Individual Constituents from Essential Oils Inhibit Biofilm Mass Production by Multi-Drug Resistant Staphylococcus aureus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Selection of Effective Compounds According to Their MICs
| SC-01 | USA300 | UAMS-1 | Newman | |
|---|---|---|---|---|
| Carvacrol | 200 (100) | 200 (100) | 200 (100) | 200 (100) |
| Citral | 500 (200) | 500 (200) | 500 (200) | 500 (200) |
| (+)-Limonene | 5000 (2000) | 5000 (2000) | 5000 (2000) | 5000 (2000) |
2.2. Biofilm Development throughout Time of S. aureus Strains in the Absence and the Presence of ICs
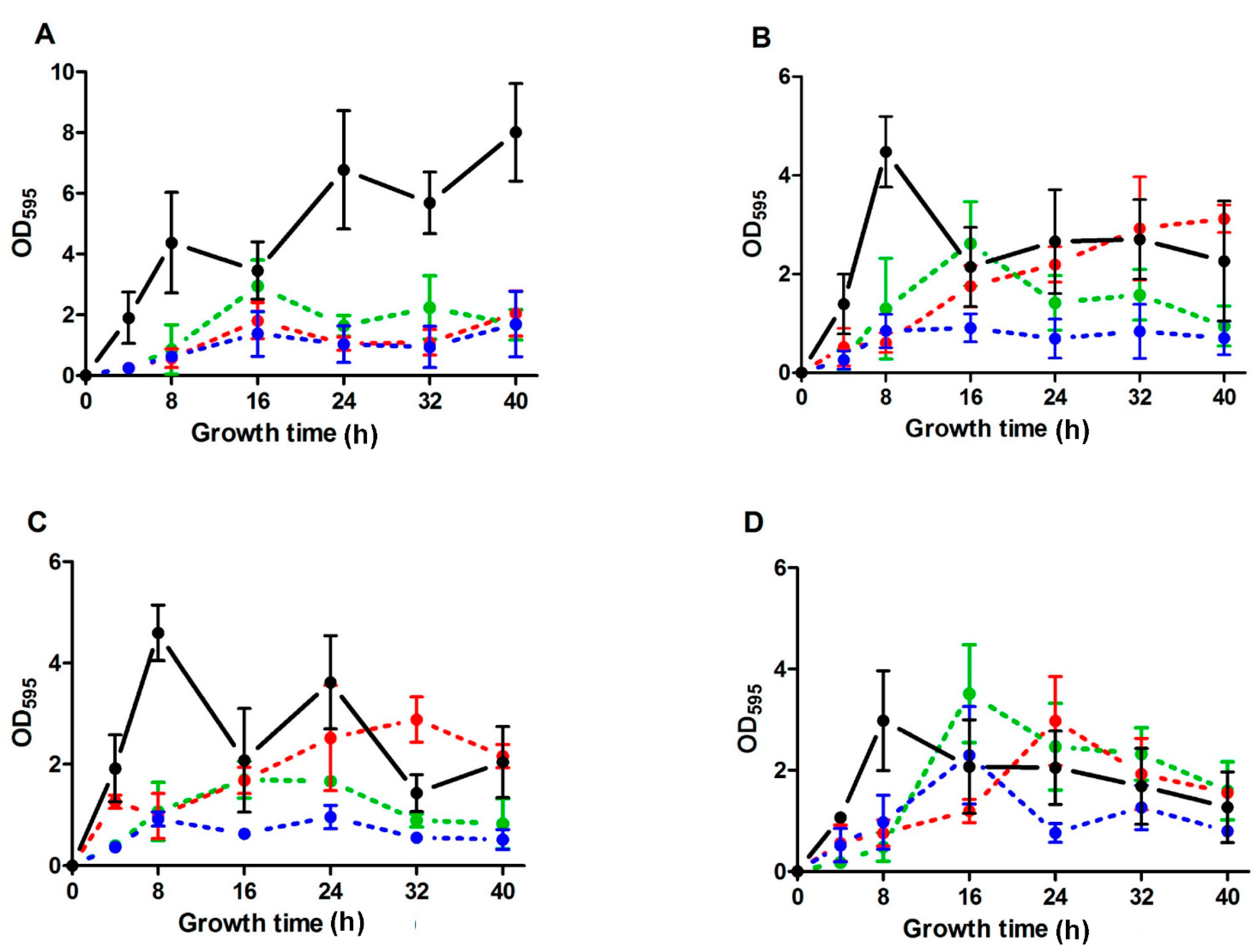
| SC-01 | USA300 | UAMS-1 | Newman | |
|---|---|---|---|---|
| Carvacrol | 81.51 *,a1 ± 11.16 | 74.62 *,a1 ± 12.23 | 74.66 *,a1 ± 2.79 | 33.28 *,b1 ± 17.34 |
| Citral | 65.00 *,a1 ± 15.30 | 47.34 *,a1,2 ± 21.50 | 57.10 *,a2 ± 6.75 | 0.21 b1 ± 29.78 |
| (+)-Limonene | 77.77 *,a1 ± 6.06 | 28.54 b2 ± 19.45 | 26.46 b2 ± 11.83 | 11.19 b1 ± 9.42 |
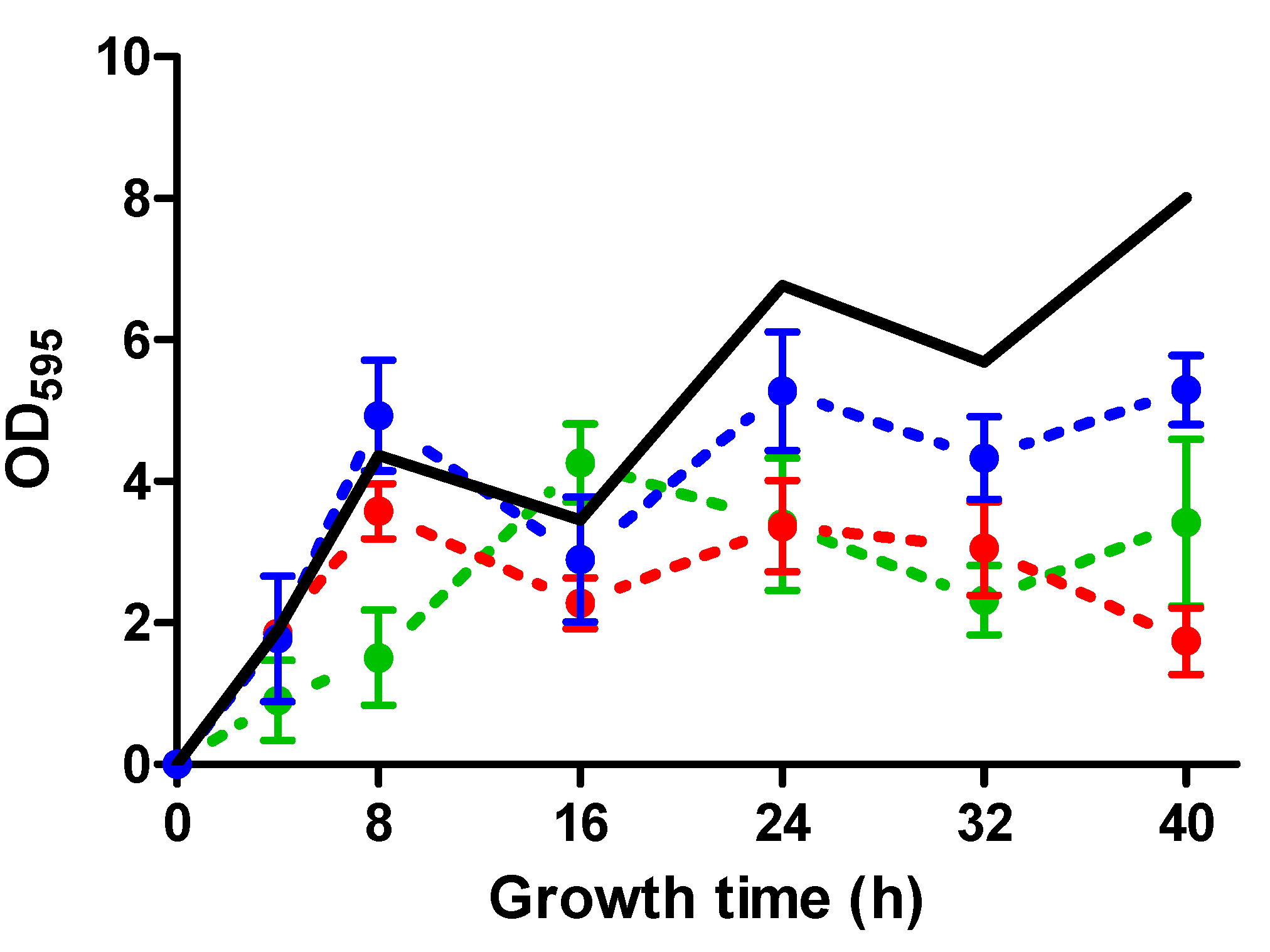
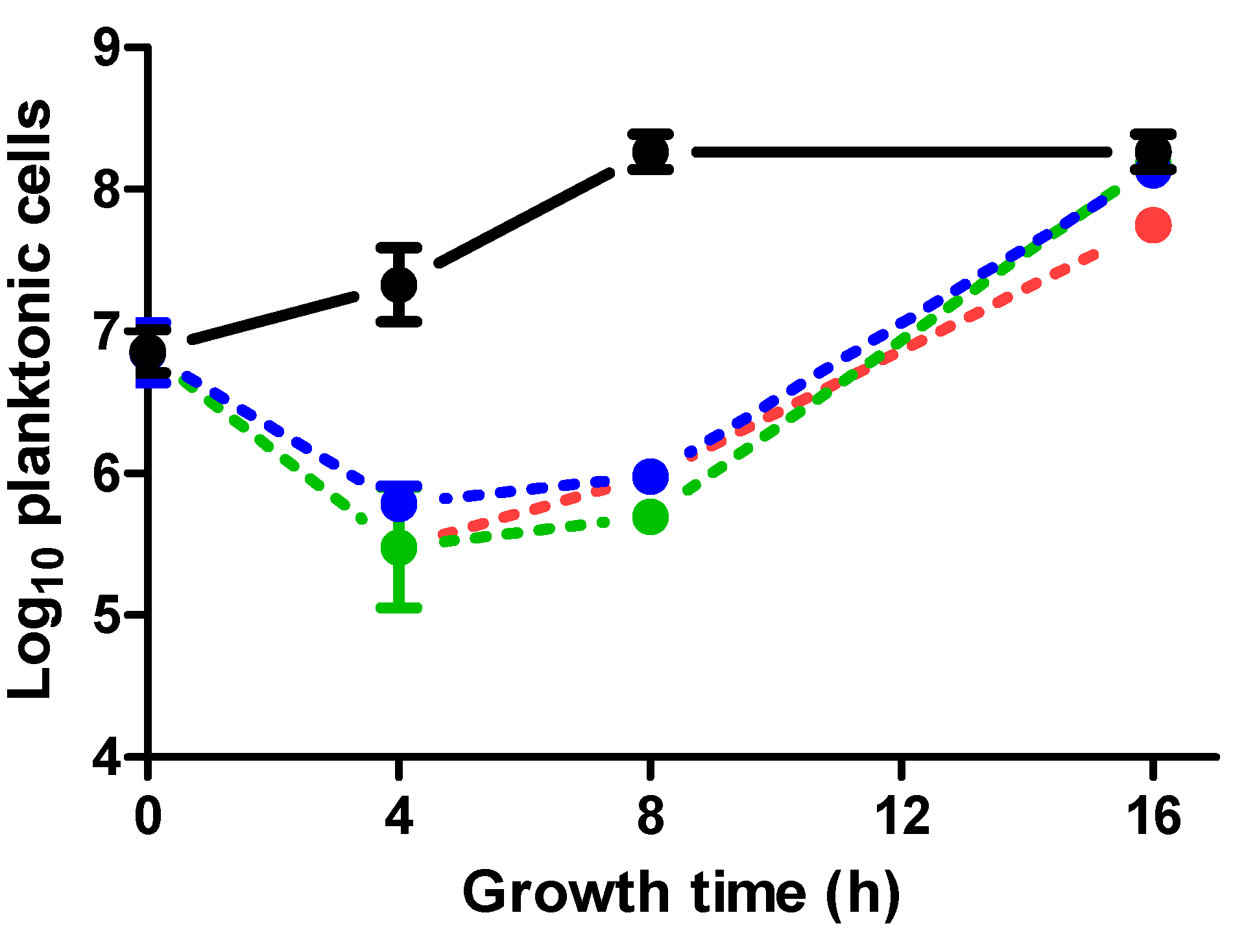
2.3. Effect of ICs on Planktonic Cells in Relation to Biofilm Formation
2.4. Discussion
3. Experimental Section
3.1. Microorganisms and Growth Conditions
3.2. Procedure for Biofilm Formation
3.3. Determination of Minimum Inhibitory Concentration (MIC) and Quantification of the Effect of Sub-MIC Concentrations of EOs and ICs
3.4. Quantification of Planktonic Cells in Biofilm-Growing Cultures
3.5. Data Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Costerton, J.; Stewart, P.S.; Greenberg, E. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef] [PubMed]
- Srey, S.; Jahid, I.K.; Ha, S.D. Biofilm formation in food industries: A food safety concern. Food Control 2013, 31, 572–585. [Google Scholar] [CrossRef]
- Smith, K.; Perez, A.; Ramage, G.; Lappin, D.; Gemmell, C.G.; Lang, S. Biofilm formation by Scottish clinical isolates of Staphylococcus aureus. J. Med. Microbiol. 2008, 57, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Otto, M. Basis of virulence in community-associated methicillin-resistant Staphylococcus aureus. Annu. Rev. Microbiol. 2010, 64, 143–162. [Google Scholar] [CrossRef] [PubMed]
- Lopez, D.; Vlamakis, H.; Kolter, R. Biofilms. Cold Spring Harb. Perspect. Biol. 2010, 2, a000398. [Google Scholar] [CrossRef] [PubMed]
- Van Houdt, R.; Michiels, C.W. Biofilm formation and the food industry, a focus on the bacterial outer surface. J. Appl. Microbiol. 2010, 109, 1117–1131. [Google Scholar] [CrossRef] [PubMed]
- Kluytmans, J.A.J.W. Methicillin-resistant Staphylococcus aureus in food products: Cause for concern or case for complacency? Clin. Microbiol. Infect. 2010, 16, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Fratamico, P.M.; Annous, B.A.; Guenther, N. Biofilm formation by Gram-positive bacteria. In Biofilms in the Food and Beverage Industries; Woodhead Publishing Limited: Cambridge, UK, 2009. [Google Scholar]
- Karatan, E.; Watnick, P. Signals, regulatory networks, and materials that build and break bacterial biofilms. Microbiol. Mol. Biol. Rev. 2009, 73, 310–347. [Google Scholar] [CrossRef] [PubMed]
- Köck, R.; Becker, K.; Cookson, B.; van Gemert-Pijnen, J.; Harbarth, S.; Kluytmans, J.; Mielke, M.; Peters, G.; Skov, R.; Struelens, M. Methicillin-resistant Staphylococcus aureus (MRSA): Burden of disease and control challenges in europe. Eurosurveillance 2010, 15, 19688. [Google Scholar] [PubMed]
- Kumar, C.G.; Anand, S. Significance of microbial biofilms in food industry: A review. Int. J. Food Microbiol. 1998, 42, 9–27. [Google Scholar] [CrossRef]
- Caballero Gómez, N.; Abriouel, H.; Grande, M.J.; Pérez Pulido, R.; Gálvez, A. Combined treatments of enterocin AS-48 with biocides to improve the inactivation of methicillin-sensitive and methicillin-resistant Staphylococcus aureus planktonic and sessile cells. Int. J. Food Microbiol. 2013, 163, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Simoes, M.; Simoes, L.C.; Vieira, M.J. A review of current and emergent biofilm control strategies. LWT Food Sci. Technol. 2010, 43, 573–583. [Google Scholar] [CrossRef]
- Lang, G.; Buchbauer, G. A review on recent research results (2008–2010) on essential oils as antimicrobials and antifungals. A review. Flavour Fragr. J. 2012, 27, 13–39. [Google Scholar] [CrossRef]
- Adukwu, E.C.; Allen, S.C.; Phillips, C.A. The anti-biofilm activity of lemongrass (Cymbopogon flexuosus) and grapefruit (Citrus paradisi) essential oils against five strains of Staphylococcus aureus. J. Appl. Microbiol. 2012, 113, 1217–1227. [Google Scholar] [CrossRef] [PubMed]
- Kerekes, E.B.; Deák, É.; Takó, M.; Tserennadmid, R.; Petkovits, T.; Vágvölgyi, C.; Krisch, J. Anti-biofilm forming and anti-quorum sensing activity of selected essential oils and their main components on food-related micro-organisms. J. Appl. Microbiol. 2013, 115, 933–942. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef] [PubMed]
- Fisher, K.; Phillips, C. Potential antimicrobial uses of essential oils in food: Is citrus the answer? Trends Food Sci. Technol. 2008, 19, 156–164. [Google Scholar] [CrossRef]
- Espina, L.; Somolinos, M.; Lorán, S.; Conchello, P.; García, D.; Pagán, R. Chemical composition of commercial citrus fruit essential oils and evaluation of their antimicrobial activity acting alone or in combined processes. Food Control 2011, 22, 896–902. [Google Scholar] [CrossRef]
- Ait-Ouazzou, A.; Espina, L.; Gelaw, T.; Lamo-Castellví, S.; Pagán, R.; García-Gonzalo, D. New insights in mechanisms of bacterial inactivation by carvacrol. J. Appl. Microbiol. 2013, 114, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Laird, K.; Armitage, D.; Phillips, C. Reduction of surface contamination and biofilms of Enterococcus sp. and Staphylococcus aureus using a citrus-based vapour. J. Hosp. Infect. 2012, 80, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Landini, P.; Antoniani, D.; Burgess, J.G.; Nijland, R. Molecular mechanisms of compounds affecting bacterial biofilm formation and dispersal. Appl. Microbiol. Biotechnol. 2010, 86, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Tong, Z.; Linghu, D.; Lin, Y.; Tao, R.; Liu, J.; Tian, Y.; Ni, L. Effects of sub-minimum inhibitory concentrations of antimicrobial agents on Streptococcus mutans biofilm formation. Int. J. Antimicrob. Agents 2012, 39, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, L.R.; D’Argenio, D.A.; MacCoss, M.J.; Zhang, Z.; Jones, R.A.; Miller, S.I. Aminoglycoside antibiotics induce bacterial biofilm formation. Nature 2005, 436, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Aiemsaard, J.; Aiumlamai, S.; Aromdee, C.; Taweechaisupapong, S.; Khunkitti, W. The effect of lemongrass oil and its major components on clinical isolate mastitis pathogens and their mechanisms of action on Staphylococcus aureus dmst 4745. Res. Vet. Sci. 2011, 91, e31–e37. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Roccaro, A.S.; Bisignano, G.; Marino, A.; Cannatelli, M.A.; Pizzimenti, F.C.; Cioni, P.L.; Procopio, F.; Blanco, A.R. Effects of oregano, carvacrol and thymol on Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Med. Microbiol. 2007, 56, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Soni, K.A.; Oladunjoye, A.; Nannapaneni, R.; Schilling, M.; Silva, J.L.; Mikel, B.; Bailey, R. Inhibition and inactivation of Salmonella Typhimurium biofilms from polystyrene and stainless steel surfaces by essential oils and phenolic constituent carvacrol. J. Food Prot. 2013, 76, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Rode, T.M.; Langsrud, S.; Holck, A.; Møretrø, T. Different patterns of biofilm formation in Staphylococcus aureus under food-related stress conditions. Int. J. Food Microbiol. 2007, 116, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Sánchez, D.; Habimana, O.; Holck, A. Impact of food-related environmental factors on the adherence and biofilm formation of natural Staphylococcus aureus isolates. Curr. Microbiol. 2013, 66, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Lebert, I.; Leroy, S.; Talon, R. Effect of industrial and natural biocides on spoilage, pathogenic and technological strains grown in biofilm. Food Microbiol. 2007, 24, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Al-Shuneigat, J.; Cox, S.D.; Markham, J.L. Effects of a topical essential oil-containing formulation on biofilm-forming coagulase-negative staphylococci. Lett. Appl. Microbiol. 2005, 41, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Scaffaro, R.; D’Arrigo, M.; Botta, L.; Filocamo, A.; Marino, A.; Bisignano, G. Study on carvacrol and cinnamaldehyde polymeric films: Mechanical properties, release kinetics and antibacterial and antibiofilm activities. Appl. Microbiol. Biotechnol. 2012, 96, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, D.; Napoli, E.M.; Cusimano, M.G.; Vitale, M.; Ruberto, A. Origanum vulgare subsp. hirtum essential oil prevented biofilm formation and showed antibacterial activity against planktonic and sessile bacterial cells. J. Food Prot. 2013, 76, 1747–1752. [Google Scholar]
- Lee, K.; Lee, J.-H.; Kim, S.-I.; Cho, M.; Lee, J. Anti-biofilm, anti-hemolysis, and anti-virulence activities of black pepper, cananga, myrrh oils, and nerolidol against Staphylococcus aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9447–9457. [Google Scholar] [CrossRef] [PubMed]
- Chorianopoulos, N.G.; Giaouris, E.D.; Skandamis, P.N.; Haroutounian, S.A.; Nychas, G.J.E. Disinfectant test against monoculture and mixed-culture biofilms composed of technological, spoilage and pathogenic bacteria: Bactericidal effect of essential oil and hydrosol of Satureja thymbra and comparison with standard acid–base sanitizers. J. Appl. Microbiol. 2008, 104, 1586–1596. [Google Scholar] [CrossRef] [PubMed]
- Burt, S.A.; Ojo-Fakunle, V.T.A.; Woertman, J.; Veldhuizen, E.J.A. The natural antimicrobial carvacrol inhibits quorum sensing in Chromobacterium violaceum and reduces bacterial biofilm formation at sub-lethal concentrations. PLoS ONE 2014, 9, e93414. [Google Scholar] [CrossRef] [PubMed]
- Giaouris, E.; Chorianopoulos, N.; Nychas, G.J. Acquired acid adaptation of Listeria monocytogenes during its planktonic growth enhances subsequent survival of its sessile population to disinfection with natural organic compounds. Food Res. Int. 2014, 64, 896–900. [Google Scholar] [CrossRef]
- Kavanaugh, N.L.; Ribbeck, K. Selected antimicrobial essential oils eradicate Pseudomonas spp. and Staphylococcus aureus biofilms. Appl. Environ. Microbiol. 2012, 78, 4057–4061. [Google Scholar] [CrossRef] [PubMed]
- Niu, C.; Gilbert, E. Colorimetric method for identifying plant essential oil components that affect biofilm formation and structure. Appl. Environ. Microbiol. 2004, 70, 6951–6956. [Google Scholar] [CrossRef] [PubMed]
- Sandasi, M.; Leonard, C.; Viljoen, A. The effect of five common essential oil components on Listeria monocytogenes biofilms. Food Control 2008, 19, 1070–1075. [Google Scholar] [CrossRef]
- Sandasi, M.; Leonard, C.; Van Vuuren, S.; Viljoen, A. Peppermint (Mentha piperita) inhibits microbial biofilms in vitro. S. Afr. J. Bot. 2011, 77, 80–85. [Google Scholar] [CrossRef]
- Harvey, J.; Keenan, K.; Gilmour, A. Assessing biofilm formation by Listeria monocytogenes strains. Food Microbiol. 2007, 24, 380–392. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.W.; Suzuki, K.; Oakford, L.; Simecka, J.W.; Hart, M.E.; Romeo, T. Biofilm formation and dispersal under the influence of the global regulator Csra of Escherichia coli. J. Bacteriol. 2002, 184, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Kreiswirth, B.; Kornblum, J.; Arbeit, R.D.; Eisner, W.; Maslow, J.N.; McGeer, A.; Low, D.E.; Novick, R.P. Evidence for a clonal origin of methicillin resistance in Staphylococcus aureus. Science 1993, 259, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Klevens, R.M.; Morrison, M.A.; Nadle, J.; Petit, S.; Gershman, K.; Ray, S.; Harrison, L.H.; Lynfield, R.; Dumyati, G.; Townes, J.M.; et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. J. Am. Med. Assoc. 2007, 298, 1763–1771. [Google Scholar] [CrossRef] [PubMed]
- Diep, B.A.; Chambers, H.F.; Graber, C.J.; Szumowski, J.D.; Miller, L.G.; Han, L.L.; Chen, J.H.; Lin, F.; Lin, J.; Phan, T.H.; et al. Emergence of multidrug-resistant, community-associated, methicillin-resistant Staphylococcus aureus clone USA300 in men who have sex with men. Ann. Intern. Med. 2008, 148, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Sacchetti, G.; Maietti, S.; Muzzoli, M.; Scaglianti, M.; Manfredini, S.; Radice, M.; Bruni, R. Comparative evaluation of 11 essential oils of different origin as functional antioxidants, antiradicals and antimicrobials in foods. Food Chem. 2005, 91, 621–632. [Google Scholar] [CrossRef]
- Kuorwel, K.K.; Cran, M.J.; Sonneveld, K.; Miltz, J.; Bigger, S.W. Essential oils and their principal constituents as antimicrobial agents for synthetic packaging films. J. Food Sci. 2011, 76, R164–R177. [Google Scholar] [CrossRef] [PubMed]
- Koch, G.; Yepes, A.; Forstner, K.U.; Wermser, C.; Stengel, S.T.; Modamio, J.; Ohlsen, K.; Foster, K.R.; Lopez, D. Evolution of resistance to a last-resort antibiotic in Staphylococcus aureus via bacterial competition. Cell 2014, 158, 1060–1071. [Google Scholar] [CrossRef] [PubMed]
- Ohno, T.; Kita, M.; Yamaoka, Y.; Imamura, S.; Yamamoto, T.; Mitsufuji, S.; Kodama, T.; Kashima, K.; Imanishi, J. Antimicrobial activity of essential oils against Helicobacter pylori. Helicobacter 2003, 8, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Duthie, E.S.; Haughton, G. Purification of free staphylococcal coagulase. Biochem. J. 1958, 70, 125–134. [Google Scholar] [PubMed]
- Gillaspy, A.F.; Hickmon, S.G.; Skinner, R.A.; Thomas, J.R.; Nelson, C.L.; Smeltzer, M.S. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect. Immun. 1995, 63, 3373–3380. [Google Scholar] [PubMed]
- Beenken, K.E.; Blevins, J.S.; Smeltzer, M.S. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect. Immun. 2003, 71, 4206–4211. [Google Scholar] [CrossRef] [PubMed]
- Diep, B.A.; Gill, S.R.; Chang, R.F.; Phan, T.H.; Chen, J.H.; Davidson, M.G.; Lin, F.; Lin, J.; Carleton, H.A.; Mongodin, E.F. Complete genome sequence of USA300, an epidemic clone of community-acquired meticillin-resistant Staphylococcus aureus. Lancet 2006, 367, 731–739. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998, 30, 295–304. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A. Microtiter dish biofilm formation assay. J. Vis. Exp. 2011. [Google Scholar] [CrossRef] [PubMed]
- Kwasny, S.M.; Opperman, T.J. Static biofilm cultures of gram-positive pathogens grown in a microtiter format used for anti-biofilm drug discovery. Curr. Protoc. Pharmacol. 2010, 50, 13A.18.11–13A.18.23. [Google Scholar]
- Pruessner, J.C.; Kirschbaum, C.; Meinlschmid, G.; Hellhammer, D.H. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 2003, 28, 916–931. [Google Scholar] [CrossRef]
- Sample Availability: Commercially available.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Espina, L.; Pagán, R.; López, D.; García-Gonzalo, D. Individual Constituents from Essential Oils Inhibit Biofilm Mass Production by Multi-Drug Resistant Staphylococcus aureus. Molecules 2015, 20, 11357-11372. https://doi.org/10.3390/molecules200611357
Espina L, Pagán R, López D, García-Gonzalo D. Individual Constituents from Essential Oils Inhibit Biofilm Mass Production by Multi-Drug Resistant Staphylococcus aureus. Molecules. 2015; 20(6):11357-11372. https://doi.org/10.3390/molecules200611357
Chicago/Turabian StyleEspina, Laura, Rafael Pagán, Daniel López, and Diego García-Gonzalo. 2015. "Individual Constituents from Essential Oils Inhibit Biofilm Mass Production by Multi-Drug Resistant Staphylococcus aureus" Molecules 20, no. 6: 11357-11372. https://doi.org/10.3390/molecules200611357
APA StyleEspina, L., Pagán, R., López, D., & García-Gonzalo, D. (2015). Individual Constituents from Essential Oils Inhibit Biofilm Mass Production by Multi-Drug Resistant Staphylococcus aureus. Molecules, 20(6), 11357-11372. https://doi.org/10.3390/molecules200611357






