Bioaccumulation of Trace Metals in Mussel (Mytilus galloprovincialis) from Mali Ston Bay during DSP Toxicity Episodes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Correlation between MBA DSP Results and Trace Metal Concentrations in Mussels
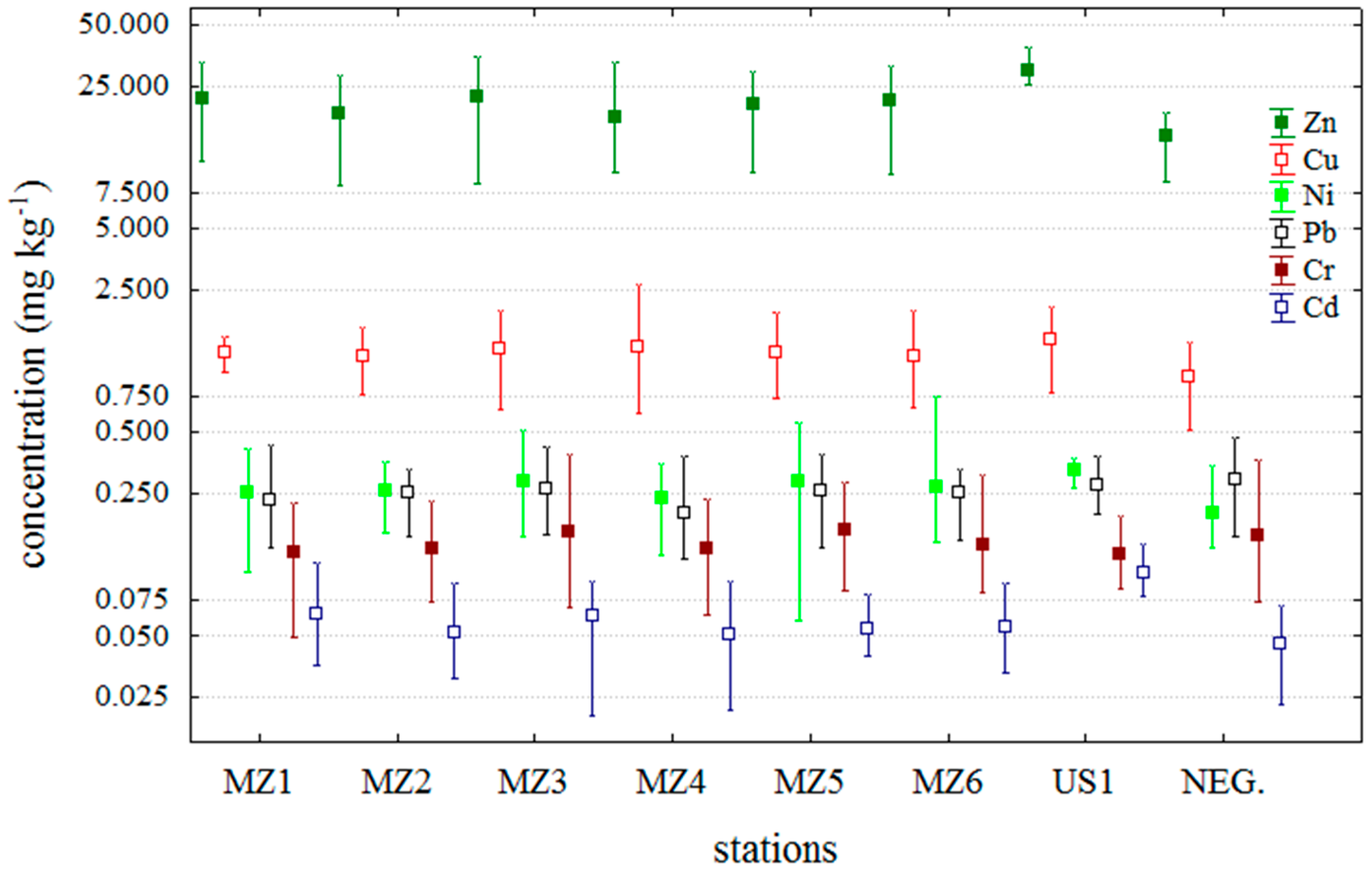
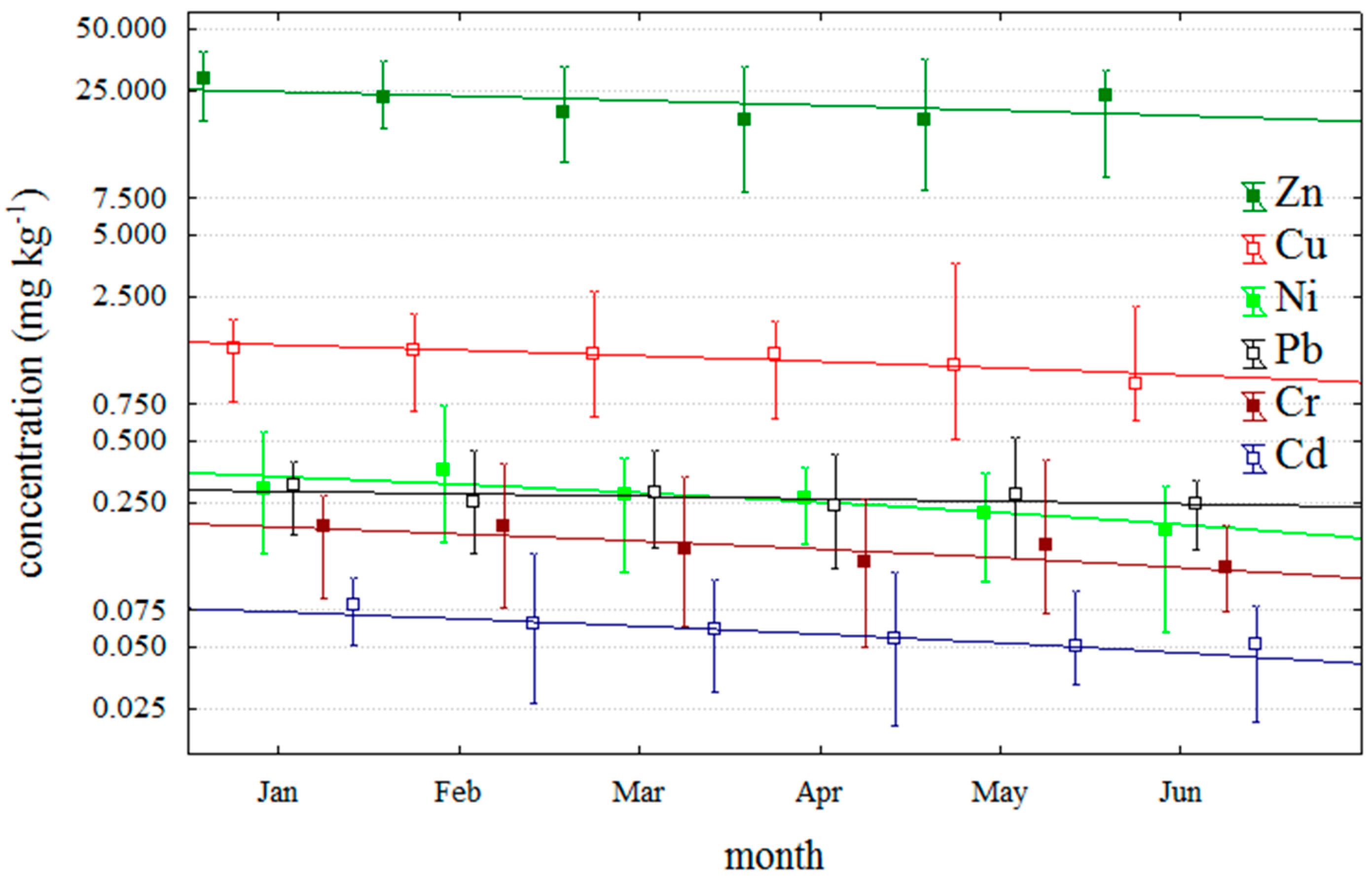
2.2. Temporal Distribution of Trace Metals during the Period of DSP Shellfish Toxicity
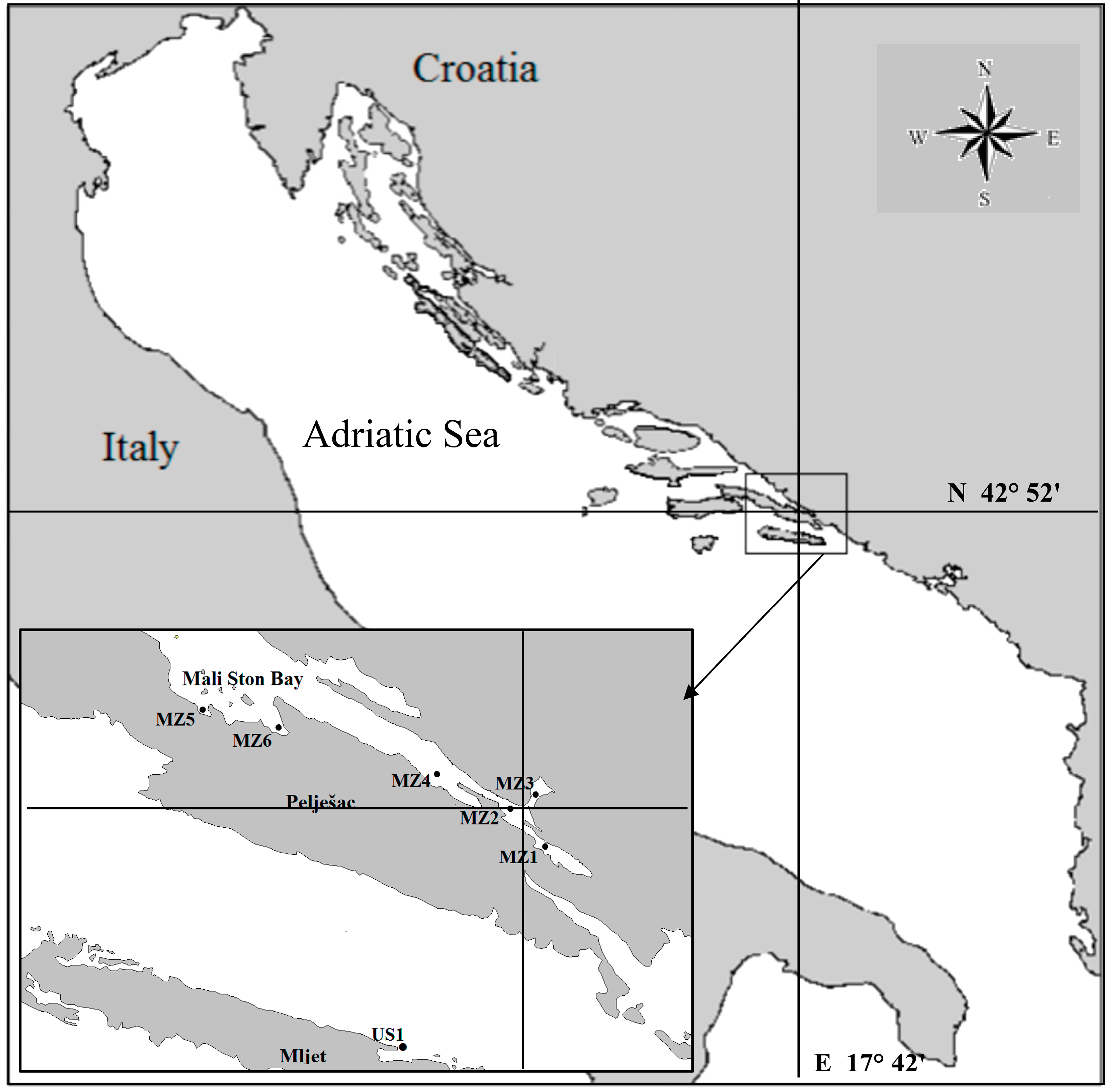
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Philip, S.R. Trace metal concentrations in aquatic invertebrates: Why and so what? Environ. Pollut. 2002, 120, 497–507. [Google Scholar]
- Khor, S.; Wood, S.A.; Salvitti, L.; Taylor, D.I.; Adamson, J.; McNabb, P.; Cary, S.C. Investigating Diet as the Source of Tetrodotoxin in Pleurobranchaea maculata. Mar. Drugs 2014, 12, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kljaković-Gašpić, Z.; Odžak, N.; Ujević, I.; Zvonarić, T.; Horvat, M.; Barić, A. Biomonitoring of mercury in polluted coastal area using transplanted mussels. Sci. Total Environ. 2006, 1, 199–209. [Google Scholar] [CrossRef] [PubMed]
- McCulloch, A.W.; Body, R.K.; de Freitas, A.S.; Foxall, R.A.; Jamieson, W.D.; Laycock, M.V.; Quilliam, M.A.; Wright, J.L.; Boyko, V.J.; McLaren, J.W. Zinc from Oyster Tissue as Causative Factor in Mouse Deaths in Official Bioassay for Paraytic Shellfish Poison. J. Assoc. Off. Anal. Chem. 1989, 72, 384–386. [Google Scholar] [PubMed]
- Park, D.L.; Adams, W.N.; Graham, S.L.; Jackson, R.C. Variability of Mouse Bioassay for Determination of paralytic Shellfish Poisoning. J. Assoc. Off. Anal. Chem. 1986, 69, 547–550. [Google Scholar] [PubMed]
- Rhodes, L.; Selwood, A.; McNabb, P.; Briggs, L.; Adamson, J.; van Ginkel, R.; Laczka, O. Trace metal effects on the production of biotoxins by microalgae. Afr. J. Mar. Sci. 2006, 28, 393–397. [Google Scholar] [CrossRef]
- Bogdanović, T.; Ujević, I.; Sedak, M.; Listeš, E.; Šimat, V.; Petričević, S.; Poljak, V. As, Cd, Hg and Pb in four edible shelfish species from breeding and harvesting areas along the eastern Adriatic Coast, Croatia. Food Chem. 2014, 146, 197–2013. [Google Scholar] [CrossRef] [PubMed]
- Kljaković-Gašpić, Z.; Odžak, N.; Ujević, I.; Zvonarić, T.; Barić, A. Biomonitoring of trace metals (Cu, Cd, Cr, Hg, Pb, Zn) in eastern Adriatic using the Mediterranean blue mussel (2001–2005). Fresenius Environ. Bull. 2006, 15, 1041–1048. [Google Scholar]
- Gavrilović, A.; Srebočan, E.; Petrinec, Z.; Pompe-Gotal, J.; Prevendar-Crnić, A. Evaluation of concentrations of heavy metals in oysters and mussels of Mali Ston region. Naše More 2004, 51, 50–58. [Google Scholar]
- Cullaj, A.; Lazo, P.; Duka, S. Heavy metals and metallonine levels in mussel samples from the Albanian sea coast, MAP/Med Pol. In In Biological Effects Monitoring Programme—Achievements and Future Orientations,Proceedings of the Workshop, Alessandria, Italy, 20–21 December 2006, Athens, Greece; UNEP/MAP, 2007. Technical Reports Serial No. 166. pp. 141–151. [Google Scholar]
- Cardellicchio, N.; Buccolieri, A.; di Leo, A.; Giandomenico, S.; Spada, L. Levels of metals in reared mussels from Taranto Gulf (Ionian Sea, Southern Italy). Food Chem. 2008, 107, 890–896. [Google Scholar] [CrossRef]
- Ščančar, J.; Zuliani, T.; Turk, T.; Milačić, R. Organotin compounds and selected metals in the marine environment of Northern Adriatic Sea. Environ. Monit. Assess. 2007, 127, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Joksimović, D.; Tomić, I.; Stanković, A.R.; Jović, M.; Stanković, S. Trace metal concentrations in Mediterranean blue mussel and surface sediments and evaluation of the mussels quality and possible risks of high human consumption. Food Chem. 2011, 127, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Stanković, S.; Jović, M.; Milanov, R.; Joksimović, D. Trace elements concentrations (Zn, Cu, Pb, Cd, As and Hg) in the mediterranean mussel (Mytilus galloprovincialis) and evaluation of mussel quality and possible human health risk from cultivated and wild sites of the southeastern Adriatic Sea, Montenegro. J. Serb. Chem. Soc. 2011, 76, 1725–1737. [Google Scholar] [CrossRef]
- Marković, J.; Joksimović, D.; Stanković, S. Trace Element Concentrations in Wild Mussels from the Coastal area of the Southeastern Adriatic, Montenegro. Arch. Biol. Sci. 2012, 64, 265–275. [Google Scholar] [CrossRef]
- Ramšak, A.; Ščančar, J.; Horvat, M. Evaluation of metallothioneins in blue mussels (Mytilus galloprovincialis) as a biomarker of mercury and cadmium exposure in the Slovenian waters (Gulf of Trieste): A long-term field study. Acta Adriat. 2012, 53, 71–86. [Google Scholar]
- Nincevic Gladan, Ž.; Ujevic, I.; Milandri, A.; Marasovic, I.; Ceredi, A.; Pigozzi, S.; Arapov, J.; Skejic, S. Lipophilic Toxin Profile in Mytilus galloprovincialis during Episodes of Diarrhetic Shellfish Poisoning (DSP) in the N.E. Adriatic Sea in 2006. Molecules 2011, 16, 888–899. [Google Scholar]
- Ujević, I.; Nazlić, N.; Ninčević-Gladan, Ž; Marasović, I. Gymnodimine and spirolide in shellfish during DSP toxicity in central and southern Adriatic Sea. In Proceedings of the ICHA; Wellington, New Zealand, October 2014; pp. 27–31. [Google Scholar]
- Ciminiello, P.; Dell’Aversano, C.; Fattorusso, E.; Magno, S.; Tartaglione, L.; Cangini, M.; Pompei, M.; Guerrini, F.; Boni, L.; Pistocchi, R. Toxin profile of Alexandrium ostenfeldii (Dinophyceae) from the Northern Adriatic Sea revealed by liquid chromatography–mass spectrometry. Toxicon 2006, 47, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Espina, B.; Otero, P.; Louzao, M.C.; Alfonso, A.; Botana, L.M. 13-Desmethyl spirolide-c 13,19-didesmethyl spirolide-c trans-epithelial permeabilities: Human intestinal permeability modeling. Toxicology 2011, 287, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yoshizawa, R.; Kawamura, T.; Yamasaki, M. Interference of Free Fatty Acids from the Hepatopancreas of Mussels with the Mouse Bioassay for Shellfish Toxins. Lipids 1996, 31, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Hayashi, K.; ltabashi, Y. Toxic Effect of Free Unsaturated Fatty Acids in the Mouse Bioassay of Diarrhetic Shellfish Toxin by Intraperitoneal Injection. Bull. Jpn. Soc. Sci. Fish. 1984, 50, 1413–1418. [Google Scholar] [CrossRef]
- Stabell, O.B.; Yndestad, M.; Heidenreich, B. Paralytic shellfish toxins seems absent in extracts of diarrhetic shellfish toxins. Environ. Toxicol. Chem. 1991, 10, 331–334. [Google Scholar] [CrossRef]
- Lawrence, J.F.; Chadha, R.K.; Ratnayake, W.M.; Truelove, J.F. An Incident of Elevated Levels of Unsaturated Free Fatty Acids in Mussels from Nova Scotia and Their Toxic Effect in Mice after Intraperitoneal Injection. Nat. Toxins 1994, 2, 318–321. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Commission Regulation (EC) No. 853/2004 of the European Parliament and of the Council of 29 April 2004 laying down specific hygiene rules for food of animal origin. Off. J. Eur. Union 2004, 139, 55–205. [Google Scholar]
- Borchardt, T.; Burchert, S.; Krabe, L.; Zeitner, R. Enhanced heavy metal concentrations in Mytilus edulis from central North Sea. Sci. Mar. 1989, 53, 725–728. [Google Scholar]
- Cossa, D. A review of the use of Mytilus spp. as quantitative indicators of cadmium and mercury contamination in coastal waters. Oceanogr. Acta 1989, 12, 417–432. [Google Scholar]
- Odžak, N.; Zvonarić, T.; Kljaković-Gašpić, Z.; Barić, A. Biomonitoring of copper, cadmium, lead, zinc and chromium in the Kaštela Bay using transplanted mussels. Fresenius Environ. Bull. 2001, 10, 37–41. [Google Scholar]
- Regoli, F.; Orlando, E. Seasonal variation of trace metal concentration in the digestive gland of the Mediterranean mussel Mytilus galloprovincialis: Comparison between polluted and non-polluted site. Arch. Environ. Contam. Toxicol. 1994, 27, 36–43. [Google Scholar] [CrossRef] [PubMed]
- United States Environmental Protection Agency. Microwave Assisted Digestion of Siliceous and Organically Based Matrices; EPA Method 3052; National Technical Information Services: Springfield, VA, USA, 1996. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ujević, I.; Vuletić, N.; Lušić, J.; Nazlić, N.; Kušpilić, G. Bioaccumulation of Trace Metals in Mussel (Mytilus galloprovincialis) from Mali Ston Bay during DSP Toxicity Episodes. Molecules 2015, 20, 13031-13040. https://doi.org/10.3390/molecules200713031
Ujević I, Vuletić N, Lušić J, Nazlić N, Kušpilić G. Bioaccumulation of Trace Metals in Mussel (Mytilus galloprovincialis) from Mali Ston Bay during DSP Toxicity Episodes. Molecules. 2015; 20(7):13031-13040. https://doi.org/10.3390/molecules200713031
Chicago/Turabian StyleUjević, Ivana, Nenad Vuletić, Jelena Lušić, Nikša Nazlić, and Grozdan Kušpilić. 2015. "Bioaccumulation of Trace Metals in Mussel (Mytilus galloprovincialis) from Mali Ston Bay during DSP Toxicity Episodes" Molecules 20, no. 7: 13031-13040. https://doi.org/10.3390/molecules200713031
APA StyleUjević, I., Vuletić, N., Lušić, J., Nazlić, N., & Kušpilić, G. (2015). Bioaccumulation of Trace Metals in Mussel (Mytilus galloprovincialis) from Mali Ston Bay during DSP Toxicity Episodes. Molecules, 20(7), 13031-13040. https://doi.org/10.3390/molecules200713031




