, 344, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Moylan, C.; Sweed, A.M.K.; Shaker, Y.M.; Scanlan, E.M.; Senge, M.O. Lead structures for applications in photodynamic therapy. Efficient synthesis of amphiphilic glycosylated lipid porphyrin derivatives: Refining linker conjugation for potential PDT applications. Tetrahedron 2015, 71, 4145–4153. [Google Scholar] [CrossRef]
- O’Connor, A.E.; Gallagher, W.M.; Byrne, A.T. Porphyrin and nonporphyrin photosensitizers in oncology: Preclinical and clinical advances in photodynamic therapy. Photochem. Photobiol. 2009, 85, 1053–1074. [Google Scholar] [CrossRef] [PubMed]
- Stockert, J.C.; Cañete, M.; Juarranz, A.; Villanueva, A.; Horobin, R.W.; Borrell, J.I.; Teixidó, J.; Nonell, S. Porphycenes: Facts and prospects in photodynamic therapy of cancer. Curr. Med. Chem. 2007, 14, 997–1026. [Google Scholar] [CrossRef] [PubMed]
- Grosseweiner, L.I. The Science of Phototherapy; CRC Press: London, UK, 1994; Chapter 8; pp. 139–155. [Google Scholar]
- Schweiter, C.; Schmidt, R. Physical mechanisms of generation and deactivation of singlet oxygen. Chem. Rev. 2003, 103, 1685–1758. [Google Scholar] [CrossRef] [PubMed]
- Kessel, D. Correlation between subcellular localization and photodynamic efficacy. J. Porph. Phthal. 2004, 8, 1009–1014. [Google Scholar] [CrossRef]
- Shakiba, M.; Chen, J.; Zheng, G. Porphyrin nanoparticles in photomedicine, in Applicat. Nanosci. Photomed. 2015, 24, 511–526. [Google Scholar]
- Tovmasyan, A.; Babayan, N.; Poghosyan, D.; Margaryan, K.; Harutyunyan, B.; Grigoryan, R.; Sarkisyan, N.; Spasojevic, I.; Mamyan, S.; Sahakyan, L.; et al. Novel amphiphilic cationic porphyrin and its Ag(II) complex as potential anticancer agents. J. Inorg. Biochem. 2014, 140, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.W.; Huynh, E.; MacDonald, T.D.; Zheng, G. Porphyrins for Imaging, Photodynamic Therapy and Photothermal Therapy. In Cancer Theranostics; Chen, X., Wong, S., Eds.; Academic Press: Waltham, MA, USA, 2014; pp. 229–254. [Google Scholar]
- Goncalves, P.J.; Correa, D.S.; Franzen, P.L.; De Boni, L.; Almeida, L.M.; Mendonca, C.R.; Borissevitch, I.E.; Zilio, S.C. Effect of interaction with micelles on the excited-state optical properties of zinc porphyrins and J-aggregates formation. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 112, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Phukan, S.; Mishra, B.; Chandra Shekar, K.P.; Kumar, A.; Kumar, D.; Mitra, S. Fluorescence spectroscopic studies on substituted porphyrins in homogeneous solvents and cationic micellar medium. J. Lumin. 2013, 134, 232–239. [Google Scholar] [CrossRef]
- Kee, H.L.; Bhaumik, J.; Diers, J.R.; Mroz, P.; Hamblin, M.R.; Bocian, D.F.; Lindsey, J.S.; Holten, D. Photophysical characterization of imidazolium-substituted Pd(II), In(III), and Zn(II) porphyrins as photosensitizers for photodynamic therapy. J. Photochem. Photobiol. A Chem. 2008, 200, 346–355. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, F. Bilirubin Copper-Porphyrins and the Bronze-Baby Syndrome. J. Pediatr. 2011, 1, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Rosenkranz, A.A.; Jans, D.A.; Sobolev, A.S. Targeted intracellular delivery of photosensitizers to enhance photodynamic efficiency. Immunol. Cell Biol. 2002, 78, 452–464. [Google Scholar] [CrossRef] [PubMed]
- Osterloh, J.; Vicente, M.G.H. Mechanisms of porphyrinoid localization in tumors. J. Porph. Phthal. 2002, 5, 305–325. [Google Scholar] [CrossRef]
- Boscencu, R.; Socoteanu, R.; Oliveira, A.S.; Vieira Ferreira, L.F.; Nacea, V.; Patrinoiu, G. Synthesis and characterization of some unsymmetrically-substituted mesoporphyrinic mono-hydroxyphenyl complexes of Copper(II). Pol. J. Chem. 2008, 82, 509–522. [Google Scholar]
- Boscencu, R.; Socoteanu, R.; Oliveira, A.S.; Ferreira, L.F.V. Studies on Zn(II) monohydroxyphenyl mesoporphyrinic complexes. Synthesis and characterization. J. Serb. Chem. Soc. 2008, 73, 713–726. [Google Scholar] [CrossRef]
- Boscencu, R.; Ilie, M.; Socoteanu, R.; Oliveira, A.S.; Constantin, C.; Neagu, M.; Manda, G.; Ferreira, L.F.V. Microwave synthesis, basic spectral and biological evaluation of some Copper(II) mesoporphyrinic complexes. Molecules 2010, 15, 3731–3743. [Google Scholar] [CrossRef] [PubMed]
- Boscencu, R. Unsymmetrical mesoporphyrinic complexes of Copper(II) and Zinc(II). Microwave-assisted synthesis, spectral characterization and cytotoxicity evaluation. Molecules 2011, 16, 5604–5617. [Google Scholar] [CrossRef]
- Boscencu, R.; Oliveira, A.S.; Ferreira, D.P.; Ferreira, L.F.V. Synthesis and spectral evaluation of some unsymmetrical mesoporphyrinic complexes. Int. J. Mol. Sci. 2012, 13, 8112–8125. [Google Scholar] [CrossRef] [PubMed]
- Boscencu, R.; Socoteanu, R.; Vasiliu, G.; Nacea, V. Synthesis under solvent free conditions of some unsymmetrically substituted porphyrinic compounds. Rev. Chim. 2014, 65, 888–891. [Google Scholar]
- Boscencu, R. Microwave Synthesis under Solvent-Free Conditions and Spectral Studies of Some Mesoporphyrinic Complexes. Molecules 2012, 17, 5592–5603. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, L.F.V.; Ferreira, D.P.; Oliveira, A.S.; Boscencu, R.; Socoteanu, R.; Ilie, M.; Constantin, C.; Neagu, M. Synthesis, Photophysical and Cytotoxicity Evaluation of A3B Type Mesoporphyrinic Compounds. Dyes Pigment. 2012, 95, 296–303. [Google Scholar] [CrossRef]
- Gouterman, M. Optical Spectra and Electronic Structure of Porphyrins and Related Rings. In The Porphyrins; Dolphin, D., Ed.; Academic Press: New York, NY, USA, 1978; Volume 3, pp. 11–87. [Google Scholar]
- Gouterman, M.; Wagniere, G.H.; Snyder, L.C. Spectra of porphyrins: Part II. Four orbital model. J. Mol. Spectrosc. 1963, 11, 108–127. [Google Scholar]
- Rosa, A.; Ricciardi, G. Biophysical and Physiochemical Studies of Tetrapyrroles. In Handbook of Porphyrin Science; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; World Scientific Publishing Co.: Singapore, 2012; Volume 22, pp. 170–236. [Google Scholar]
- Sample Availability: Not available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).