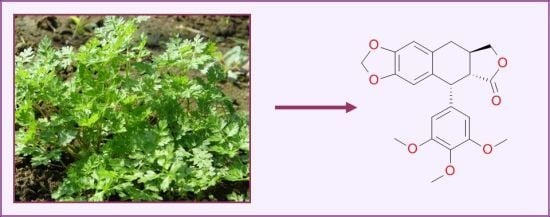
Ethnomedicinal, Phytochemical and Pharmacological Profile of Anthriscus sylvestris as an Alternative Source for Anticancer Lignans
Abstract
:1. Introduction
2. Botany
2.1. Taxonomical Classification
2.2. Etymology
2.3. Distribution and Habitat
2.4. Botanical Description
3. Ethnomedicinal Uses
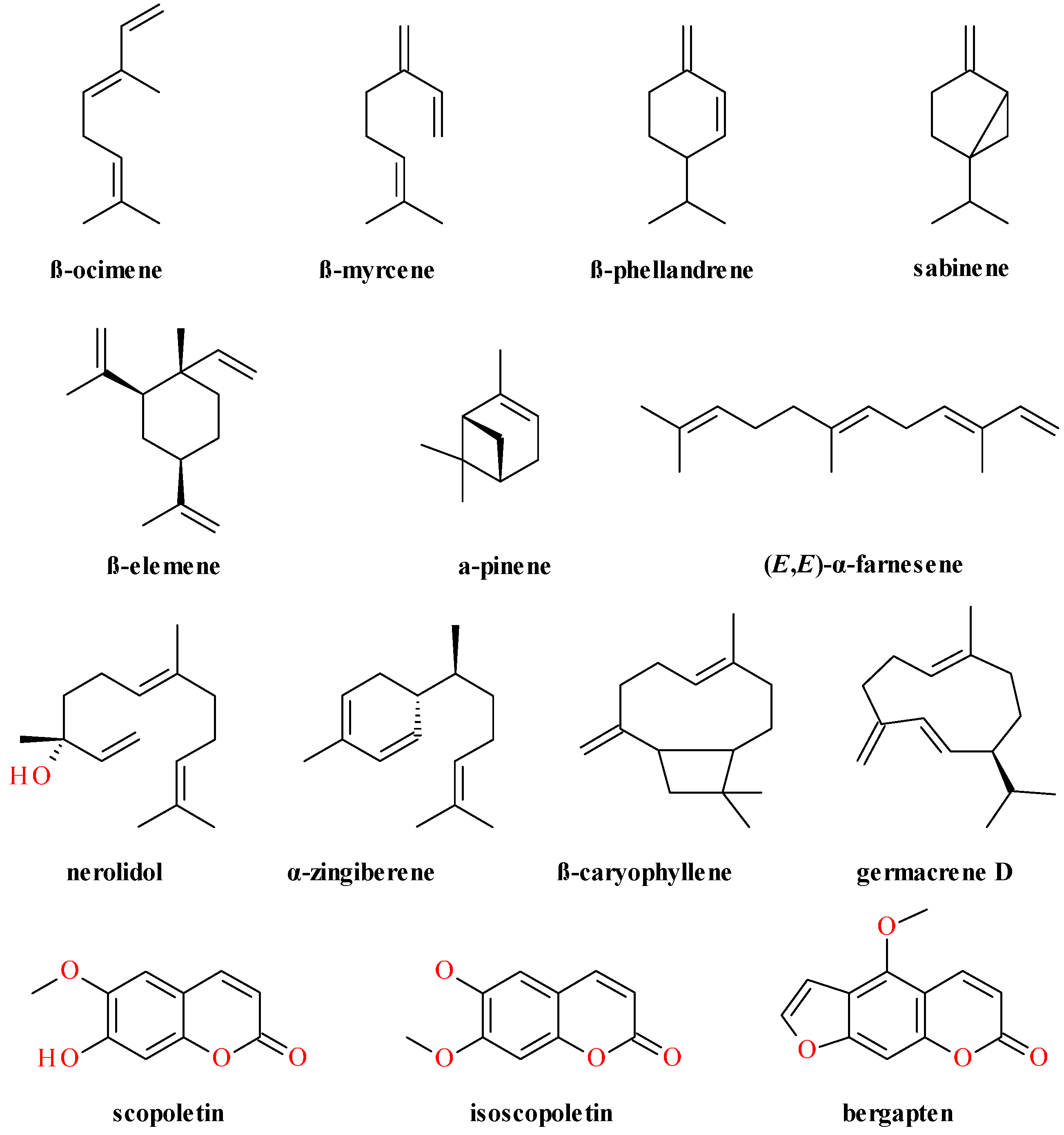
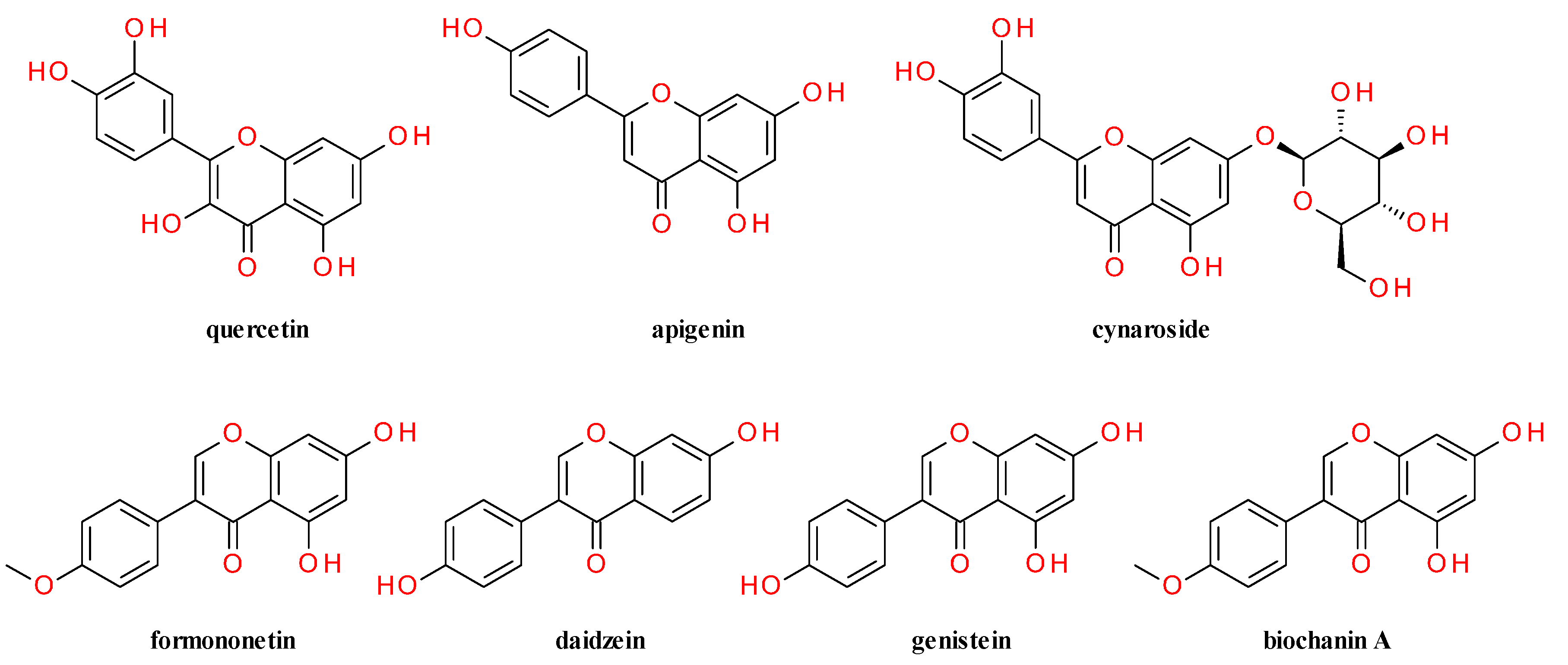
4. Phytochemical Profile
4.1. Root
4.2. Aerial Parts
4.3. Fruits
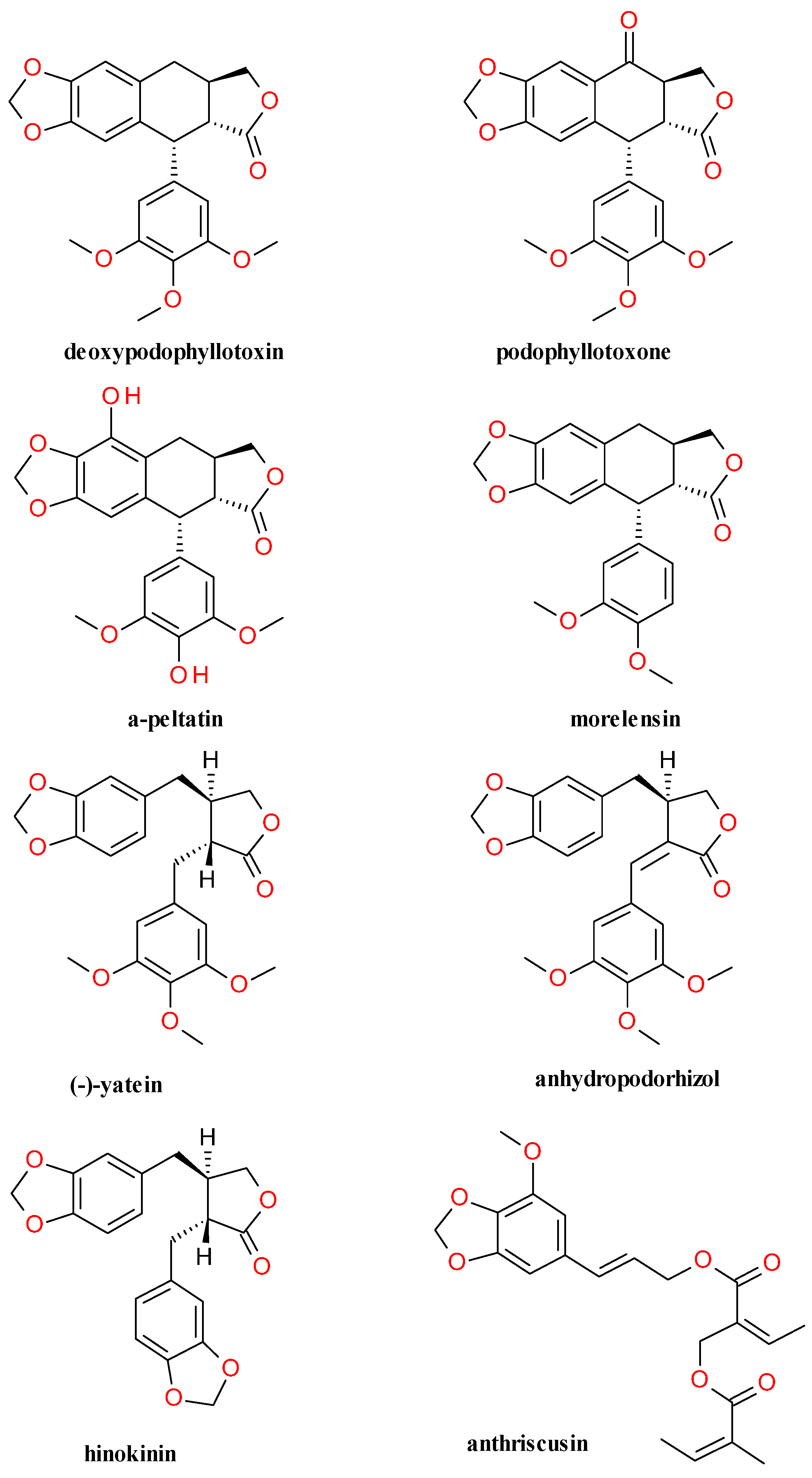
4.4. Lignans Profile and Content
| Genus and Family | Species | Part Used and Content |
|---|---|---|
| Juniperus (Cupressaceae) | J. taxifolia | Leaves (0.004%) [77] |
| J. sabina | Leaves (0.125%) [78,79] | |
| J. davurica | Leaves (0.727%) [69] | |
| J. communis | Leaves and stems (0.007%) [80,81] Stems (0.008–0.017%) [70] | |
| J. blaaws | Stems (0.008%) [70] | |
| J. x-media | Stems (0.024%) [70] | |
| J. squamata | Stems (0.025%) [70] | |
| J. recurva | Stems (0.011%) [70] | |
| J. bermudiana | Leaves (0.44%) [82] | |
| Callitris (Cupressaceae) | C. endlicheri | Leaves (0.392%–0.577%); stems (0.198%); cones (0.053%) [83]. |
| C. columellaris | Leaves (0.062%) [84,85] | |
| C. rhomboidea. | Leaves (0.178%–0.350%); stems (0.119%); cones (0.066%) [83] | |
| C. preissii | Leaves (0.010%–0.015%); stems (0.008%); cones (0.003%) [83] | |
| C. drummondii | Leaves (0.010%–0.015%) [83,86]; stems (0.008%); cones (0.003%) [83] | |
| Bridelia (Euphorbiaceae) | B. ferruginea | Roots (0.001%) [87] |
| B. microphylla | Stems (0.09%) [88]; fruit [89] | |
| B. morelensis | Stems (0.31%) [84,88] | |
| B. permollis | Stems (0.004%) [84,88] | |
| Macrococculus (Menispermaceae) | M. pomiferus | Dried stems (0.001%) [90] |
| Podophyllum (Berberidaceae) | P. peltatum | Roots (0.023%) [84,88,91] |
| Sinopodophyllum (Berberidaceae) | S. hexandrum | Roots and rhizomes (0.008%) [92,93] |
| Dysosma (Berberidaceae) | Dysosma versipellis | Roots (0.504%) [94] |
5. Pharmacology
5.1. Antitumor Activity
5.1.1. Deoxypodophyllotoxin
5.1.2. Semisynthetic Derivatives
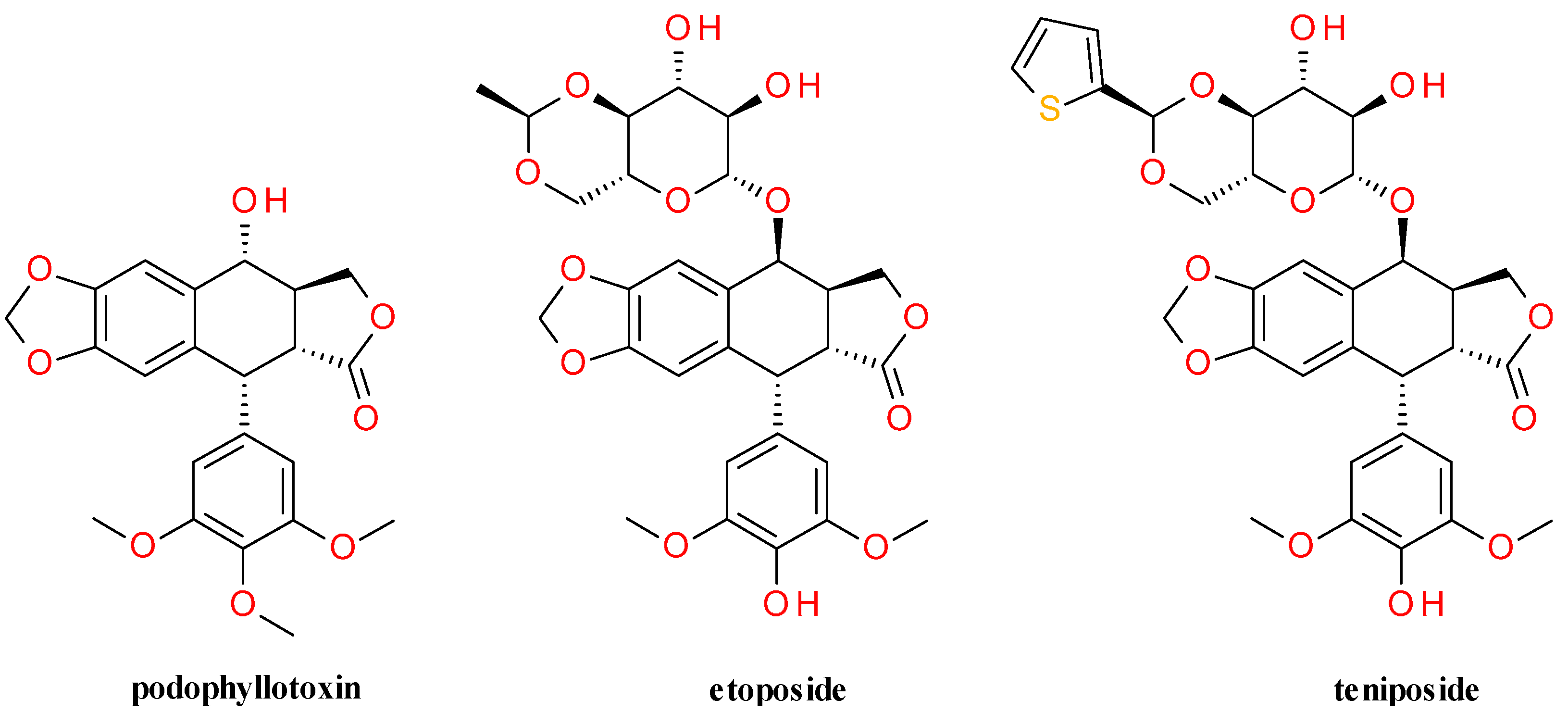
5.2. Antimicrobial Activity
5.3. Anti-Inflammatory Activity
5.4. Antioxidant Activity
6. Biotechnology Applications
6.1. Callus Tissues
6.2. Heterologous Expression System
7. Conclusions
Acknowledgements
Author Contributions
Conflicts of Interest
References
- Plunkett, G.M.; Soltis, D.E.; Soltis, P.S. Evolutionary patterns in Apiaceae: Inferences based on matK sequence data. Syst. Bot. 1996, 21, 477–495. [Google Scholar] [CrossRef]
- Hultén, E.; Fries, M. Atlas of North European Vascular Plants North of the Tropic of Cancer; Koeltz Scientific Books: Königstein, Federal Republic of Germany, 1984; Volume 2, p. 694. [Google Scholar]
- Kozawa, M.; Baba, K.; Matsuyama, Y.; Kido, T.; Sakai, M.; Takemoto, T. Components of the root of Anthriscus sylvestris Hoffm. II. Insecticidal activity. Chem. Pharm. Bull. (Tokyo) 1982, 30, 2885–2888. [Google Scholar] [CrossRef]
- Ikeda, R.; Nagao, T.; Okabe, H.; Nakano, Y.; Matsunaga, H.; Katano, M.; Mori, M. Antiproliferative constituents in umbelliferae plants. III. Constituents in the root and the ground part of Anthriscus sylvestris Hoffm. Chem. Pharm. Bull. (Tokyo) 1998, 46, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Chang, Y.L.; Teng, C.M.; Chen, I.S. Anti-platelet aggregation alkaloids and lignans from Hernandia nymphaeifolia. Planta Med. 2000, 66, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Sudo, K.; Konno, K.; Shigeta, S.; Yokota, T. Inhibitory effects of podophyllotoxin derivatives on herpes simplex virus replication. Antivir. Chem. Chemother. 1998, 9, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, E.; Abad, A.; Montenegro, G.; Del Olmo, E.; López-Pérez, J.L.; San Feliciano, A. Analgesic and anti-inflammatory activity of podophyllotoxin derivatives. Pharm. Biol. 2013, 51, 566–72. [Google Scholar] [CrossRef] [PubMed]
- Khaled, M.; Jiang, Z.Z.; Zhang, L.Y. Deoxypodophyllotoxin: A promising therapeutic agent from herbal medicine. J. Ethnopharmacol. 2013, 149, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Spalik, K.; Jarvis, C.E. Typification of Linnaean Names Now in Anthriscus (Apiaceae). Taxon 1989, 38, 288–293. [Google Scholar] [CrossRef]
- Spalik, K. Typification of the Boissier names in Anthriscus Pers. (Apiaceae). Bot. J. Linn. Soc. 1995, 119, 77–86. [Google Scholar] [CrossRef]
- Downie, S.R.; Katz-Downie, D.S.; Spalik, K.A. Phylogeny of Apiaceae Tribe Scandiceae: Evidence from Nuclear Ribosomal DNA Internal Transcribed Spacer Sequences. Am. J. Bot. 2000, 87, 76–95. [Google Scholar] [CrossRef] [PubMed]
- Haines, A. Flora Novae Angliae: A Manual for the Identification of Native and Naturalized Higher Plants of New England; Yale University Press: New Haven, UK, 2011; pp. 336–338. [Google Scholar]
- Reduron, J.P.; Spalik, K. Le genre Anthriscus (Apiaceae) dans la flore française. Acta Bot. Gallica 1995, 142, 55–96. [Google Scholar] [CrossRef]
- Bojnanský, V.; Fargašová, A. Atlas of Seeds and Fruits of Central and East-European Flora: The Carpathian Mountains Region; Springer: Dordrecht, The Netherlands, 2007; pp. 443–474. [Google Scholar]
- Spalik, K. Species boundaries, phylogenetic-relationships, and ecological differentiation in anthriscus (Apiaceae). Plant Syst. Evol. 1996, 199, 17–32. [Google Scholar] [CrossRef]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea: Rosaceae to Umbelliferae; Cambridge University Press: New York, NY, USA, 1968; Volume 2, p. 326. [Google Scholar]
- Nickavar, B.; Mojab, F.; Mojahedi, A. Composition of the essential oil from Anthriscus nemorosa. Chem. Nat. Compd. 2009, 45, 443–444. [Google Scholar] [CrossRef]
- Magnusson, S.H. Invasive Alien Species Fact Sheet Anthriscus sylvestris. European Network on Invasive Alien Species. Available online: www.nobanis.org/globalassets/speciesinfo (accessed on 10 April 2015).
- Gledhill, D. The Names of the Plants; Cambridge University Press: New York, NY, USA, 2008; p. 51. [Google Scholar]
- Marafioti, R.L. The meaning of generic names of important economic plants. Econ. Bot. 1970, 24, 189–207. [Google Scholar] [CrossRef]
- Deforce, K. The historical use of laudanum. Palynological evidence from 15th and 16th century cesspits in northern Belgium. Veg. Hist. Archaeobot. 2006, 15, 145–148. [Google Scholar] [CrossRef]
- Bailey, L.H. How Plants Get Their Names; Dover Publications, Inc.: New York, NY, USA, 1963; pp. 126–176. [Google Scholar]
- Baskin, C.C.; Milberg, P.; Andersson, L.; Baskin, J.M. Deep complex morphophysiological dormancy in seeds of Anthriscus sylvestris (Apiaceae). Flora 2000, 195, 245–251. [Google Scholar]
- Walton, D.W.H. European weeds and other alien species in the Subantarctic. Weed Res. 1975, 15, 271–282. [Google Scholar] [CrossRef]
- Webb, C.J.; Sykes, W.R.; Garnock-Jones, P.J.; Given, D.R.; Brownsey, P.J. Checklist of dicotyledons, gymnosperms, and pteridophytes naturalised in New Zealand: Additional records and corrections. New Zeal. J. Bot. 1989, 27, 139–162. [Google Scholar] [CrossRef]
- Townsend, C.C. One New and One Disjunct Variety of Umbelliferae from East Africa. Kew Bull. 1984, 39, 603–605. [Google Scholar] [CrossRef]
- Ullmann, I.; Bannister, P.; Wilson, J.B. The vegetation of roadside verges with respect to environmental gradients in southern New Zealand. J. Veg. Sci. 1995, 6, 131–142. [Google Scholar] [CrossRef]
- Hansson, M.L.; Goransson, A. Growth and Biomass Partitioning of Anthriscus sylvestris (L) Hoffm and Festuca ovina (L.) at Different Relative Addition Rates of Nitrogen. Plant Soil 1993, 155, 187–190. [Google Scholar] [CrossRef]
- Smith, R.S.; Shiel, R.S.; Millward, D.; Corkhill, P.; Sanderson, R.A. Soil seed banks and the effects of meadow management on vegetation change in a 10-year meadow field trial. J. Appl. Ecol. 2002, 39, 279–293. [Google Scholar] [CrossRef]
- Hansson, M.L.; Persson, T.S. Anthriscus sylvestris—A growing conservation problem. Ann. Bot. Fenn. 1994, 31, 205–213. [Google Scholar]
- Vanmierlo, J.E.M.; Vangroenendael, J.M. A Population-Dynamic Approach to the Control of Anthriscus sylvestris (L) Hoffm. J. Appl. Ecol. 1991, 28, 128–139. [Google Scholar] [CrossRef]
- Chang, J.; Guan, B.; Ge, Y.; Chan, Y.G. Comparative studies on phenotypic plasticity of two herbs, Changium smyrnioides and Anthriscus sylvestris. J. Zhejiang Univ. Sci. 2004, 5, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Sheng, H. Spatial distribution patterns and environmental interpretation of Anthriscus sylvestris clonal buds. Front. For. China 2008, 3, 449–455. [Google Scholar] [CrossRef]
- Hadač, E. Ruderal vegetation of the Broumov basin, NE. Bohemia. Folia Geobot. Phytotaxon. 1978, 13, 129–163. [Google Scholar] [CrossRef]
- Silvertown, J.; Tremlett, M. Interactive Effects of Disturbance and Shade upon Colonization of Grassland: An Experiment with Anthriscus sylvestris (L.) Hoffm., Conium maculatum L., Daucus carota L. and Heracleum sphondylium L. Funct. Ecol. 1989, 3, 229–235. [Google Scholar] [CrossRef]
- Zarzycki, K.; Trzcinska-Tacik, H.; Rózanski, W.; Szelag, Z.; Wolek, J.; Korzeniak, U. Ecological Indicator Values of Vascular Plant of Poland; W. Szafer Institute of Botany, Polish Academy of Sciences: Kraków, Poland, 2002; p. 183. [Google Scholar]
- Sell, P.; Murrell, G. Flora of Great Britain and Ireland: Mimosaceae to Lentibulariaceae; Cambridge University Press: New York, NY, USA, 2009; Volume 3, pp. 253, 258, 269. [Google Scholar]
- Darbyshire, S.; Hoeg, R.; Haverkort, J. The biology of Canadian weeds. 111. Anthriscus sylvestris (L.) Hoffm. Can. J. Plant Sci. 1999, 79, 671–682. [Google Scholar] [CrossRef]
- De Craene, R.L.P. Floral Diagrams: An Aid to Understanding Flower Morphology and Evolution; Cambridge University Press: New York, NY, USA, 2010; pp. 341–343. [Google Scholar]
- Butcher, R.W. A New Illustrated British Flora; Leonard Hill Limited: London, UK, 1961; pp. 100–102. [Google Scholar]
- Spalik, K.; Wojewódzka, A.; Downie, S.R. The evolution of fruit in Scandiceae subtribe Scandicinae (Apiaceae). Can. J. Bot. 2001, 79, 1358–1374. [Google Scholar]
- Allen, D.E.; Hatfield, G. Medicinal Plants in Folk Tradition: An Ethnobotany of Britain and Ireland; Timber Press: Cambridge, UK, 2004; pp. 182–183. [Google Scholar]
- Wahida, B.; Amor, M.; Nabil, C. An Inventory of Ethnomedicinal Plants Used in Tunisia. In Ethnomedicinal Plants: Revitalization of Traditional Knowledge of Herbs; Rai, M., Acharya, D., Rios, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 2011; p. 336. [Google Scholar]
- Milovanovic, M.; Banjac, N.; Vucelic-Radovic, B. Functional food: Rare herbs, seeds and vegetable oils as sources of flavors and phytosterols. J. Agric. Sci. (Belgrade) 2009, 54, 81–94. [Google Scholar] [CrossRef]
- Yong, Y.; Shin, S.Y.; Lee, Y.H.; Lim, Y. Antitumor activity of deoxypodophyllotoxin isolated from Anthriscus sylvestris: Induction of G2/M cell cycle arrest and caspase-dependent apoptosis. Bioorg. Med. Chem. Lett. 2009, 19, 4367–4371. [Google Scholar] [CrossRef] [PubMed]
- Gairola, S.; Sharma, J.; Bedi, Y.S. A cross-cultural analysis of Jammu, Kashmir and Ladakh (India) medicinal plant use. J. Ethnopharmacol. 2014, 155, 925–986. [Google Scholar] [CrossRef] [PubMed]
- Özqeltk, H. Notes on economic plants. Econ. Bot. 1994, 48, 214–221. [Google Scholar] [CrossRef]
- Borg-Karlson, A.K.; Valterová, I.; Nilsson, L.A. Volatile compounds from flowers of six species in the family Apiaceae: Bouquets for different pollinators? Phytochemistry 1993, 35, 111–119. [Google Scholar] [CrossRef]
- Chen, H.; Jiang, H.Z.; Li, Y.C.; Wei, G.Q.; Geng, Y.; Ma, C.Y. Antitumor constituents from Anthriscus sylvestris (L.) Hoffm. Asian Pac. J. Cancer Prev. 2014, 15, 2803–2807. [Google Scholar] [CrossRef] [PubMed]
- Chupakhina, G.N.; Maslennikov, P.V. Plant adaptation to oil stress. Russ. J. Ecol. 2004, 35, 290–295. [Google Scholar] [CrossRef]
- Bos, R.; Koulman, A.; Woerdenbag, H.J.; Quax, W.J.; Pras, N. Volatile components from Anthriscus sylvestris (L.) Hoffm. J. Chromatogr. A 2002, 966, 233–238. [Google Scholar] [CrossRef]
- Jeong, G.S.; Kwon, O.K.; Park, B.Y.; Oh, S.R.; Ahn, K.S.; Chang, M.J.; Oh, W.K.; Kim, J.C.; Min, B.S.; Kim, Y.C.; et al. Lignans and coumarins from the roots of Anthriscus sylvestris and their increase of caspase-3 activity in HL-60 cells. Biol. Pharm. Bull. 2007, 30, 1340–1343. [Google Scholar] [CrossRef] [PubMed]
- Kramer, M.; Mühleis, A.; Conrad, J.; Leitenberger, M.; Beifuss, U.; Carle, R.; Kammerer, D.R. Quantification of Polyacetylenes in Apiaceous Plants by High-Performance Liquid Chromatography Coupled with Diode Array Detection. Z. Naturforsch. C. 2011, 66, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Hendrawati, O.; Woerdenbag, H.J.; Michiels, P.J.A.; Aantjes, H.G.; Van Dam, A.; Kayser, O. Identification of lignans and related compounds in Anthriscus sylvestris by LC-ESI-MS/MS and LC-SPE-NMR. Phytochemistry 2011, 72, 2172–2179. [Google Scholar] [CrossRef] [PubMed]
- Kamil, W.M.; Dewick, P.M. Biosynthetic relationship of aryltetralin lactone lignans to dibenzylbutyrolactone lignans. Phytochemistry 1986, 25, 2093–2102. [Google Scholar] [CrossRef]
- Koulman, A.; Kubbinga, M.E.; Batterman, S.; Woerdenbag, H.J.; Pras, N.; Woolley, J.G.; Quax, W.J.A. Phytochemical study of lignans in whole plants and cell suspension cultures of Anthriscus sylvestris. Planta Med. 2003, 69, 733–738. [Google Scholar] [PubMed]
- Kurihara, T.; Kikuchi, M. Studies on the constituents of Anthriscus sylvestris Hoffm. II. On the components of the flowers and leaves (author’s transl). Yakugaku Zasshi 1979, 99, 602–606. [Google Scholar] [PubMed]
- Milovanovic, M.; Milovanovic, M.; Picuric-Jovanovic, K.; Picuric-Jovanovic, K.; Vucelic-Radovic, B.; Vucelic-Radovic, B.; Vrbaski, Z.; Vrbaski, Z. Antioxidant Effects of Flavonoids of Anthriscus sylvestris in Lard. J. Am. Oil Chem. Soc. 1996, 73, 773–776. [Google Scholar] [CrossRef]
- Dall’Acqua, S.; Giorgetti, M.; Cervellati, R.; Innocenti, G. Deoxypodophyllotoxin content and antioxidant activity of aerial parts of Anthriscus sylvestris Hoffm. Z. Naturforsch. Sect. C J. Biosci. 2006, 61, 658–662. [Google Scholar] [CrossRef]
- Žemlička, L.; Fodran, P.; Lukeš, V.; Vagánek, A.; Slováková, M.; Staško, A.; Dubaj, T.; Liptaj, T.; Karabín, M.; Birošová, L.; et al. Physicochemical and biological properties of luteolin-7-O-β-d-glucoside (cynaroside) isolated from Anthriscus sylvestris (L.) Hoffm. Monatshefte Chem. Chem. Mon. 2014, 145, 1307–1318. [Google Scholar] [CrossRef]
- Svendsen, A.B. Isolation of luteolin-7-glycoside from the flowers of Anthriscus sylvestris (L.) Hoffm. Pharm. Acta Helv. 1959, 34, 29–32. [Google Scholar] [PubMed]
- Abdulmanea, K.; Prokudina, E.A.; Lanková, P.; Vaníčková, L.; Koblovská, R.; Zelený, V.; Lapčík, O. Immunochemical and HPLC identification of isoflavonoids in the Apiaceae family. Biochem. Syst. Ecol. 2012, 45, 237–243. [Google Scholar] [CrossRef]
- Suzuki, S.; Sakakibara, N. Survey and enzymatic formation of lignans of Anthriscus sylvestris. J. Wood Sci. 2002, 48, 536–541. [Google Scholar] [CrossRef]
- Koulman, A.; Batterman, S.; Van Putten, F.M.S.; Bos, R.; Quax, W.J. Lignan Profiles of Indoor-Cultivated Anthriscus sylvestris. Planta Med. 2003, 69, 959–961. [Google Scholar] [PubMed]
- Hendrawati, O.; Woerdenbag, H.J.; Hille, J.; Quax, W.J.; Kayser, O. Seasonal variations in the deoxypodophyllotoxin content and yield of Anthriscus sylvestris L. (Hoffm.) grown in the field and under controlled conditions. J. Agric. Food Chem. 2011, 59, 8132–8139. [Google Scholar] [CrossRef] [PubMed]
- Koulman, A.; Hendrawati, O.; Batterman, S.; Van Putten, F.M.S.; Bos, R.; Kayser, O. The Seasonal Variations of Lignan Profiles in Anthriscus sylvestris (L.) Hoffm. Planta Med. 2007, 73, P_112. [Google Scholar] [CrossRef]
- Yang, J.; Liang, Q.; Wang, M.; Jeffries, C.; Smithson, D.; Tu, Y.; Boulos, N.; Jacob, M.R.; Shelat, A.A.; Wu, Y.; et al. UPLC-MS-ELSD-PDA as a powerful dereplication tool to facilitate compound identification from small-molecule natural product libraries. J. Nat. Prod. 2014, 77, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Woo, K.W.; Choi, S.U.; Park, J.C.; Lee, K.R. A new lignan glycoside from Juniperus rigida. Arch. Pharm. Res. 2011, 34, 2043–2049. [Google Scholar] [CrossRef] [PubMed]
- Och, M.; Och, A.; Cieśla, Ł.; Kubrak, T.; Pecio, Ł.; Stochmal, A.; Kocki, J.; Bogucka-Kocka, A. Study of cytotoxic activity, podophyllotoxin, and deoxypodophyllotoxin content in selected Juniperus species cultivated in Poland. Pharm. Biol. 2015, 53, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Lamshöft, M.; Spiteller, M. Aspergillus fumigatus Fresenius, an endophytic fungus from Juniperus communis L. Horstmann as a novel source of the anticancer pro-drug deoxypodophyllotoxin. J. Appl. Microbiol. 2009, 107, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Hung, H.C.; Sung, P.J.; Chen, I.S.; Kuo, W.L. Aporphine alkaloids and cytotoxic lignans from the roots of Illigera luzonensis. Phytochemistry 2011, 72, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Ishikawa, T.; Duh, C.Y.; Tsai, I.L.; Chen, I.S. New dimeric aporphine alkaloids and cytotoxic constituents of Hernandia nymphaeifolia. Planta Med. 1996, 62, 528–533. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, M.; Toia, R.F.; Croft, K.D. Lignans from Barks of Hernandia nymphaefolia and H. peltata. Planta Med. 1995, 61, 487–488. [Google Scholar] [CrossRef] [PubMed]
- Udino, L.; Abaul, J.; Bourgeois, P.; Gorrichon, L.; Duran, H.; Zedde, C. Lignans from the Seeds of Hernandia sonora. Planta Med. 1999, 65, 279–281. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Sepúlveda, A.M.; Mendieta-Serrano, M.; Mojica, M.Y.A.; Salas-Vidal, E.; Marquina, S.; Villarreal, M.L.; Puebla, A.M.; Delgado, J.I.; Alvarez, L. Cytotoxic podophyllotoxin type-lignans from the steam bark of Bursera fagaroides var. fagaroides. Molecules 2012, 17, 9506–9519. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, S.B.; You, Y.J.; Ahn, B.Z. Deoxypodophyllotoxin; The cytotoxic and antiangiogenic component from Pulsatilla koreana. Planta Med. 2002, 68, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Muto, N.; Tomokuni, T.; Haramoto, M.; Tatemoto, H.; Nakanishi, T.; Inatomi, Y.; Murata, H.; Inada, A. Isolation of apoptosis- and differentiation-inducing substances toward human promyelocytic leukemia HL-60 cells from leaves of Juniperus taxifolia. Biosci. Biotechnol. Biochem. 2008, 72, 477–484. [Google Scholar] [CrossRef] [PubMed]
- San Feliciano, A.; Miguel Del Corral, J.M.; Gordaliza, M.; Castro, M.A. Acetylated lignans from Juniperus sabina. Phytochemistry 1989, 28, 659–660. [Google Scholar] [CrossRef]
- Gao, R.; Gao, C.; Tian, X.; Yu, X.; Di, X.; Xiao, H.; Zhang, X. Insecticidal activity of deoxypodophyllotoxin, isolated from Juniperus sabina L, and related lignans against larvae of Pieris rapae L. Pest Manag. Sci. 2004, 60, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Benzina, S.; Harquail, J.; Jean, S.; Beauregard, A.P.; Colquhoun, C.D.; Carroll, M.; Bos, A.; Gray, C.A.; Robichaud, G.A. Deoxypodophyllotoxin Isolated from Juniperus Communis Induces Apoptosis in Breast Cancer Cells. Anticancer Agents Med. Chem. 2014, 15, 79–88. [Google Scholar] [CrossRef]
- Carpenter, C.D.; O’Neill, T.; Picot, N.; Johnson, J.A.; Robichaud, G.A.; Webster, D.; Gray, C.A. Anti-mycobacterial natural products from the Canadian medicinal plant Juniperus communis. J. Ethnopharmacol. 2012, 143, 695–700. [Google Scholar] [CrossRef] [PubMed]
- Renouard, S.; Lopez, T.; Hendrawati, O.; Dupre, P.; Doussot, J.; Falguieres, A.; Ferroud, C.; Hagege, D.; Lamblin, F.; Laine, E.; et al. Podophyllotoxin and deoxypodophyllotoxin in Juniperus bermudiana and 12 other Juniperus species: Optimization of extraction, method validation, and quantification. J. Agric. Food Chem. 2011, 59, 8101–8107. [Google Scholar] [CrossRef] [PubMed]
- Renouard, S.; Corbin, C.; Colas, C.; Fidel, T.; Lopez, T.; Leclerc, E.A.; Hendrawati, O.; Falguières, A.; Doussot, J.; et al. Aerial parts of Callitris species as a rich source of deoxypodophyllotoxin. Ind. Crops Prod. 2015, 63, 53–57. [Google Scholar] [CrossRef]
- Koulman, A. Podophyllotoxin. Doctoral dissertation, Dissertation, Rijksuniversiteit: Groningen, The Netherlands, 2003. Available online: https://www.rug.nl/research/ (accessed on 24 July 2015).
- Aynehchi, Y. Desoxypodophyllotoxin, the cytotoxic principle of Callitris columellaris F. Muell. J. Pharm. Sci. 1971, 60, 121–122. [Google Scholar] [CrossRef] [PubMed]
- Van Uden, W.; Pras, N.; Maingré, T.M. The accumulation of podophyllotoxin-β-d-glucoside by cell suspension cultures derived from the conifer Callitris drummondii. Plant Cell Rep. 1990, 9, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.A.; Gustafson, K.R.; Cardellina, J.H.; Boyd, M.R. A New Podophyllotoxin Derivative from Bridelia ferruginea. Nat. Prod. Lett. 2000, 14, 285–292. [Google Scholar] [CrossRef]
- Bianchi, E.; Caldwell, M.E.; Cole, J.R. Antitumor agents from Bursera microphylla (Burseraceae) I. Isolation and characterization of deoxypodophyllotoxin. J. Pharm. Sci. 1968, 57, 696–697. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Xiao, J.J.; Meng, S.C.; Dong, X.M.; Ge, Y.W.; Wang, R.F.; Shang, M.Y.; Cai, S.Q. A new cytotoxic flavonoid from the fruit of Sinopodophyllum hexandrum. Fitoterapia 2010, 81, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Su, B.N.; Jones, W.P.; Cuendet, M.; Kardono, L.B.S.; Ismail, R.; Riswan, S.; Fong, H.H.S.; Farnsworth, N.R.; Pezzuto, J.M.; Kinghorn, A.D. Constituents of the stems of Macrococculus pomiferus and their inhibitory activities against cyclooxygenases-1 and -2. Phytochemistry 2004, 65, 2861–2866. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.E.; Dewick, P.M. Aryltetralin lignans from Podophyllum hexandrum and Podophyllum peltatum. Phytochemistry 1984, 23, 1147–1152. [Google Scholar] [CrossRef]
- Sun, Y.J.; Li, Z.L.; Chen, H.; Liu, X.Q.; Zhou, W.; Hua, H.M. Three new cytotoxic aryltetralin lignans from Sinopodophyllum emodi. Bioorg. Med. Chem. Lett. 2011, 21, 3794–3797. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Nagatsu, A.; Hatano, K.; Shirai, N.; Kato, S.; Ogihara, Y. New lignan glycosides from Chinese medicinal plant, Sinopodophillum emodi. Chem. Pharm. Bull. (Tokyo). 2003, 51, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.W.; Zhou, J.R.; Hon, P.M.; Li, S.L.; Zhou, Y.; Li, L.L.; Ye, W.C.; Xu, H.X.; Shaw, P.C.; But, P.P.H. Lignans from Dysosma versipellis with inhibitory effects on prostate cancer cell lines. J. Nat. Prod. 2007, 70, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, N.; Suzuki, S.; Umezawa, T.; Shimada, M. Biosynthesis of yatein in Anthriscus sylvestris. Org. Biomol. Chem. 2003, 1, 2474–2485. [Google Scholar] [CrossRef] [PubMed]
- Ragamustari, S.K.; Nakatsubo, T.; Hattori, T.; Ono, E.; Kitamura, Y.; Suzuki, S.; Yamamura, M.; Umezawa, T. A novel O-methyltransferase involved in the first methylation step of yatein biosynthesis from matairesinol in Anthriscus sylvestris. Plant Biotechnol. 2013, 30, 375–384. [Google Scholar] [CrossRef]
- Lim, Y.H.; Leem, M.J.; Shin, D.H.; Chang, H.B.; Hong, S.W.; Moon, E.Y.; Lee, D.K.; Yoon, S.J.; Woo, W.S. Cytotoxic constituents from the roots of Anthriscus sylvestris. Arch. Pharm. Res. 1999, 22, 208–212. [Google Scholar] [CrossRef] [PubMed]
- Manso-Martínez, R. Podophyllotoxin poisoning of microtubules at steady-state: Effect of substoichiometric and superstoichiometric concentrations of drug. Mol. Cell. Biochem. 1982, 45, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Loike, J.D.; Horwitz, S.B. Effects of podophyllotoxin and VP-16-213 on microtubule assembly in vitro and nucleoside transport in HeLa cells. Biochemistry 1976, 15, 5435–5443. [Google Scholar] [CrossRef] [PubMed]
- Kelleher, J.K. Tubulin binding affinities of podophyllotoxin and colchicine analogues. Mol. Pharmacol. 1977, 13, 232–241. [Google Scholar] [PubMed]
- Srivastava, V.; Negi, A.S.; Kumar, J.K.; Gupta, M.M.; Khanuja, S.P.S. Plant-based anticancer molecules: A chemical and biological profile of some important leads. Bioorg. Med. Chem. 2005, 13, 5892–908. [Google Scholar] [CrossRef] [PubMed]
- Gordaliza, M.; Castro, M.A.; del Corral, J.M.; Feliciano, A.S. Antitumor properties of podophyllotoxin and related compounds. Curr. Pharm. Des. 2000, 6, 1811–1839. [Google Scholar] [CrossRef] [PubMed]
- Loike, J.D.; Brewer, C.F.; Sternlicht, H.; Gensler, W.J.; Horwitz, S.B. Structure-activity study of the inhibition of microtubule assembly in vitro by podophyllotoxin and its congeners. Cancer Res. 1978, 38, 2688–2693. [Google Scholar] [PubMed]
- Zavala, F.; Guenard, D.; Robin, J.P.; Brown, E. Structure-antitubulin activity relationship in steganacin congeners and analogues. Inhibition of tubulin polymerization in vitro by (±)-isodeoxypodophyllotoxin. J. Med. Chem. 1980, 23, 546–549. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.R.; Xu, Y.; Jiang, Z.Z.; Guerram, M.; Wang, B.; Zhu, X.; Zhang, L.Y. Deoxypodophyllotoxin induces G2/M cell cycle arrest and apoptosis in SGC-7901 cells and inhibits tumor growth in vivo. Molecules 2015, 20, 1661–1675. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.Y.; Yong, Y.; Kim, C.G.; Lee, Y.H.; Lim, Y. Deoxypodophyllotoxin induces G2 /M cell cycle arrest and apoptosis in HeLa cells. Cancer Lett. 2010, 287, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Kim, H.; Ahn, J.; Jung, S.K.; Um, M.Y.; Son, K.H.; Kim, T.W.; Ha, T.Y. Anthricin isolated from Anthriscus sylvestris (L.) Hoffm. Inhibits the growth of breast cancer cells by inhibiting Akt/mTOR signaling, and its apoptotic effects are enhanced by autophagy inhibition. Evid. Based Complement. Altern. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Jiang, Z.; Duan, H.; Sun, L.; Zhang, S.; Chen, M.; Wang, Y.; Gao, Q.; Song, Y.; Zhu, X.; et al. Deoxypodophyllotoxin triggers necroptosis in human non-small cell lung cancer NCI-H460 cells. Biomed. Pharmacother. 2013, 67, 701–706. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wu, M.; Miao, J.; Duan, H.; Zhang, S.; Chen, M.; Sun, L.; Wang, Y.; Zhang, X.; Zhu, X.; Zhang, L. Deoxypodophyllotoxin exerts both anti-angiogenic and vascular disrupting effects. Int. J. Biochem. Cell Biol. 2013, 45, 1710–1719. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Xu, H. Recent advances in semisynthesis, biosynthesis, biological activities, mode of action, and structure-activity relationship of podophyllotoxins: An update (2008–2010). Mini Rev. Med. Chem. 2011, 11, 901–909. [Google Scholar] [CrossRef] [PubMed]
- C.T.; Xu, F.Q.; Li, G.T.; Li, Y.; Ding, Z.T.; Zhou, J.; Jiang, Z.H.; Hu, J.M. Synthesis and Anticancer Activity of Glucosylated Podophyllotoxin Derivatives Linked via 4β-Triazole Rings. Molecules 2013, 18, 13992–14012. [Google Scholar]
- Cho, E.; Choi, J.; Kim, H.; Choi, K.; Ku, J.; Park, K.W.; Kim, J.; Lee, S. Antibacterial activity and protective effect against gastric cancer by Anthriscus sylvestris fractions. Hortic. Environ. Biotechnol. 2013, 54, 326–330. [Google Scholar] [CrossRef]
- Lee, S.H.; Son, M.J.; Ju, H.K.; Lin, C.X.; Moon, T.C.; Choi, H.G.; Son, J.K.; Chang, H.W. Dual inhibition of cyclooxygenases-2 and 5-lipoxygenase by deoxypodophyllotoxin in mouse bone marrow-derived mast cells. Biol. Pharm. Bull. 2004, 27, 786–788. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Moon, T.C.; Quan, Z.; Lee, E.; Kim, Y.K.; Yang, J.H.; Suh, S.J.; Jeong, T.C.; Lee, S.H.; Kim, C.H.; et al. The naturally occurring flavolignan, deoxypodophyllotoxin, inhibits lipopolysaccharide-induced iNOS expression through the NF-kappaB activation in RAW264.7 macrophage cells. Biol. Pharm. Bull. 2008, 31, 1312–1315. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.X.; Son, M.J.; Ju, H.K.; Moon, T.C.; Lee, E.; Kim, S.H.; Kim, M.J.; Son, J.K.; Lee, S.H.; Chang, H.W. Deoxypodophyllotoxin, a naturally occurring lignan, inhibits the passive cutaneous anaphylaxis reaction. Planta Med. 2004, 70, 474–476. [Google Scholar] [PubMed]
- Giri, A.; Lakshmi Narasu, M. Production of podophyllotoxin from Podophyllum hexandrum: A potential natural product for clinically useful anticancer drugs. Cytotechnology 2000, 34, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, T. History of plant tissue culture. Methods Mol. Biol. 2012, 877, 9–27. [Google Scholar] [PubMed]
- Hendrawati, O.; Hille, J.; Woerdenbag, H.J.; Quax, W.J.; Kayser, O. In vitro regeneration of wild chervil (Anthriscus sylvestris L.). In Vitro Cell. Dev. Biol. Plant 2012, 48, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Vasilev, N.P.; Julsing, M.K.; Koulman, A.; Clarkson, C.; Woerdenbag, H.J.; Ionkova, I.; Bos, R.; Jaroszewski, J.W.; Kayser, O.; Quax, W.J. Bioconversion of deoxypodophyllotoxin into epipodophyllotoxin in E. coli using human cytochrome P450 3A4. J. Biotechnol. 2006, 126, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.H.; Lee, S.H.; Jin, C.; Kim, D.H. Mechanism-based inactivation of cytochrome P450 3A4 by methylenedioxyphenyl lignans from Acanthopanax chiisanensis. Planta Med. 2008, 74, 822–827. [Google Scholar] [CrossRef] [PubMed]
- Kondo, K.; Ogura, M.; Midorikawa, Y.; Kozawa, M.; Tsujibo, H.; Baba, K.; Inamori, Y. Conversion of deoxypodophyllotoxin to podophyllotoxin related compounds by microbes. Agric. Biol. Chem. 1989, 53, 777–782. [Google Scholar] [CrossRef]
- Sample Availability: Samples are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaru, O.T.; Niţulescu, G.M.; Orțan, A.; Dinu-Pîrvu, C.E. Ethnomedicinal, Phytochemical and Pharmacological Profile of Anthriscus sylvestris as an Alternative Source for Anticancer Lignans. Molecules 2015, 20, 15003-15022. https://doi.org/10.3390/molecules200815003
Olaru OT, Niţulescu GM, Orțan A, Dinu-Pîrvu CE. Ethnomedicinal, Phytochemical and Pharmacological Profile of Anthriscus sylvestris as an Alternative Source for Anticancer Lignans. Molecules. 2015; 20(8):15003-15022. https://doi.org/10.3390/molecules200815003
Chicago/Turabian StyleOlaru, Octavian Tudorel, George Mihai Niţulescu, Alina Orțan, and Cristina Elena Dinu-Pîrvu. 2015. "Ethnomedicinal, Phytochemical and Pharmacological Profile of Anthriscus sylvestris as an Alternative Source for Anticancer Lignans" Molecules 20, no. 8: 15003-15022. https://doi.org/10.3390/molecules200815003
APA StyleOlaru, O. T., Niţulescu, G. M., Orțan, A., & Dinu-Pîrvu, C. E. (2015). Ethnomedicinal, Phytochemical and Pharmacological Profile of Anthriscus sylvestris as an Alternative Source for Anticancer Lignans. Molecules, 20(8), 15003-15022. https://doi.org/10.3390/molecules200815003