Developments in Synthetic Application of Selenium(IV) Oxide and Organoselenium Compounds as Oxygen Donors and Oxygen-Transfer Agents
Abstract
:1. Introduction
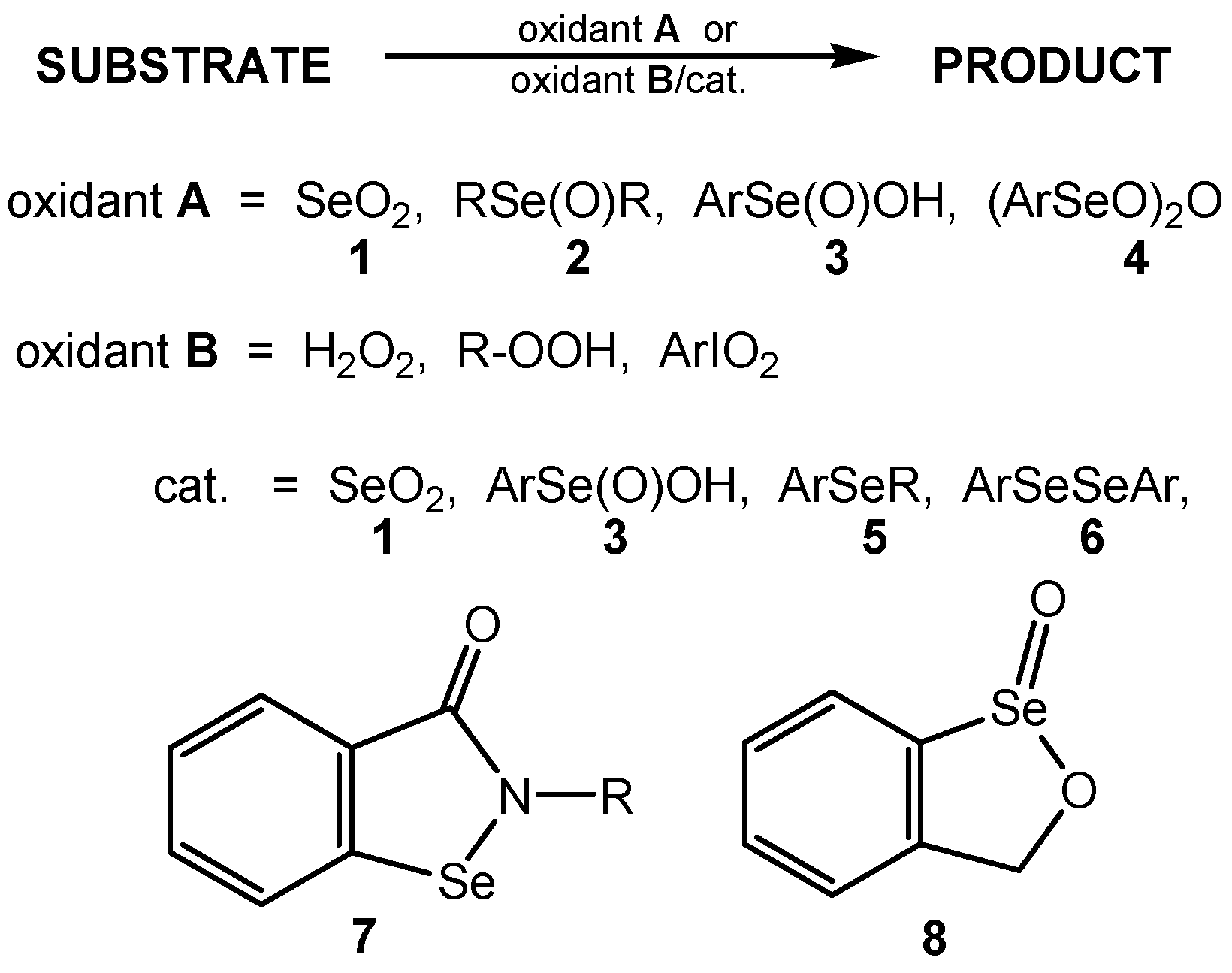
2. Selenium(IV) Oxide and Selenic(IV) Acid as Oxidizing Agents and Oxidation Catalysts
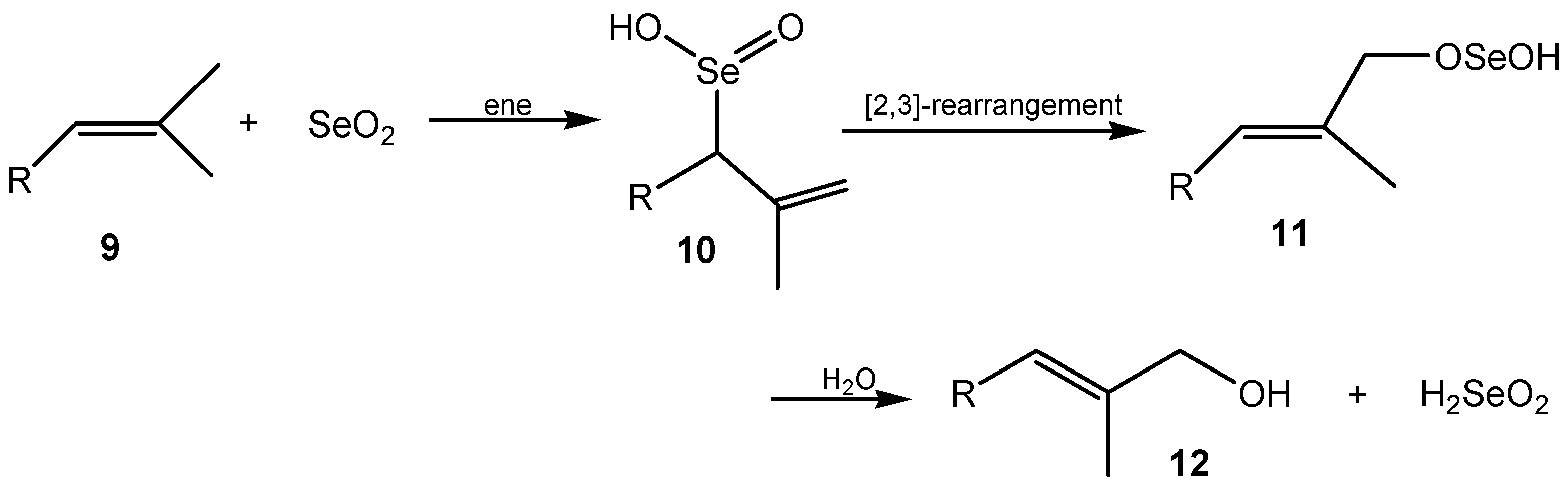
2.1. Allylic Hydroxylation
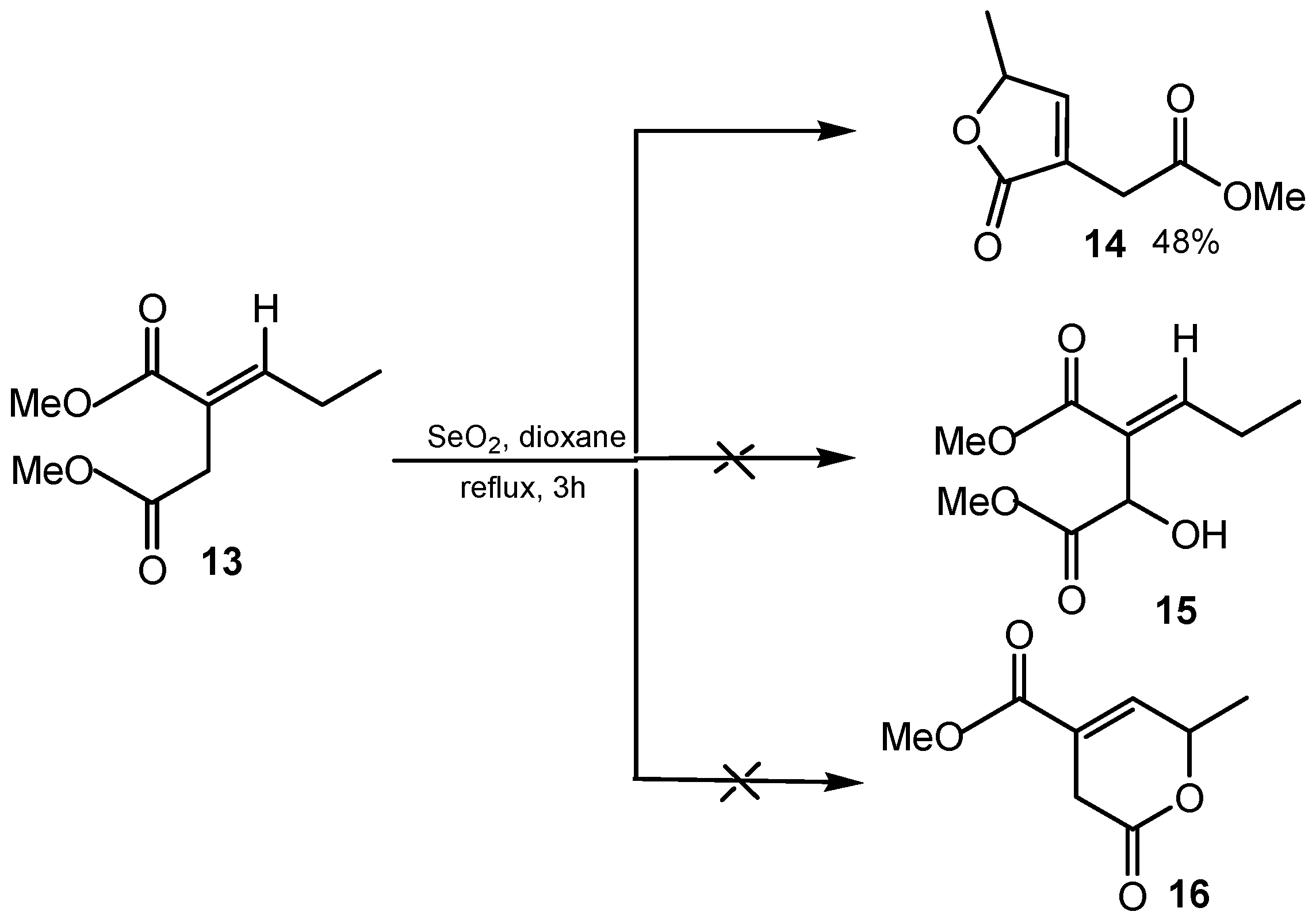
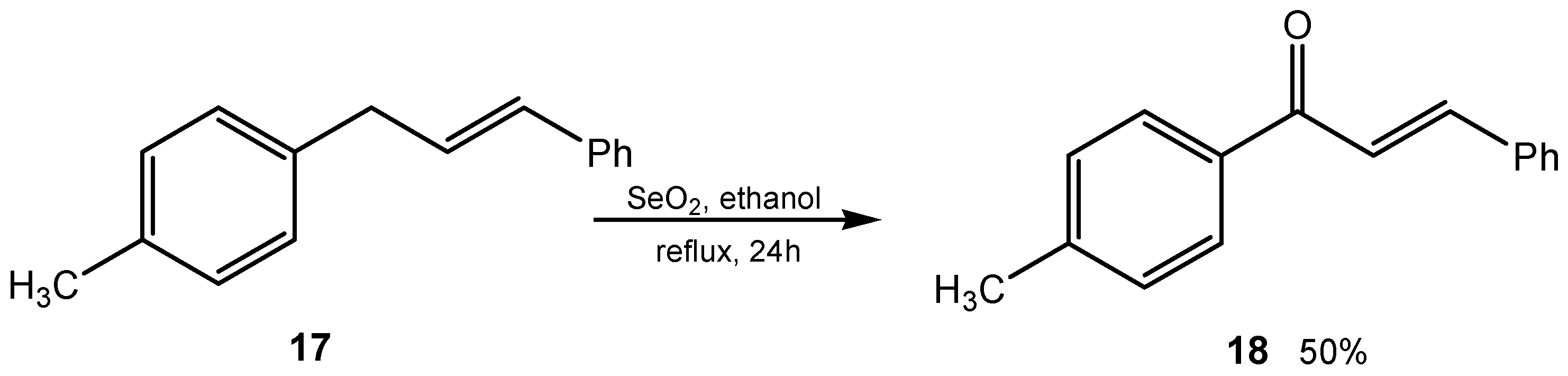
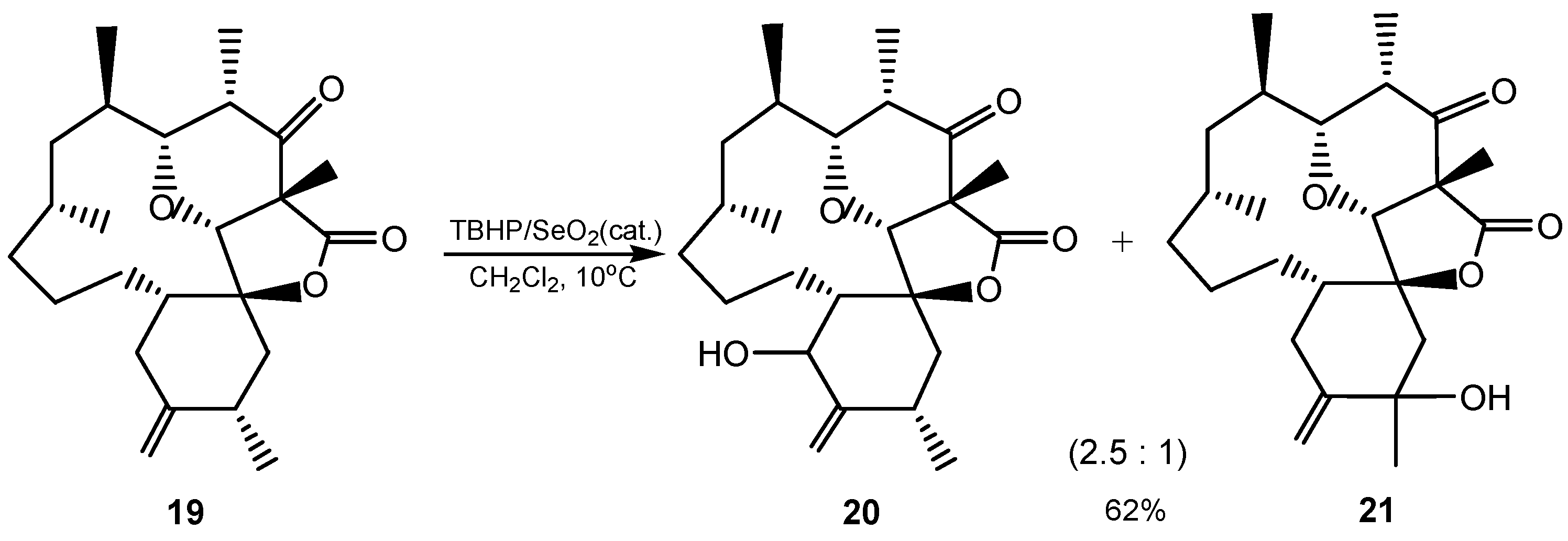
2.2. 1,2-Dihydroxylation of Alkenes
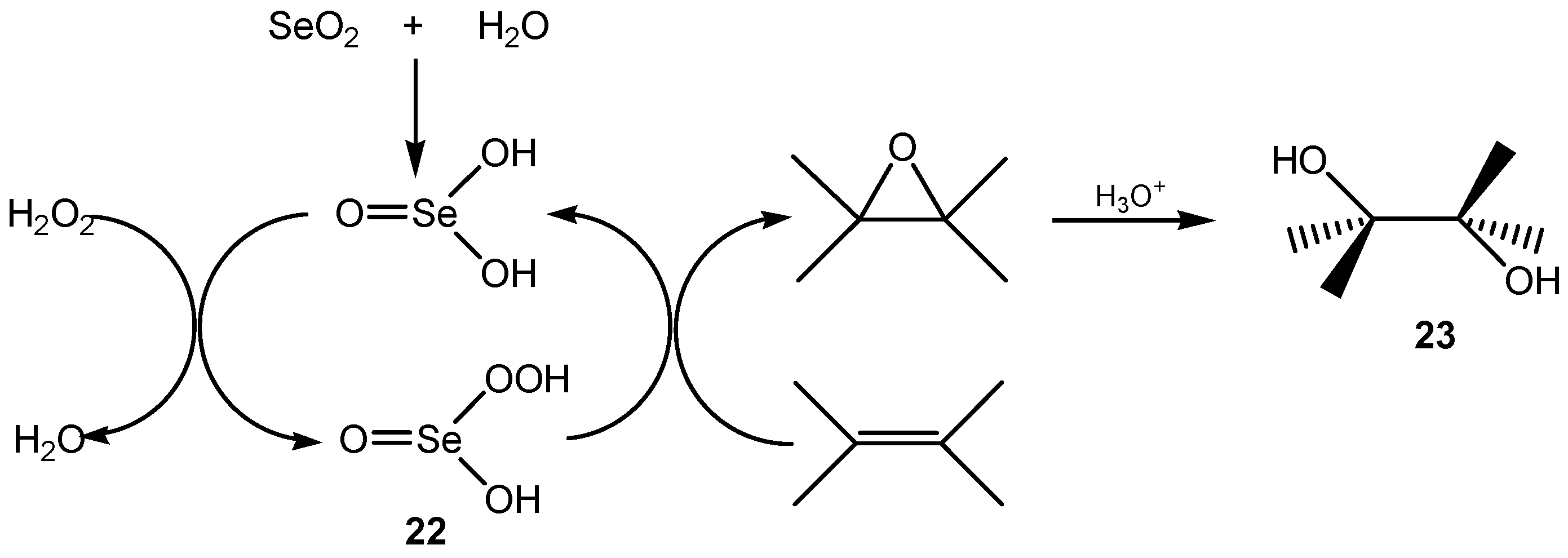
2.3. Oxidation of the Methyl and Methylene Groups
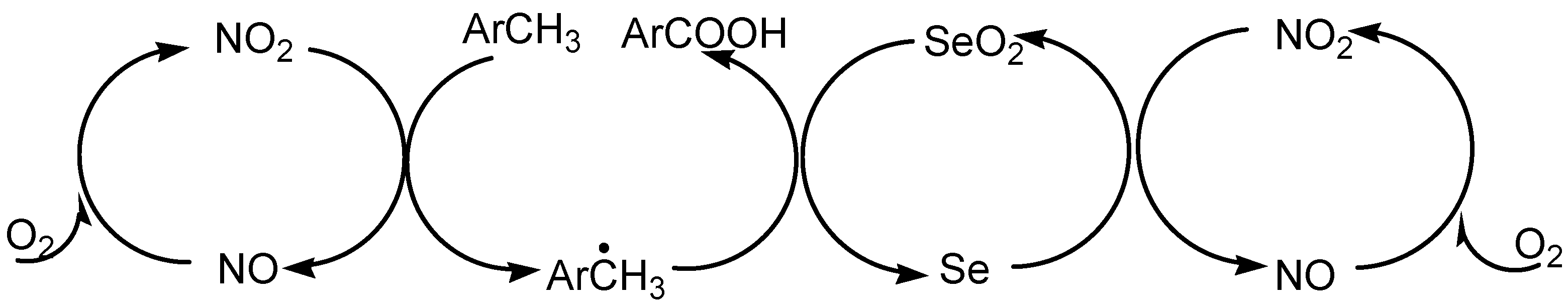
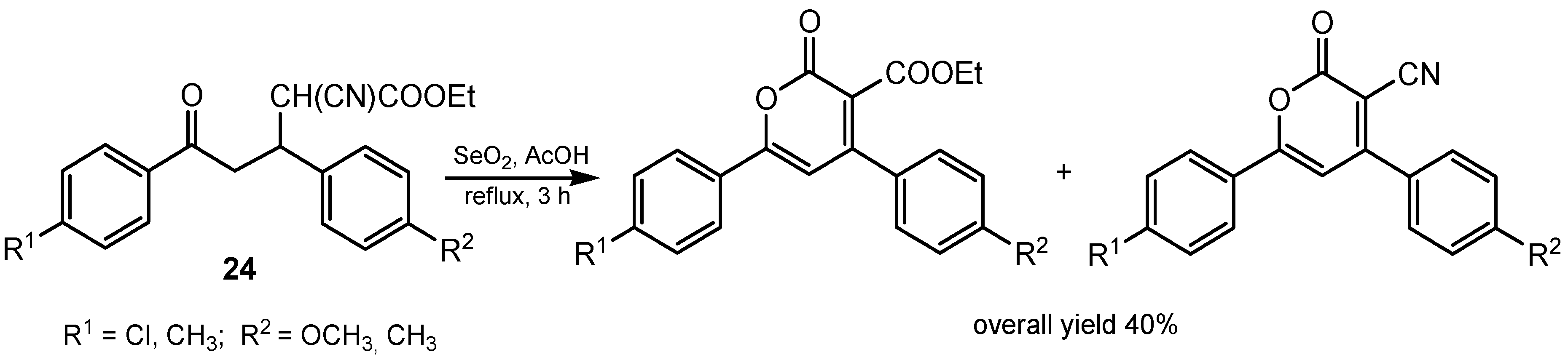
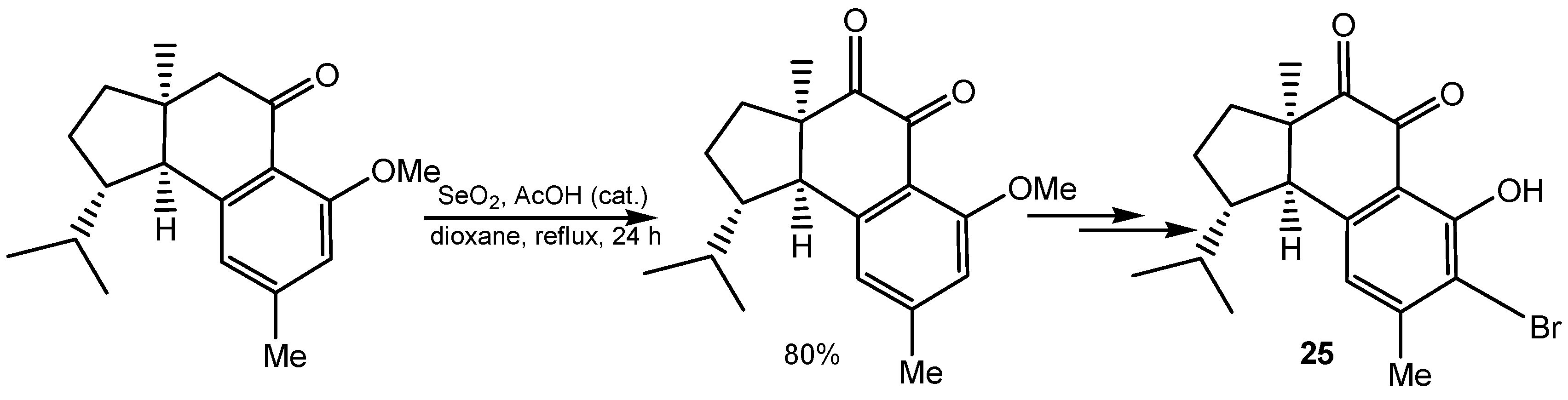
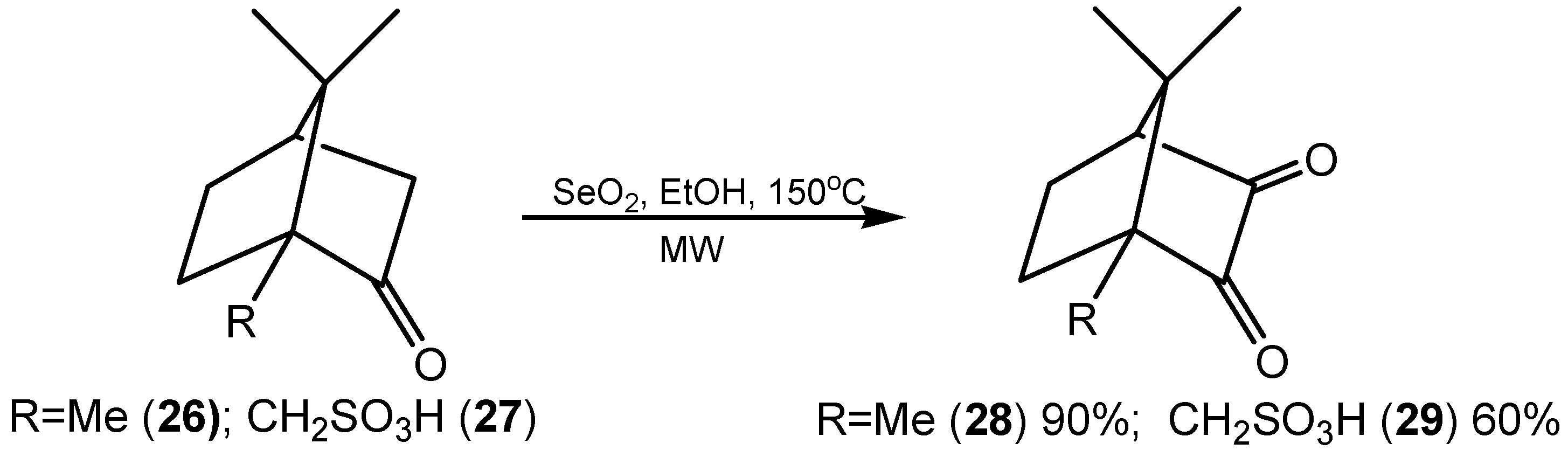
2.4. Dehydrogenation and Oxidative Bond Cleavage
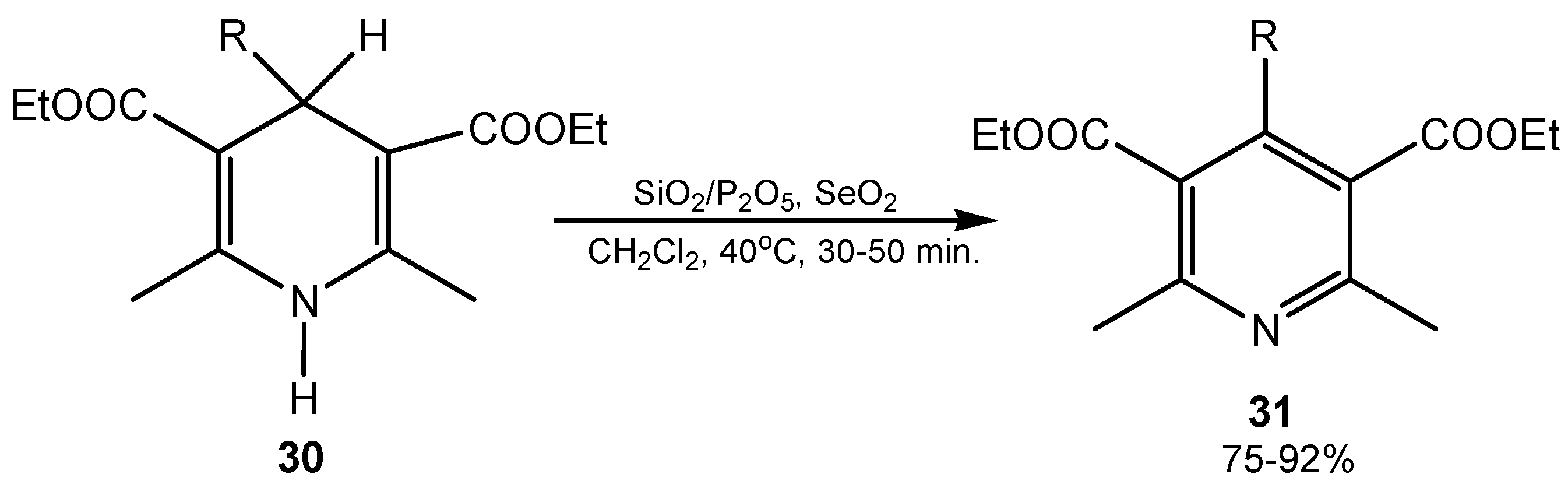
2.5. Oxidative Cyclization and Ring Transformations
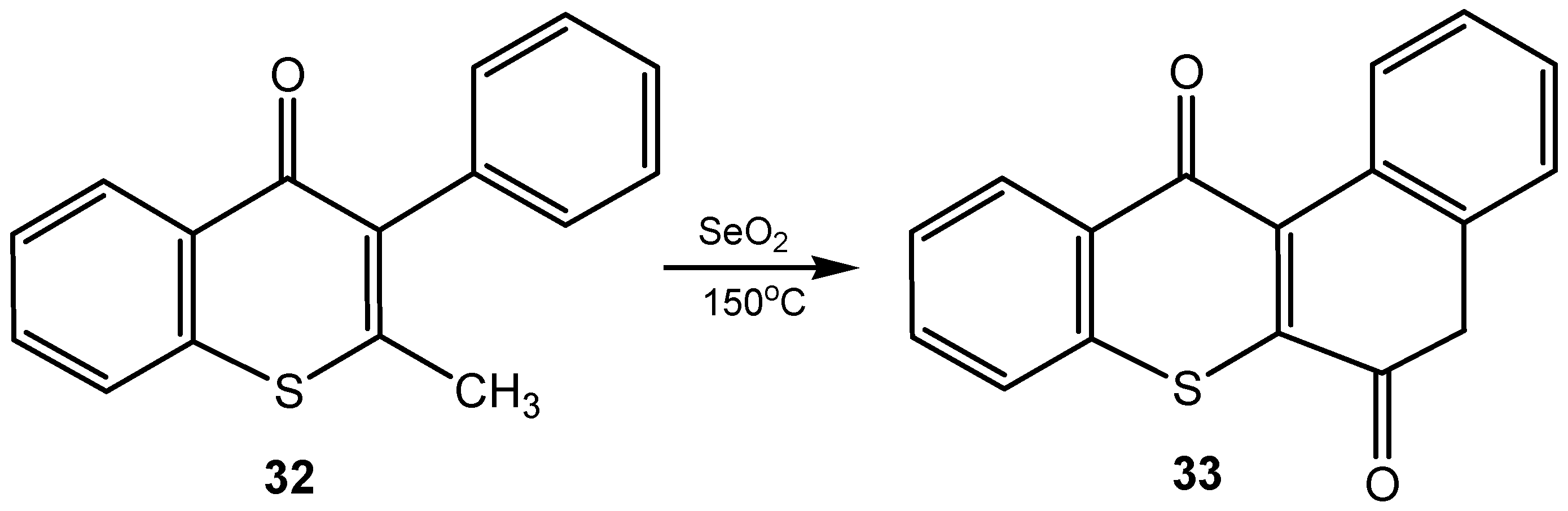
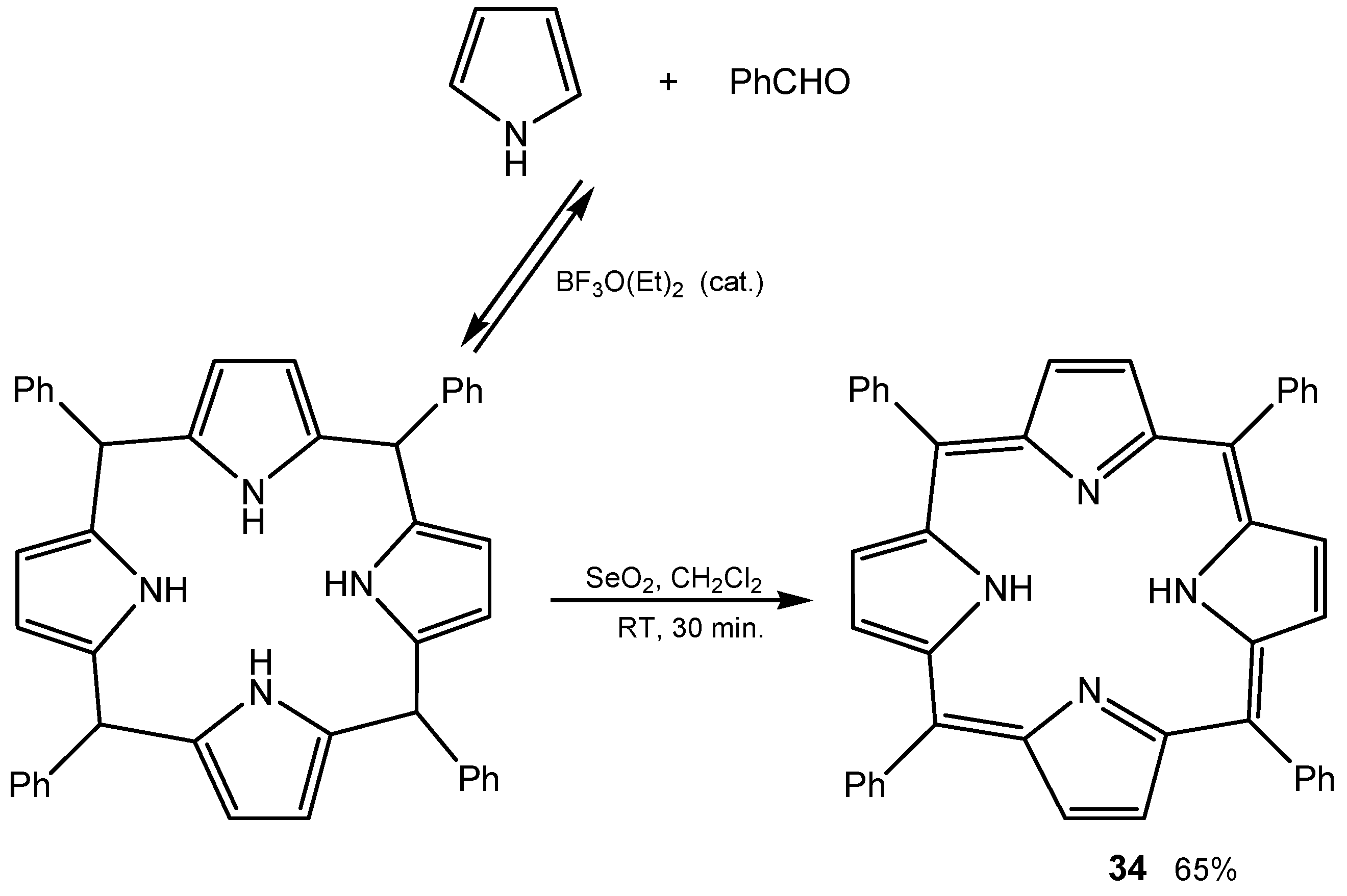
2.6. Miscellaneous Oxidative Transformations
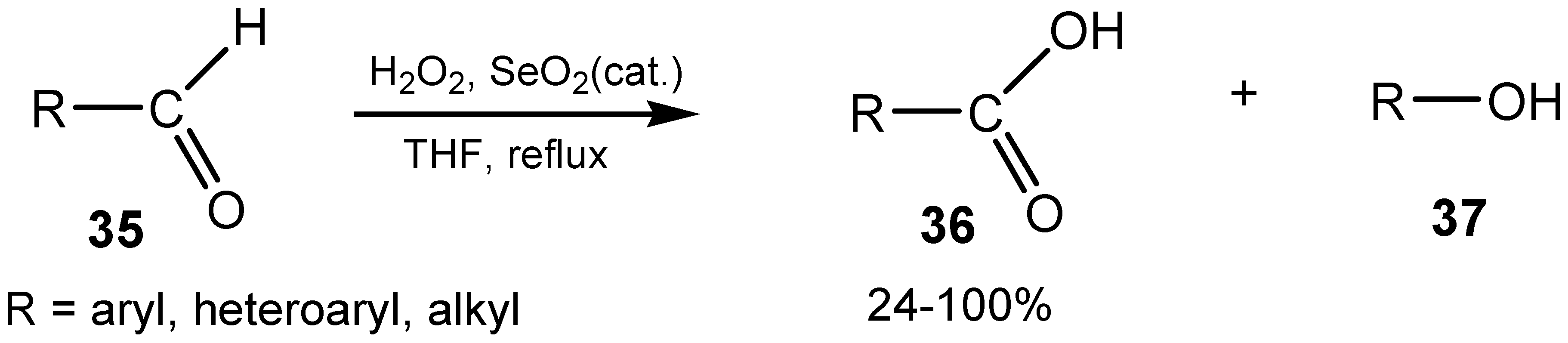
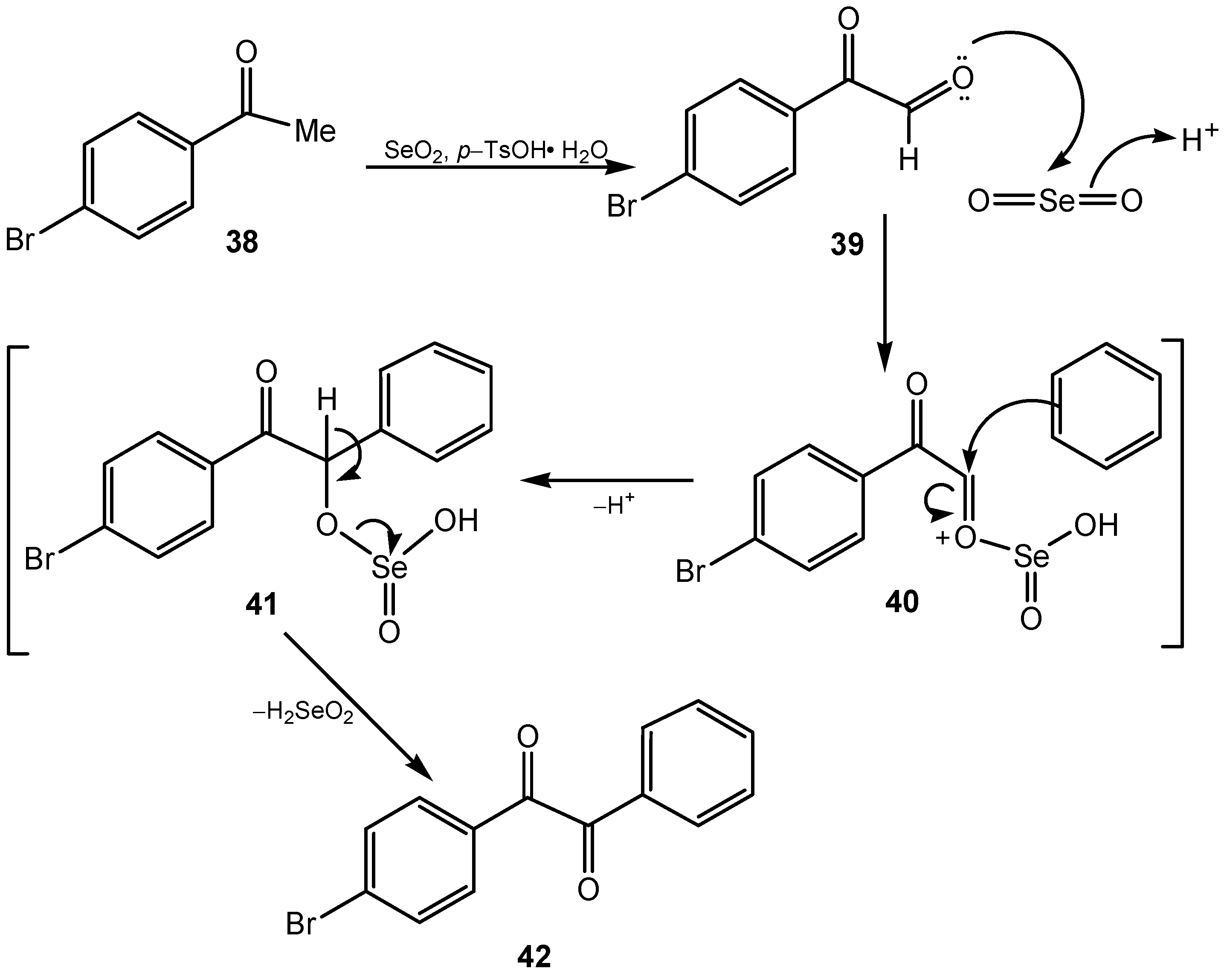
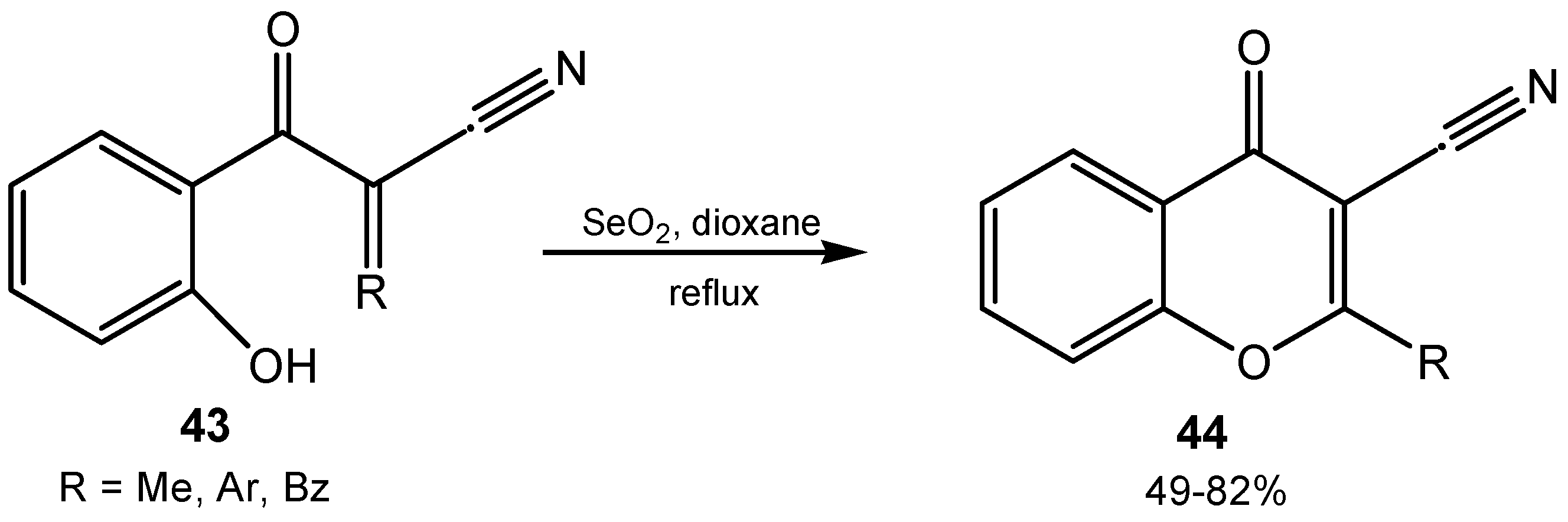
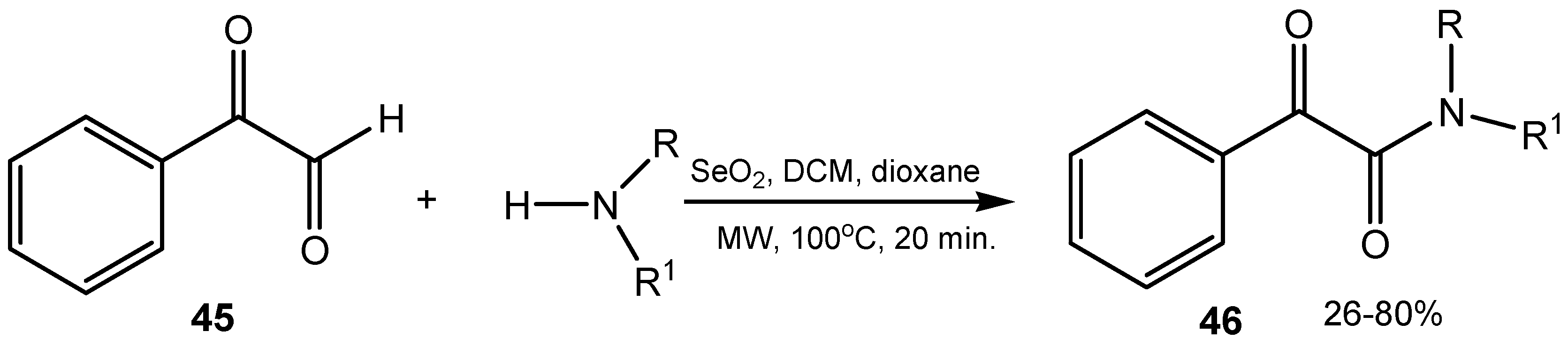
3. Organoselenium Compounds as Oxidizing Agents and Oxidation Catalysts
3.1. Selenides and Selenoxides
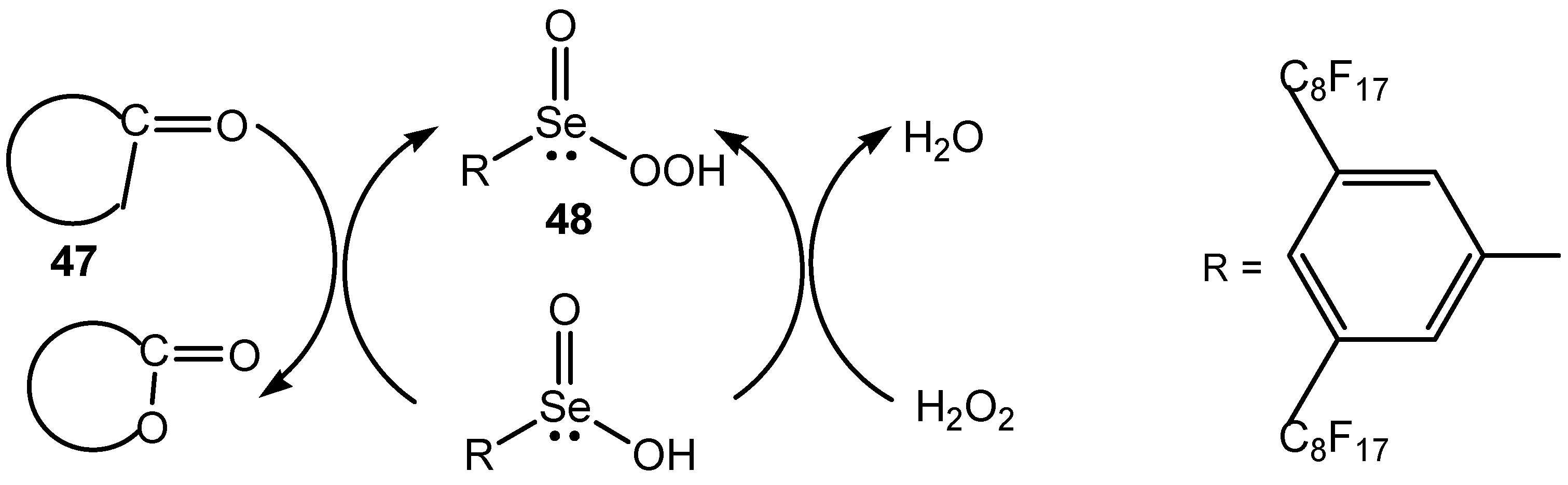
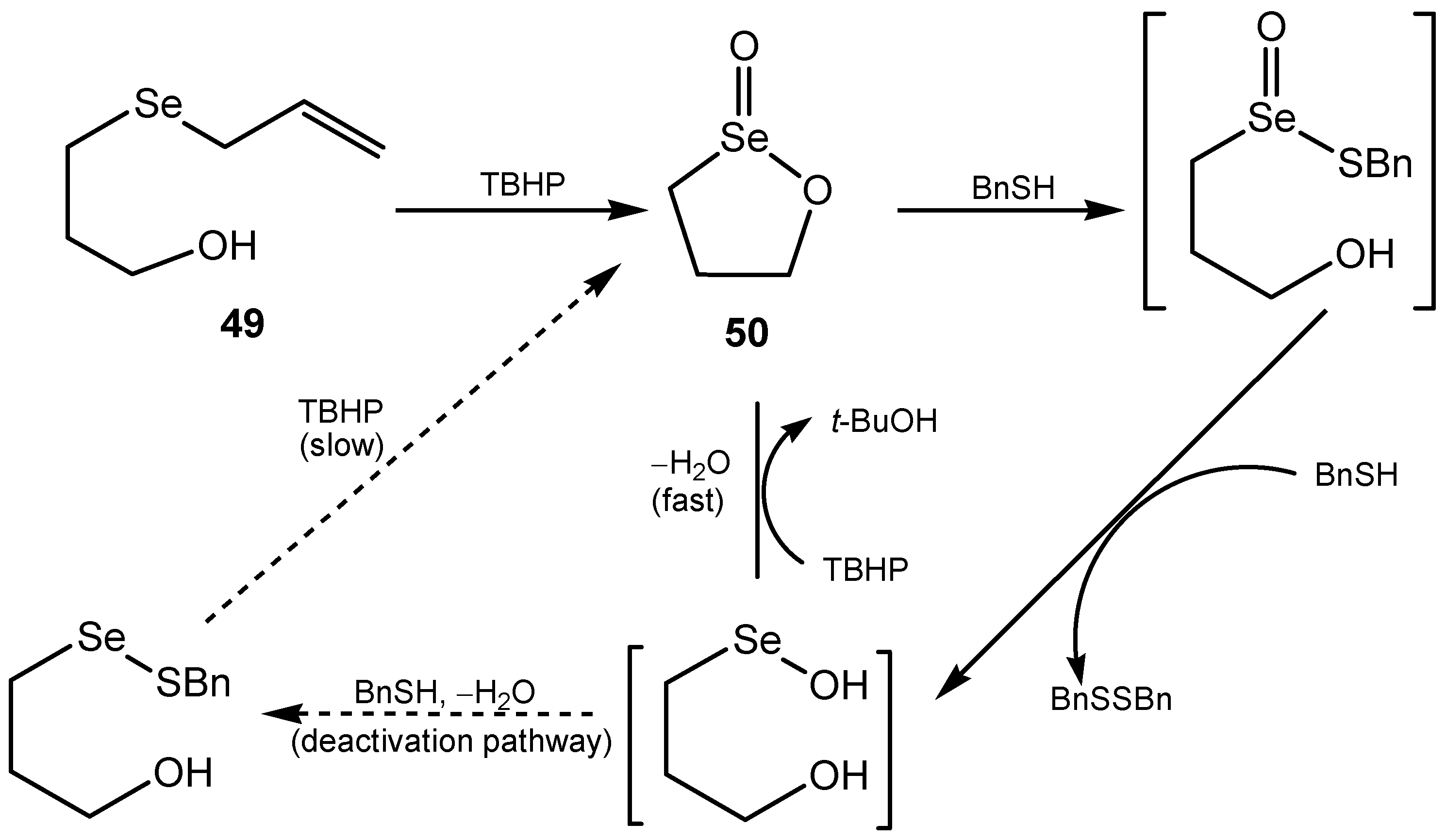
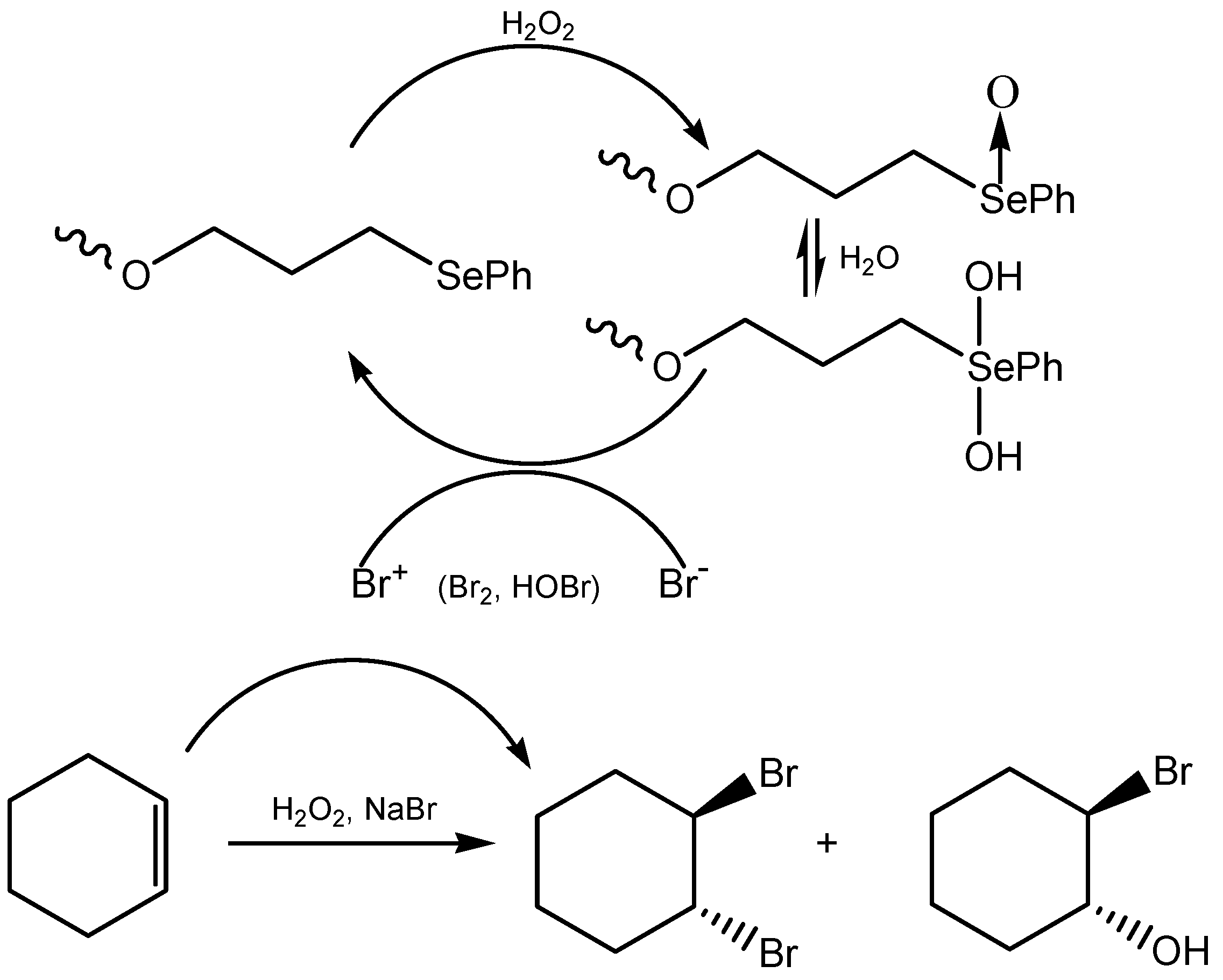
3.2. Seleninic Acids and Their Derivatives
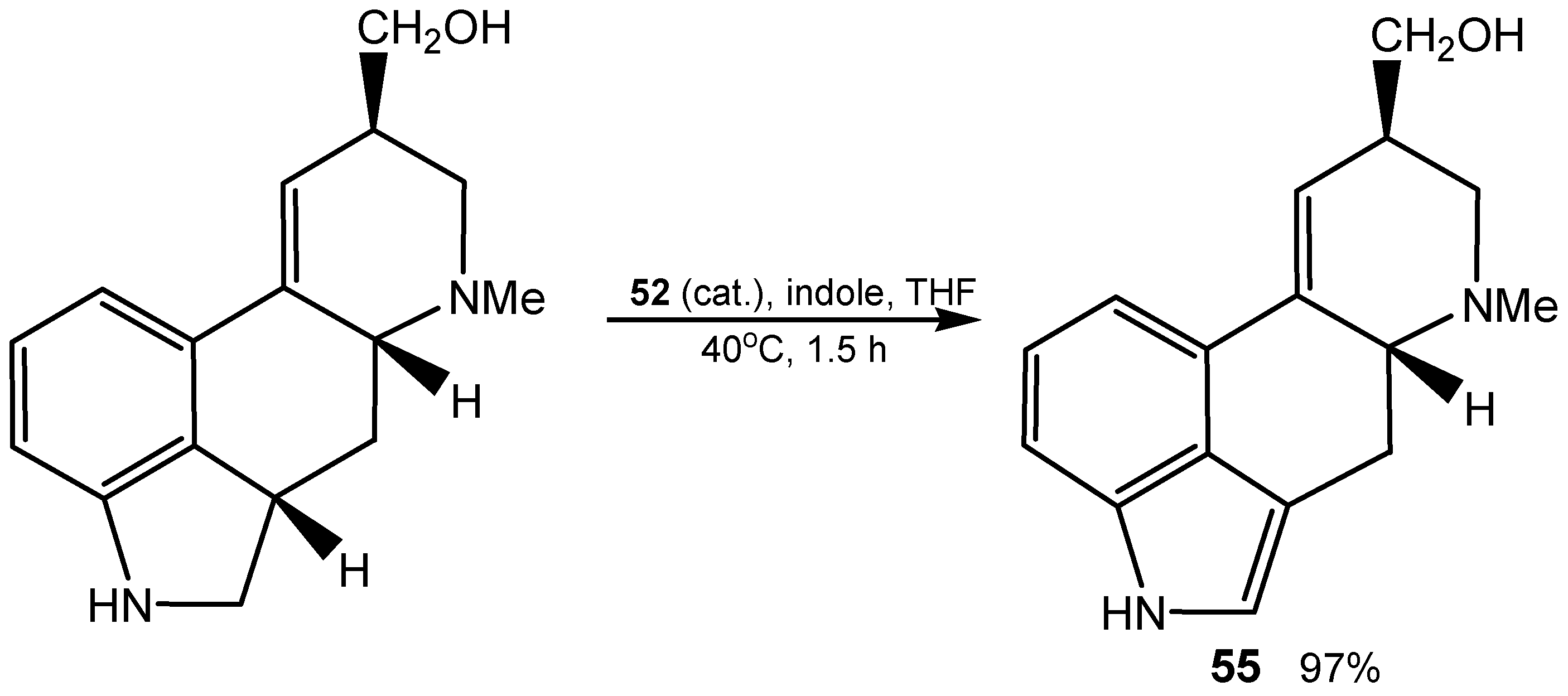
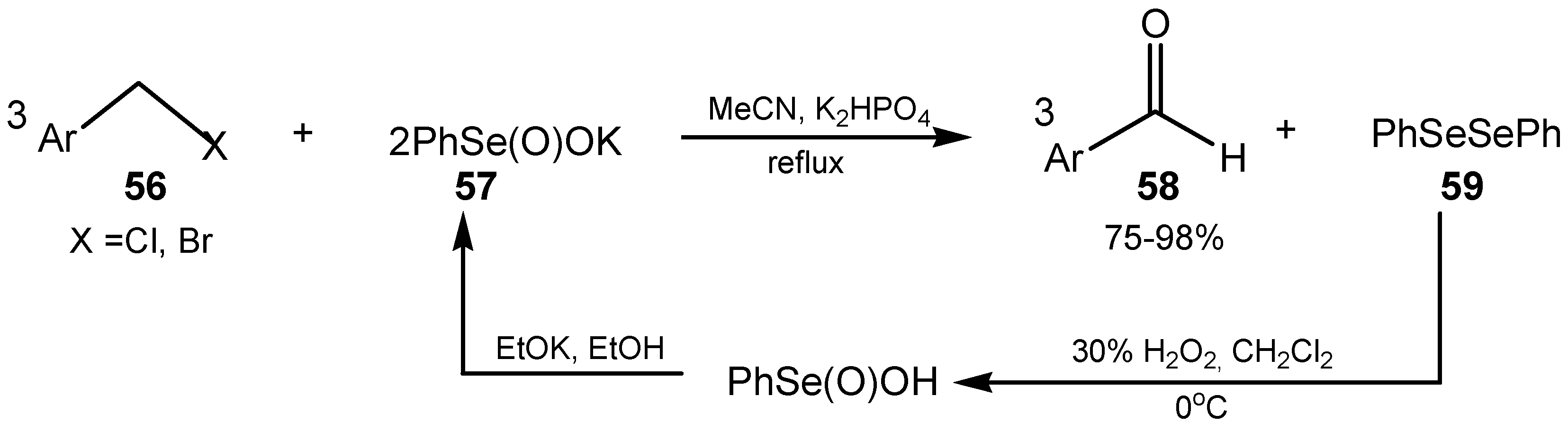
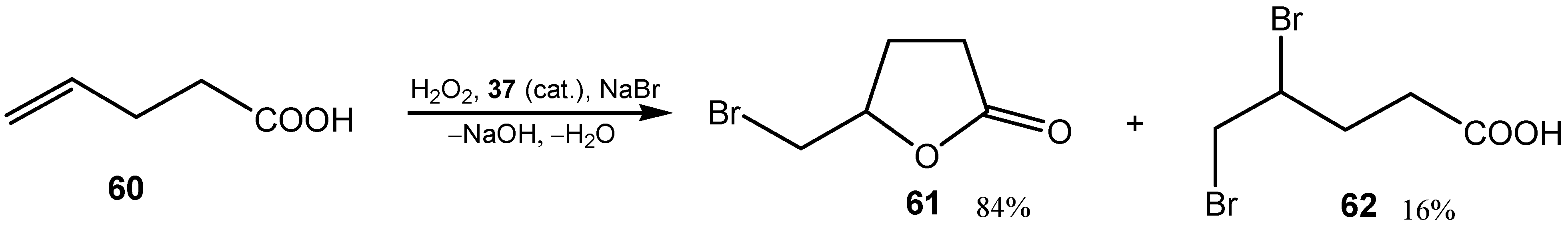
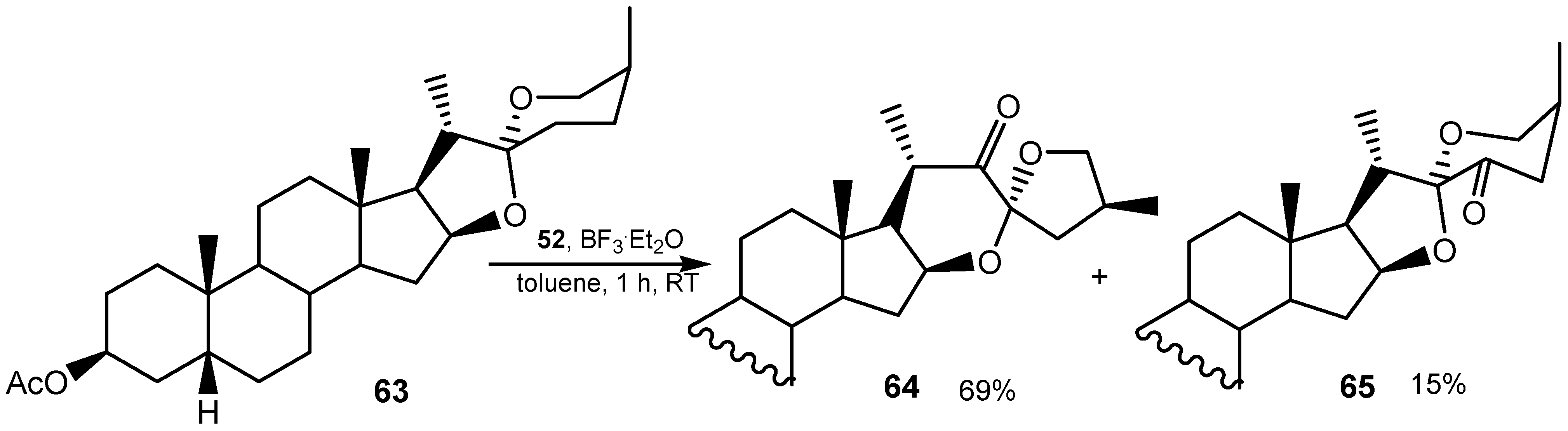
3.3. Diselenides
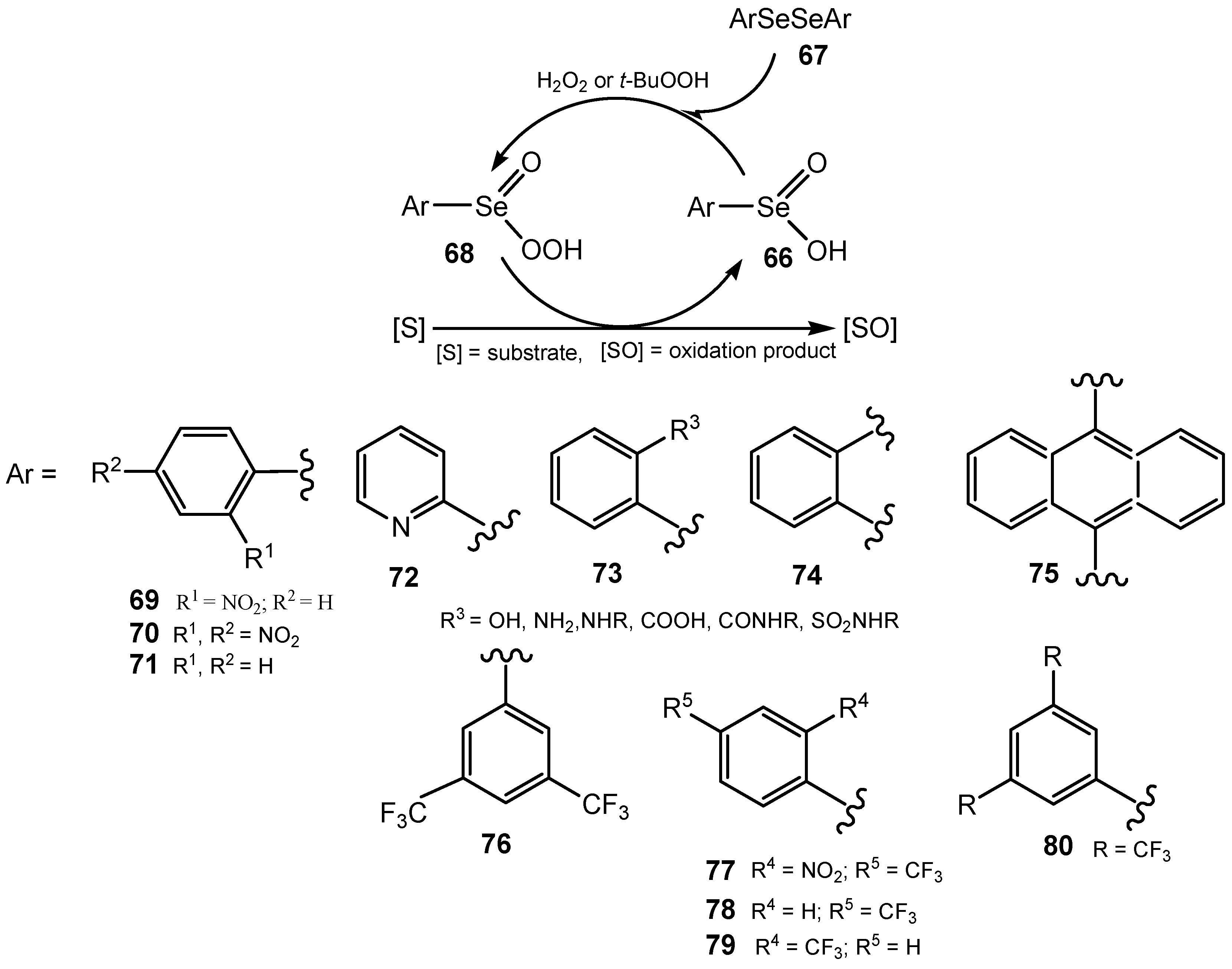
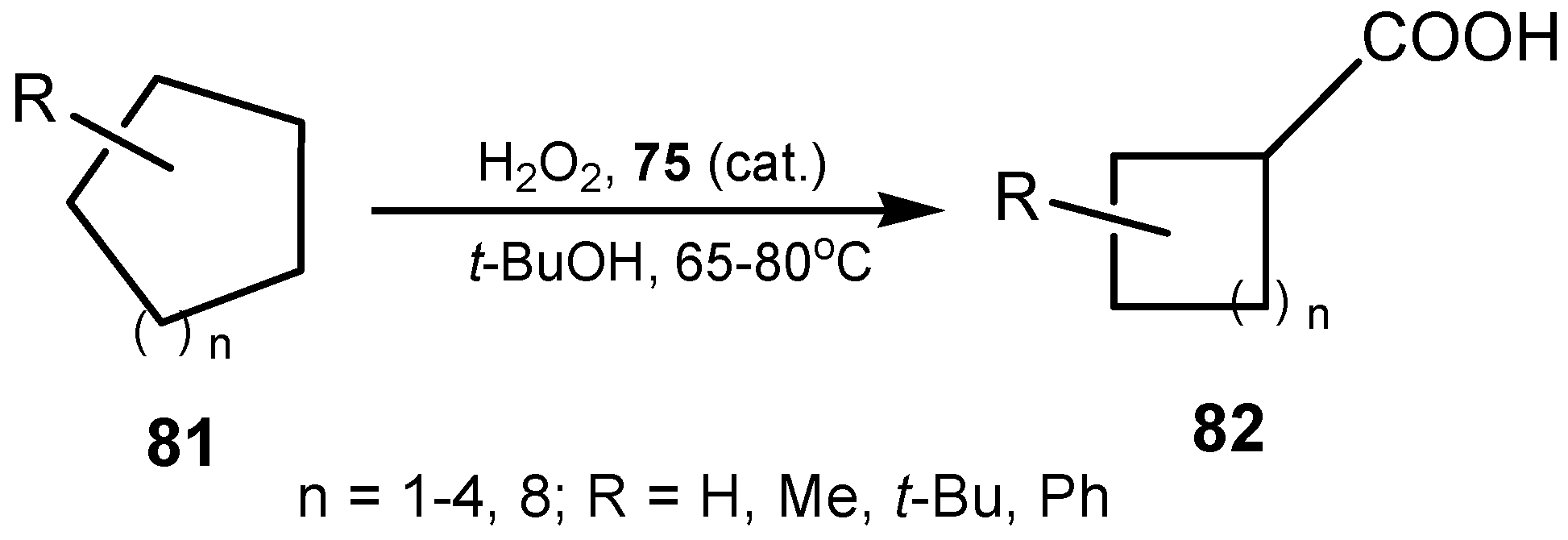
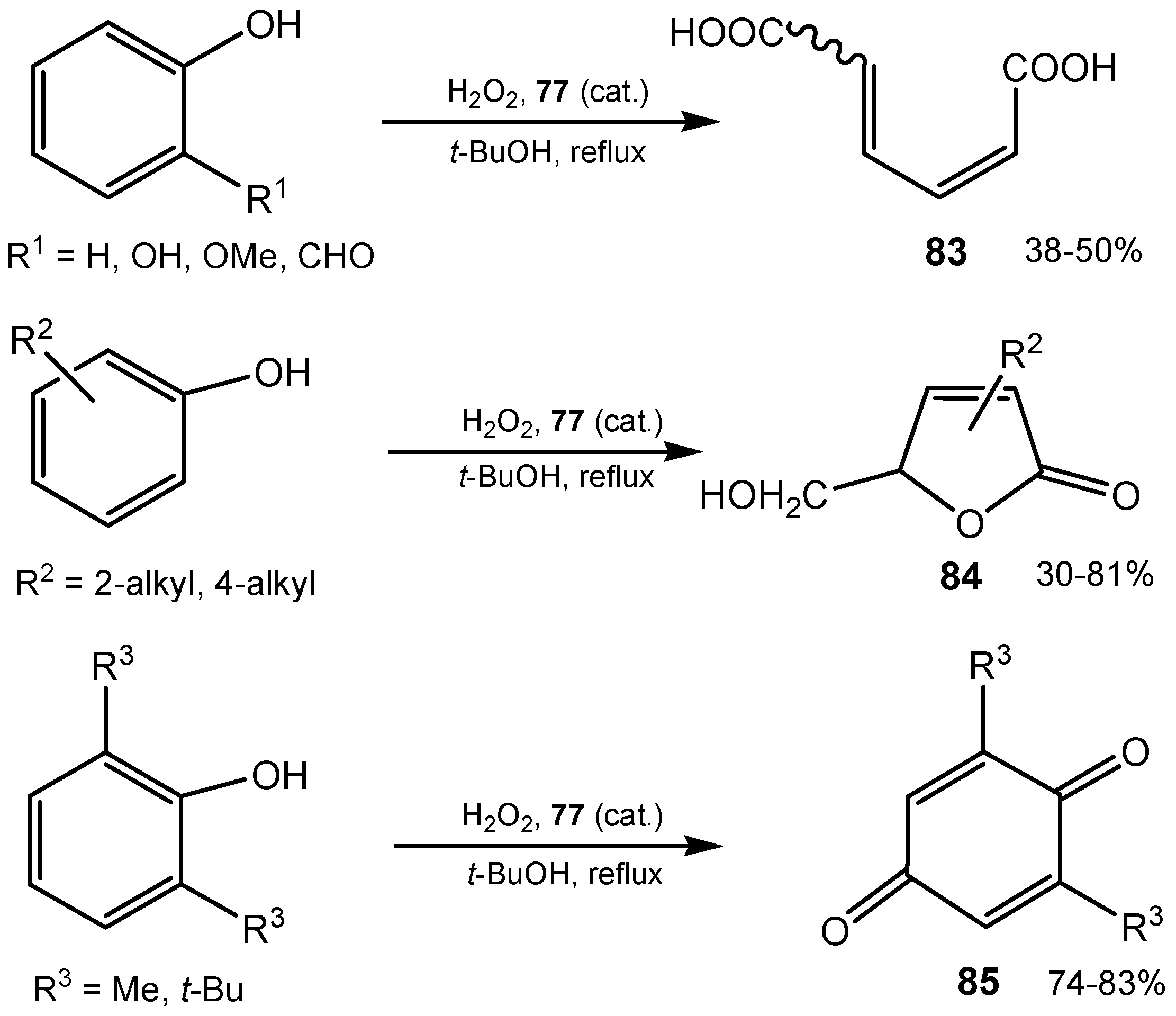
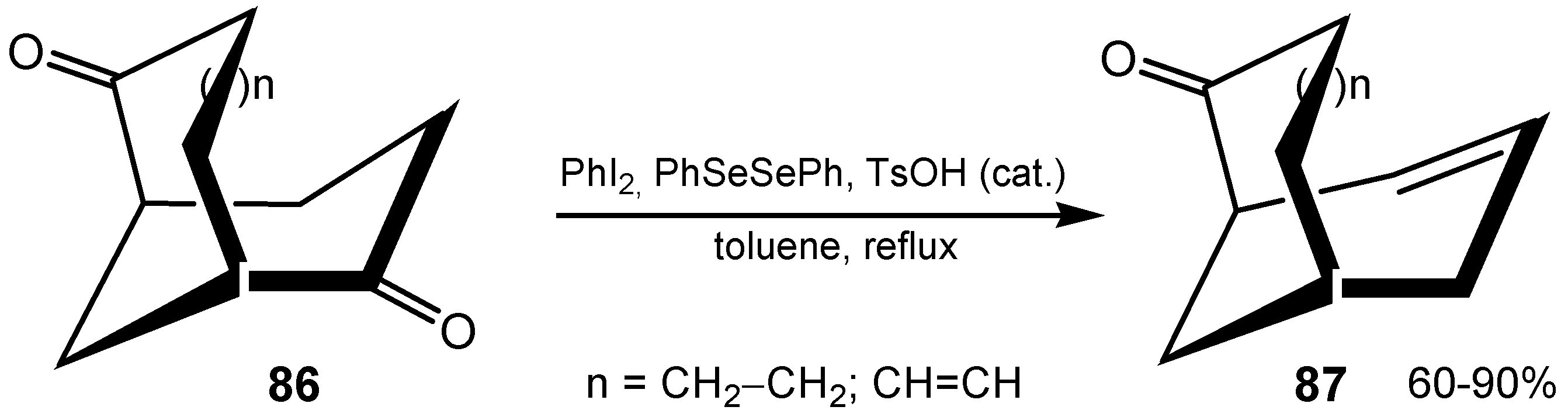
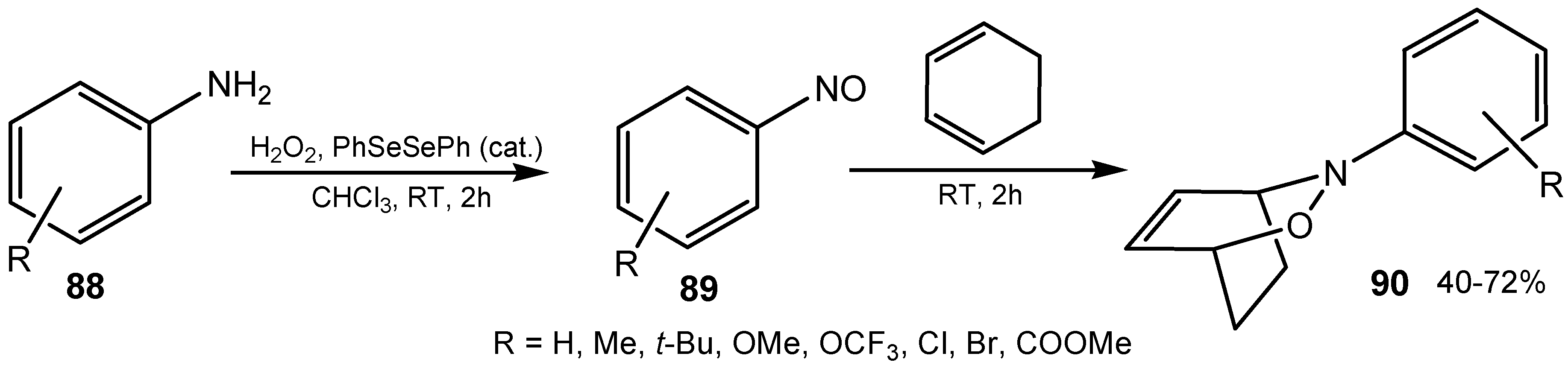
3.4. Selenenamides and Related Compounds
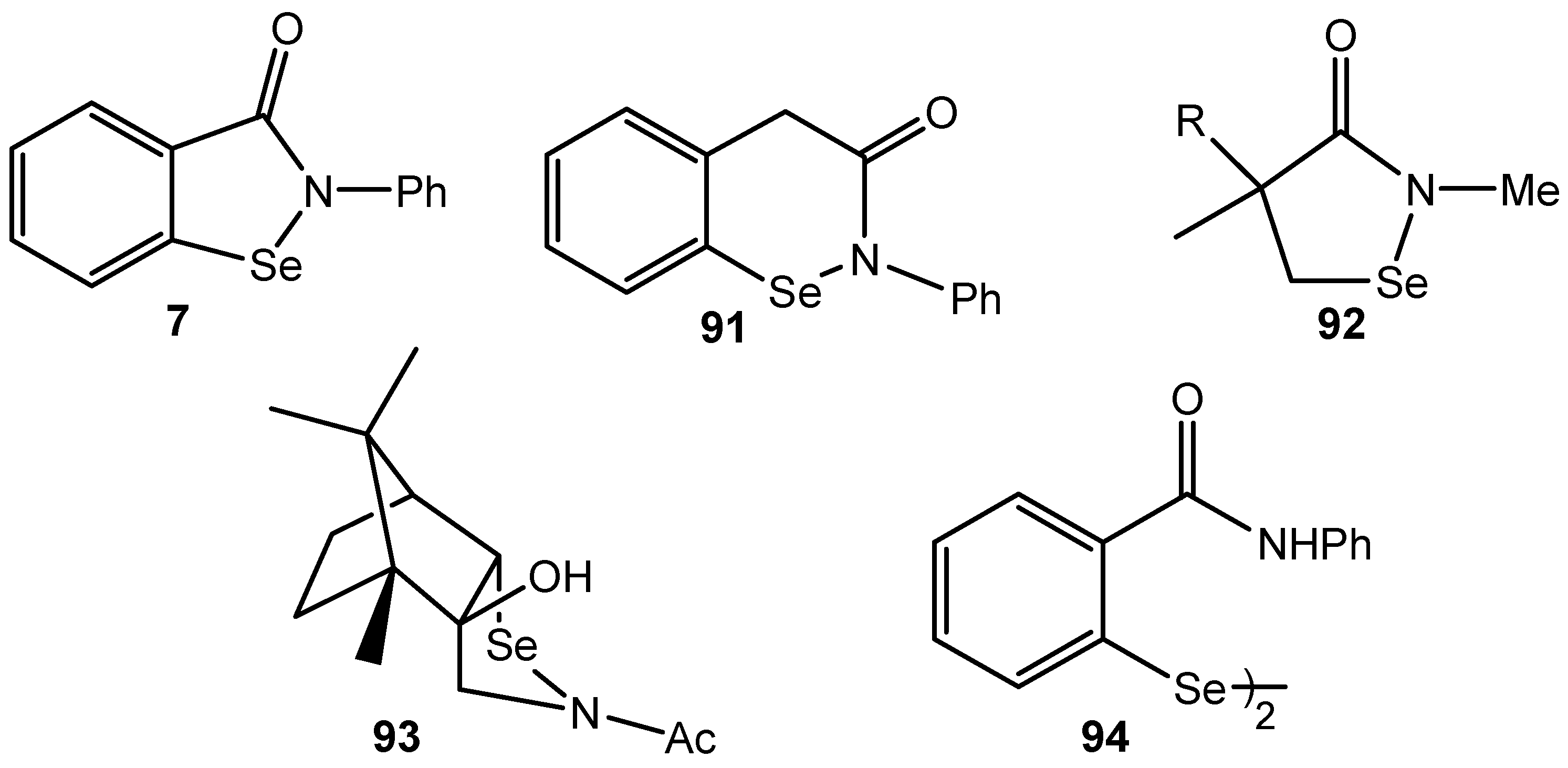
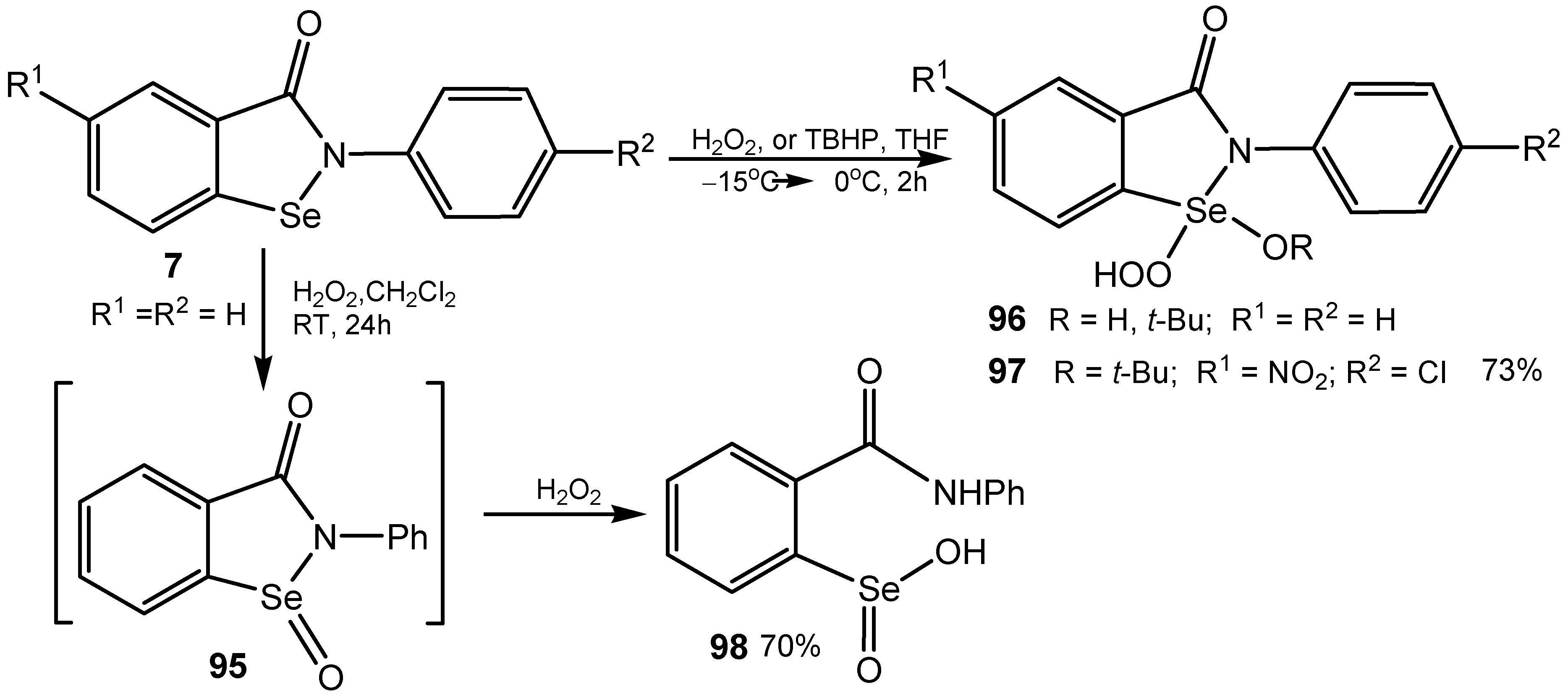
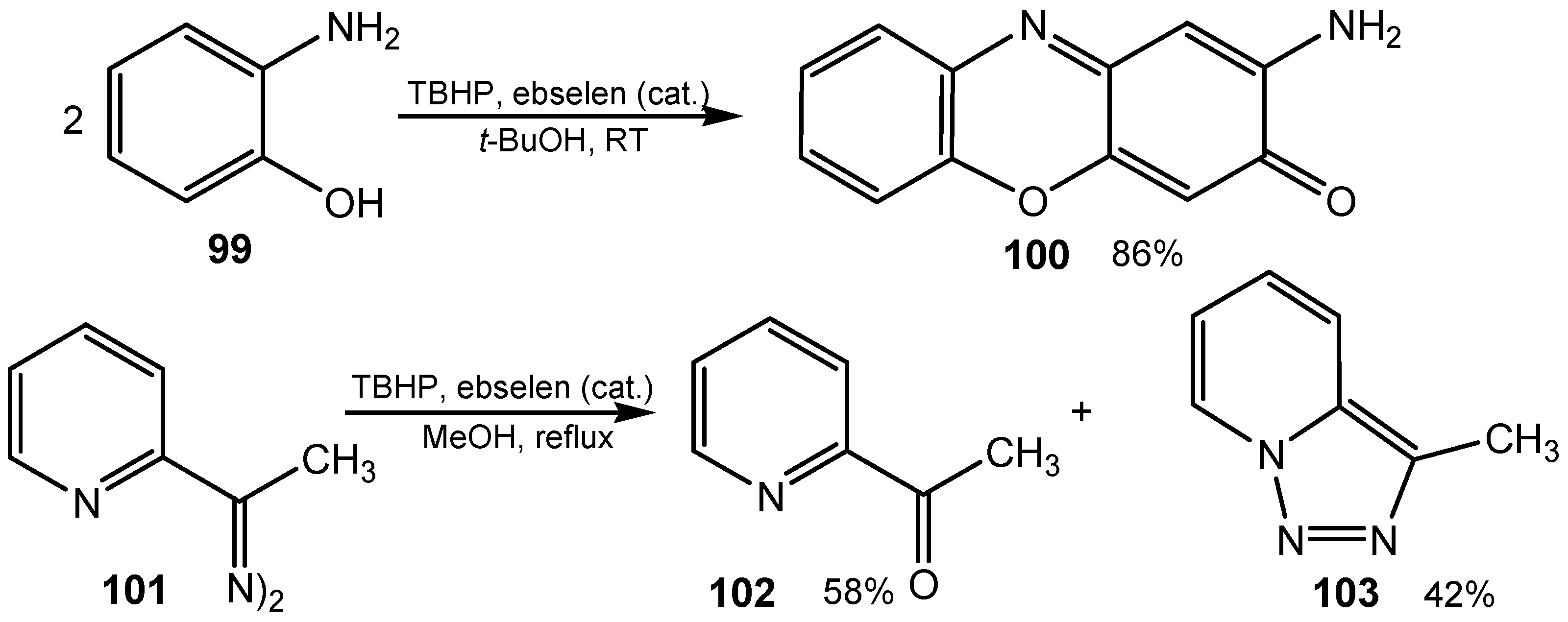
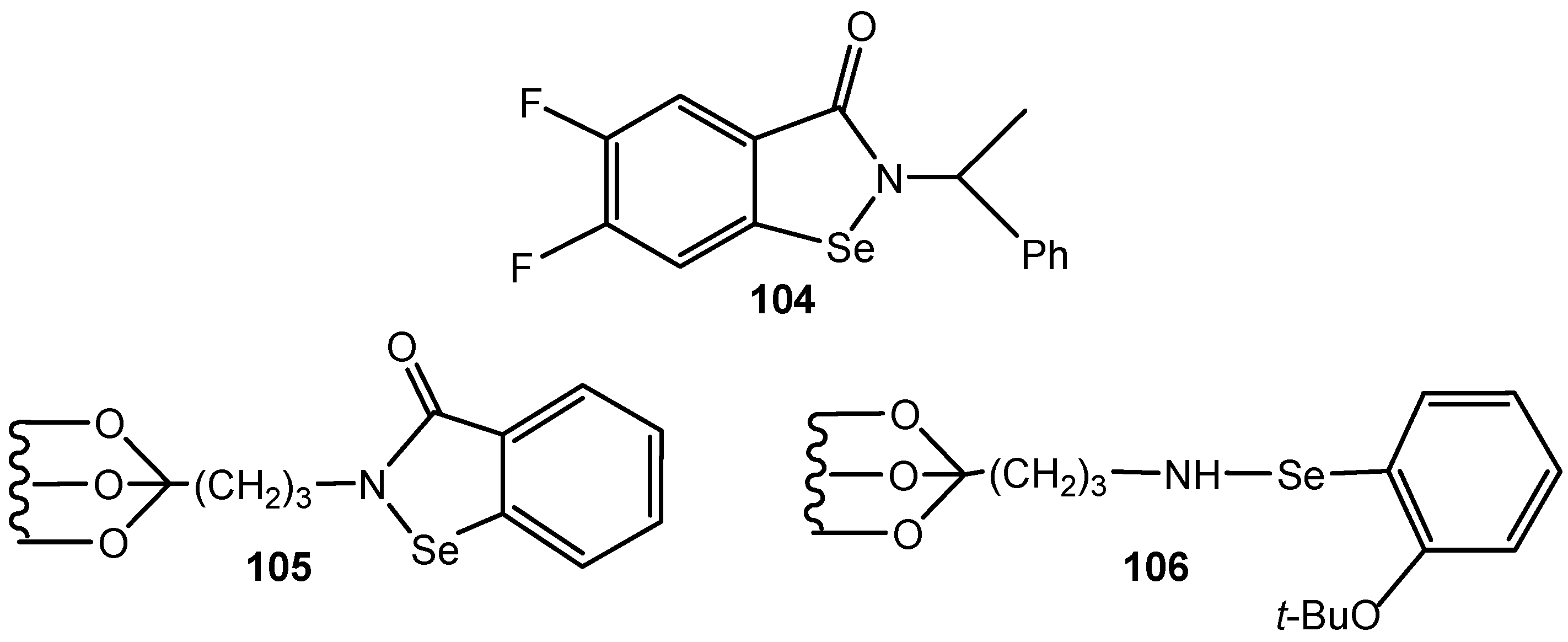
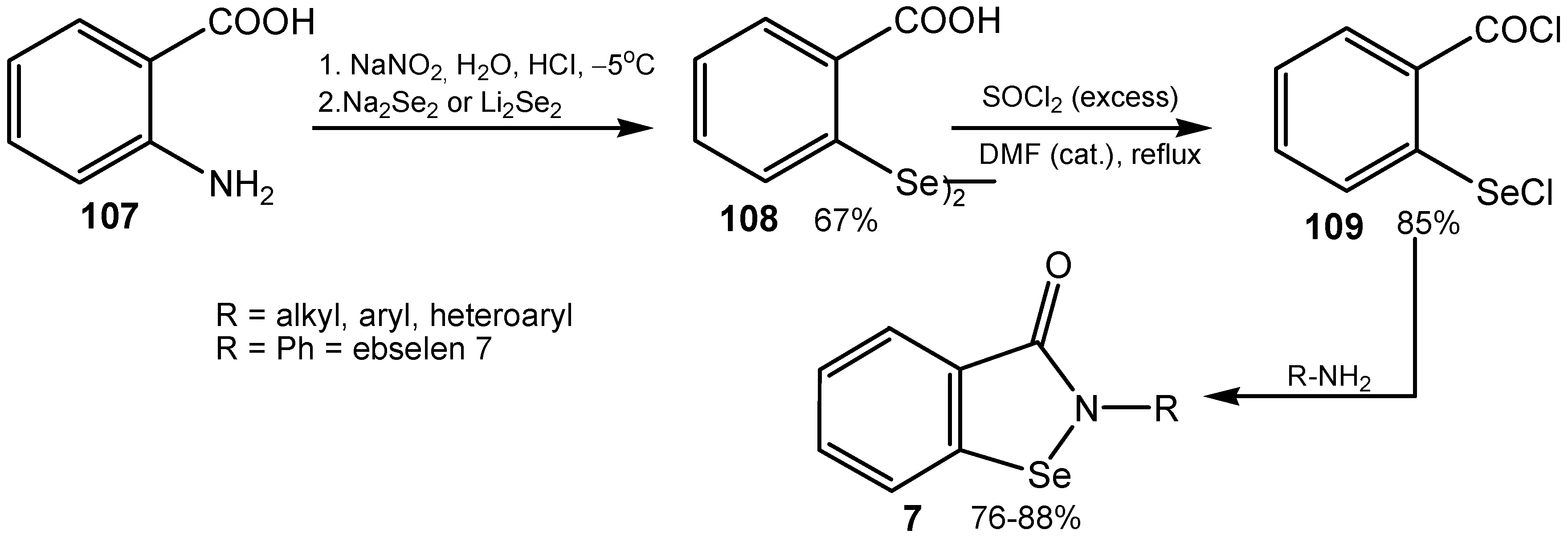
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Paulmier, C. Selenium Reagents and Intermediates in Organic Synthesis; Pergamon Press: Oxford, UK, 1986. [Google Scholar]
- Back, T.G. Preparative uses of organoselenium and organotellurium compounds. In The Chemistry of Organic Selenium and Tellurium Compounds; Patai, S., Ed.; John Wiley & Sons: Chichester, UK, 1987; Volume 2, pp. 91–213. [Google Scholar]
- Młochowski, J. Organoselenium compounds as oxidants and oxidation catalysts. Phosphorus Sulphur Silicon 1998, 136, 191–204. [Google Scholar] [CrossRef]
- Back, T.G. Oxidation with Selenium Reagents. In Organoselenium Chemistry. A Practical Approach; Back, T.G., Ed.; Oxford University Press: Oxford, UK, 1999; pp. 93–112. [Google Scholar]
- Nishibayashi, Y.; Uemura, S. Selenium Compounds as Ligands and Catalysts. Top. Curr. Chem. 2000, 208, 236–254. [Google Scholar]
- Młochowski, J.; Brząszcz, M.; Giurg, M.; Palus, J.; Wójtowicz, H. Selenium-Promoted Oxidation of Organic Compounds: Reactions and Mechanisms. Eur. J. Org. Chem. 2003, 2003, 4329–4339. [Google Scholar] [CrossRef]
- Giurg, M.; Syper, S. Diaryl Diselenides and Related Compounds as Oxygen-Transfer Agents. Phosphorus Sulphur Silicon 2008, 183, 970–985. [Google Scholar] [CrossRef]
- Freudendahl, D.M.; Santoro, S.; Shahzad, S.A.; Santi, C.; Wirth, T. Green Chemistry with Selenium Reagents: Development of Efficient Catalytic Reactions. Angew. Chem. 2009, 48, 8409–8411. [Google Scholar] [CrossRef] [PubMed]
- Młochowski, J.; Lisiak, R.; Wójtowicz-Młochowska, H. Organoselenium and organotellurium oxidation and reduction. In The Chemistry of Organic Selenium and Tellurium Compounds; Patai, S., Rappoport, Z., Liebman, J.F., Marek, I., Eds.; Wiley: Chichester, UK, 2012; Volume 3, Part 2; pp. 1083–1161. [Google Scholar]
- Nomoto, A.; Ogawa, A. Preparative uses of organoselenium and organotellurium compounds. In The Chemistry of Organic Selenium and Tellurium Compounds; Patai, S., Rappoport, Z., Liebman, J.F., Marek, I., Eds.; Wiley: Chichester, UK, 2012; Volume 3, Part 1; pp. 623–688. [Google Scholar]
- Sheldon, R.A. Homogenous and heterogenous catalytic oxidations with peroxide reagents. Top. Curr. Chem. 1993, 164, 21–43. [Google Scholar]
- Młochowski, J.; Said, S.B. Catalyzed Hydrogen Peroxide Oxidation of Organic Compounds. Pol. J. Chem. 1997, 71, 149–169. [Google Scholar]
- Sanderson, W.F. Cleaner industrial processes using hydrogen peroxide. Pure Appl. Chem. 2000, 72, 1289–1304. [Google Scholar] [CrossRef]
- Ten Brink, G.-J.; Arends, I.W.C.E.; Sheldon, R.A. The Bayer-Villiger Reaction: New developments toward greener procedures. Chem. Rev. 2004, 104, 4105–4124. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S.; White, M.C. A predictably selective aliphatic C-H oxidation reactions for complex molecule synthesis. Science 2007, 318, 783–787. [Google Scholar] [CrossRef] [PubMed]
- Gelalcha, F.G. Heterocyclic peroxides in selective oxidations. Chem. Rev. 2007, 107, 3338–3361. [Google Scholar] [CrossRef] [PubMed]
- Młochowski, J.; Peczyńska-Czoch, W.; Piętka-Ottlik, M.; Wójtowicz-Młochowska, H. Non-Metal and Enzymatic Catalysts for Hydroperoxide Oxidation of Organic Compounds. Open Catal. J. 2011, 4, 54–82. [Google Scholar] [CrossRef]
- Breder, A.; Ortgies, S. Recent developments in sulphur- and selenium-catalysed oxidative and isophysic functionalization reaction of alkenes. Tetrahedron Lett. 2015. Ahead of Print. [Google Scholar] [CrossRef]
- Lattanzi, A. Oxidation of sulfur, selenium and tellurium. In Comprehensive Organic Synthesis; Knohel, P., Molander, G.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 7, Chapter 28; pp. 837–879. [Google Scholar]
- Nogueira, C.W.; Rocha, J.B.T. Organoselenium and organotellurium compounds: Toxicology and pharmacology. In The Chemistry of Organic Selenium and Tellurium Compounds; Patai, S., Rappoport, Z., Liebman, J.F., Marek, I., Eds.; Wiley: Chichester, UK, 2012; Volume 3, Part 2; pp. 1277–1357. [Google Scholar]
- Nogueira, C.W.; Zeni, G.; Rocha, J.T.B. Organoselenium and Organotellurium Compounds: Toxicology and Pharmacology. Chem. Rev. 2004, 104, 6255–6285. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, C.W.; Rocha, J.T.B. Diphenyl diselenide a janus-faced molecule. J. Braz. Chem. Soc. 2010, 21, 2055–2071. [Google Scholar] [CrossRef]
- Meotti, F.C.; Borges, V.C.; Zeni, G.; Rocha, J.B.T.; Nogueira, C.W. Potential renal and hepatic toxicity of diphenyl diselenide, diphenyl ditelluride and Ebselen for rats and mice. Toxicol. Lett. 2003, 143, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Santoro, S.; Azaredo, J.B.; Nascimento, V.; Braga, A.L.; Sancineto, L.; Santi, C. The green side of the moon: Eco-friendly aspects of organoselenium chemistry. RCS Adv. 2014, 4, 31521–31535. [Google Scholar]
- Braga, A.L.; Schwab, R.S.; Rodrigues, O.E.D. Organoselenium Chemistry: Between Synthesis and Biochemistry; Santi, C., Ed.; Bentham Science Publishers: Oak Park, IL, USA, 2014; pp. 197–267. [Google Scholar]
- Santi, C.; Santoro, S.; Battiselli, B. Organoselenium Compounds as Catalysts in Nature and Laboratory. Curr. Org. Chem. 2010, 14, 2442–2462. [Google Scholar] [CrossRef]
- Riley, H.L.; Morley, J.F.; Friend, N.A.C. Selenium dioxide, a new oxidizing agent. Part I. Its reaction with aldehydes and ketones. J. Chem. Soc. 1932, 1875–1883. [Google Scholar] [CrossRef]
- Maity, A.C. Selenium Dioxide (SeO2)—A versatile reagent. Synlett 2008, 465–466. [Google Scholar] [CrossRef]
- McNally, J. Selenium(IV) oxide—tert-Butylhydroperoxide. In e-EROS Encyclopedia of Reagents for Organic Synthesis; Fuchs, P.L., Ed.; Wiley&Sons: Chichester, UK, 2010. [Google Scholar]
- Hoekstra, W.J. Selenium(IV) oxide. In e-EROS Encyclopedia of Reagents for Organic Synthesis; Fuchs, P.L., Ed.; Wiley&Sons: Chichester, UK, 2010. [Google Scholar]
- Gebhardt, C.; Priewisch, B.; Ivran, E.; Rueck-Braun, K. Oxidation of anilines with hydrogen peroxide and selenium dioxide as catalyst. Synthesis 2008, 1889–1894. [Google Scholar]
- Ogawa, A. Selenium and tellurium in organic synthesis. In Main Group Metals in Organic Synthesis; Yamamoto, Y., Oshima, K., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2004; Volume 2, pp. 813–866. [Google Scholar]
- Sharpless, K.B.; Lauer, R.F. Selenium dioxide oxidation of olefins. Evidence for the intermediacy of allylseleninic acids. J. Am. Chem. Soc. 1972, 94, 7154–7155. [Google Scholar] [CrossRef]
- Singelton, D.A.; Hang, C. Isotope Effects and the Mechanism of Allylic Hydroxylation of Alkenes with Selenium Dioxide. J. Org. Chem. 2000, 65, 7554–7560. [Google Scholar] [CrossRef]
- Ra, C.S.; Park, G. Ab initio studies of the allylic hydroxylation: DFT calculation on the reaction of 2-methyl-2-butene with selenium dioxide Tetrahedron. Lett. 2003, 44, 1099–1102. [Google Scholar] [CrossRef]
- Park, G.; Hwang, J.C.; Jung, W.S.; Ra, C.S. Stereochemical Course of the Allylic Hydroxylation: Reaction of 1-tert-Butyl-4-alkylidenecyclohexanes with Selenium Dioxide. Bull. Korean. Chem. Soc. 2005, 26, 1856–1860. [Google Scholar] [CrossRef]
- Patel, R.M.; Puranik, V.G.; Argade, N.P. Regio- and stereoselective selenium dioxide allylic oxidation of (E)-dialkyl alkylidenesuccinates to (Z)-allylic alcohols: Synthesis of natural and unnatural butenolides. Org. Biomol. Chem. 2011, 9, 6312–6322. [Google Scholar] [CrossRef] [PubMed]
- Khurana, J.M.; Dawra, K.; Majumdar, S. An efficient 1,3-allylic carbonyl transposition of chalcones. Monatsh. Chem. 2009, 140, 69–72. [Google Scholar] [CrossRef]
- Strommer, R.; Straus, W.; Emmert, H.; Sailer, R.; Steiner, E.; Resinger, E.W.; Haslinger, E.; Schramm, H.W. Synthesis and biological activity of oxidation products of the antiprogestine mifepristone. Monatsh. Chem. 2001, 132, 387–392. [Google Scholar] [CrossRef]
- Strommer, R.; Hoedl, C.; Strauss, W.; Sailer, R.; Haslinger, E.; Schramm, H.W.; Seger, C. Synthesis of 6-hydroxy derivatives of steroidal hormones by SeO2 mediated oxidation. Monatsh. Chem. 2004, 135, 1137–1141. [Google Scholar]
- Kim, H.S.; Kang, J.H. Selenium Dioxide Oxidation of 3β-Benzoyloxy-5α-cholest-8(14)-en-15-one: Chemical Synthesis of 3β-Hydroxy-5α-cholest-8(14),16-dien-15-one. Bull. Korean. Chem. Soc. 2001, 22, 1390–1392. [Google Scholar] [CrossRef]
- Ma, E.; Choi, T. An Efficient 4β-Hydroxylation of Steroidal 5-en-3β-ols and 1,4-Conjugation of Steroidal 4-en-3-ones Using SeO2 Oxidation. Bull. Korean. Chem. Soc. 2009, 30, 245–248. [Google Scholar]
- Czajkowska, D.; Morzycki, J.W.; Santillan, R.; Siergiejczyk, L. Synthesis of ‘glycospirostanes’ via ring-closing metathesis. Steroids 2009, 74, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, H.; Kotoku, N.; Sawama, Y.; Nagatomi, Y.; Yasuyuki Kita, Y. Concise asymmetric synthesis of a model compound, (4S,5S,6S)-6-(2,2-dimethoxy)ethyl-4,5-epoxy-6-hydroxy-2-cyclohexenone, for the cyclohexenone core of scyphostatin. Tetrahedron Lett. 2002, 43, 4825–4828. [Google Scholar] [CrossRef]
- Mushfiq, M.; Rehman, S.R. One-pot SeO2 oxidation of steroidal alkenes. Oxidation Commun. 2010, 33, 898–904. [Google Scholar]
- Kharitonov, Y.V.; Shults, E.E.; Gatilov, Y.V.; Bagryanskaya, I.Y.; Shakirov, M.M.; Tolstikov, G.A. Synthetic transformations of higher terpenoids. XXVII. Synthesis of 7-hydroxylabdanoids and their transformations. Chem. Nat. Compd. 2012, 48, 250–257. [Google Scholar] [CrossRef]
- Ernet, T.; Haufe, G. Allylic hydroxylation of vinyl fluorides. Synthesis 1997, 953–956. [Google Scholar] [CrossRef]
- Fairlamb, I.J.S.; Dickinson, J.M.; Pegg, M. Selenium dioxide E-methyl oxidation of suitably protected geranyl derivatives—Synthesis of farnesyl mimics. Tetrahedron Lett. 2001, 42, 2205–2208. [Google Scholar] [CrossRef]
- Smith, A.B., III; Bosanac, T.; Basu, K. Evolution of the Total Synthesis of (−)-Okilactomycin Exploiting a Tandem Oxy-Cope Rearrangement/Oxidation, a Petasis−Ferrier Union/Rearrangement, and Ring-Closing Metathesis. J. Am. Chem. Soc. 2009, 131, 2348–2558. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Pennington, J.D.; Williams, H.J.; Scott, A.I. Models for Taxol Biosynthesis: SeO2 Oxidation of Taxadiene. Synth. Commun. 2006, 36, 2577–2585. [Google Scholar] [CrossRef]
- Manktala, R.; Dhillon, R.S.; Chhabra, B.R. Urea-hydrogen peroxide and microwave. An eco-friendly blend for allylic oxidation of alkenes with catalytic selenium dioxide. Indian J. Chem. Sect. B 2006, 45B, 1591–1594. [Google Scholar] [CrossRef]
- Sabir, H.; Mukta, S. Selective oxygenation and plant-growth regulatory activity of sesquiterpene lactones. J. Phys. Sci. 2010, 21, 99–107. [Google Scholar]
- Sabir, H.; Mukta, S.; Meenakshi, H. Highly selective oxygenations of olefins over selenium dioxide using urea hydrogen peroxide as oxidising agent with sesquiterpene lactones. J. Glob. Pharma Technol. 2010, 2, 88–92. [Google Scholar]
- Barrero, A.F.; Quildez Del Moral, J.F.; Del Mar Herador, M.; Sanchez, E.M.; Arteaga, J.F. Regio- and Enantioselective Functionalization of Acyclic Polyprenoids. J. Mex. Chem. Soc. 2006, 50, 149–156. [Google Scholar]
- Warpehoski, M.A.; Chabaud, B.; Sharpless, K.B. Selenium dioxide oxidation of endocyclic olefins. Evidence for a dissociation-recombination pathway. J. Org. Chem. 1982, 47, 2897–2900. [Google Scholar] [CrossRef]
- Aranda, G.; Bertranne-Delahaye, M.; Azerad, R.; Maurs, M.; Cortés, M.; Ramirez, H.; Vernal, G.; Prangé, T. Practical and Efficient 1α-Hydroxylation of 4,4-Dimethyl-2-Ene Derivatives in Terpenic Series. Synth. Commun. 1997, 27, 45–60. [Google Scholar] [CrossRef]
- Chabaud, B.; Sharpless, K.B. Oxidation of acetylenes with tert-butyl hydroperoxide catalyzed by selenium dioxide. J. Org. Chem. 1979, 44, 4202–4204. [Google Scholar] [CrossRef]
- Gogoi, P.; Sharma, S.D.; Konwar, D. SeO2/H2O2/H2O-Dioxane: A new catalytic system for trans dihydroxylation of olefins. Lett. Org. Chem. 2007, 4, 249–252. [Google Scholar] [CrossRef]
- Chang, M.Y.; Lin, C.H.; Chen, Y.L. Selenium dioxide–mediated methoxyhydroxylation of cyclic arylolefin. Tetrahedron Lett. 2010, 51, 1430–1433. [Google Scholar] [CrossRef]
- Chang, M.Y.; Hsu, R.T.; Cheng, H.P.; Lin, P.J. Concise synthesis of 3-arylpiperidines. Heterocycles 2006, 68, 1173–1183. [Google Scholar] [CrossRef]
- Knothe, G.; Glass, R.S.; Schroeder, T.B.; Bagby, M.O.; Weisleder, D. Reaction of isolated double bonds with selenium dioxide/hydrogen peroxide: Formation of novel selenite esters. Synthesis 1997, 57–60. [Google Scholar] [CrossRef]
- Goswami, S.; Mukherjee, R.; Mukherjee, R.; Jana, S.; Maity, A.C.; Adak, A. Simple and EfficientSynthesis of 2,7-Difunctionalized-1,8-Naphthyridines. Molecules 2005, 10, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Monem, Y.H.A.; Elkanzi, N.A.; Mohamed, N.M.M. Synthesis of some new spirocyclic β-lactams and spirocyclic thiazolidin-4-one derivatives. Eur. J. Chem. 2013, 4, 195–202. [Google Scholar]
- Rakhimov, A.I.; Shul’man, R.B.; Fedunov, R.G. Oxidation of 6-methyl-2,4-dioxypyrimidine with selenious acid. Russ. J. Gen. Chem. 2011, 81, 2328–2331. [Google Scholar] [CrossRef]
- Goswami, S.; Adak, A.K. Microwave Assisted Improved Synthesis of 6-Formylpterin and Other Heterocyclic Mono- and Di-aldehydes. Synth. Commun. 2003, 33, 475–480. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, Y.-Y.; Yang, C.; Yu, X.; Zhi, X.Y.; Xu, H. Regioselective synthesis of fraxinellone-based hydrazone derivatives as insecticidal agents. Bioorg. Med. Chem. Lett. 2012, 22, 5384–5387. [Google Scholar] [CrossRef] [PubMed]
- Bobrov, D.N.; Tyvorskii, V.I. Facile synthesis of caerulomycin E by the formation of 2,2ʹ-bipyridine core via a 2-pyridyl substituted 4H-pyran-4-one. Formal synthesis of caerulomycin A. Tetrahedron 2010, 66, 5432–5434. [Google Scholar] [CrossRef]
- Hannesian, S.; Szychowski, J.; Maianti, J.P. Synthesis and Comparative Antibacterial Activity of Verdamicin C2 and C2a. A New Oxidation of Primary Allylic Azides in Dihydro[2H]pyrans. Org. Lett. 2009, 11, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekhar, R.; Gopalan, B.; Nanjan, M.J. Synthesis and Characterisation of 3-Hydroxy-4, 5-dihydro[1,2,3] Oxadiazolo [3,4-A]Quinolin-10-ium and its Fluoro derivative. Int. J. ChemTech Res. 2011, 3, 1125–1128. [Google Scholar]
- Tagawa, Y.; Yamashita, K.; Higuchi, Y.; Goto, Y. Improved Oxidation of Active Methyl Group of N-Heteroaromatic Compounds by Selenium Dioxide in the Presence of tert-Butyl Hydroperoxide. Heterocycles 2003, 60, 953–957. [Google Scholar] [CrossRef]
- Goswami, S.; Maity, A.C. Oxidative Removal of Heterocyclic Alkyl or Sugar Side Chain by Microwave: A Simple Step to Xanthopterin, 6-Formylpterin, and 3-Hydroxymethyl-2(1H)-Quinoxalinone. Chem. Lett. 2007, 36, 1118–1119. [Google Scholar] [CrossRef]
- Habibi, M.; Bayat, Y.; Marandi, R.; Mehrdadsharif, A.A.; Salahi, S. One-pot synthesis of 4H-pyran-4-one carboxaldehyde derivatives by using selenium dioxide as a reusable oxidant. Asian J. Chem. 2012, 24, 5239–5241. [Google Scholar]
- Remias, J.E.; Sen, A. Nitrogen oxides/selenium dioxide-mediated benzylic oxidations. J. Mol. Cat. A Chem. 2003, 201, 63–70. [Google Scholar] [CrossRef]
- Jordan, J.A.; Gribble, G.W.; Badenock, J.C. A concise total synthesis of bruceolline E. Tetrahedron Lett. 2011, 52, 6772–6774. [Google Scholar] [CrossRef]
- Sivaperuman, S.; Santhanagopalan, P.; Amali, I.B.; Shanmugam, M. Chemoselective Selenium Dioxide Oxidation of 1,4-Adducts Derived from Substituted Arylidene Acetophenones. Synth. Commun. 2009, 39, 2882–2888. [Google Scholar]
- Mehta, G.; Shinde, H.M. Enantiospecific total synthesis of 6-epi-(−)-hamigeran B. IntramolecularHeck reaction in a sterically constrained environment. Tetrahedron Lett. 2003, 44, 7049–7053. [Google Scholar] [CrossRef]
- Young, R.M.; Davies-Coleman, M.T. Microwave-assisted selenium dioxide oxidation of aryl methyl ketones to aryl glyoxals. Tetrahedron Lett. 2011, 52, 4036–4038. [Google Scholar] [CrossRef]
- Shirude, S.T.; Patel, P.; Giridhar, R.; Yadav, M.R. An efficient and time saving microwave- assisted selenium dioxide oxidation of 1,2-diarylethanones. Indian J. Chem. Sect. B 2006, 45B, 1080–1085. [Google Scholar] [CrossRef]
- Belsey, S.; Danks, T.N.; Wagner, G. Microwave-Assisted Selenium Dioxide Oxidation of Camphor Derivatives to α-Dicarbonyl Compounds and Oxoimines. Synth. Commun. 2006, 36, 1019–1024. [Google Scholar] [CrossRef]
- Taherpour, A.; Kamal, S.B. 1,2,3-trione compounds synthesis by oxidation 1,3- diketones. Asian J. Chem. 2007, 19, 4107–4109. [Google Scholar]
- Xiao-Hua, C.; Hai-Jun, Y.; Guo-Lin, Z. Aromatization of 1,4-dihydropyridines with selenium dioxide. Can. J. Chem. 2005, 83, 273–275. [Google Scholar]
- Paul, S.; Shivani, S.; Gupta, M.; Choudhary, D.; Gupta, R. Oxidative Aromatization of Hantzsch 1,4-Dihydropyridines by SiO2/P2O5-SeO2 under Mild and Heterogeneous Conditions. Bull. Korean Chem. Soc. 2007, 28, 336–338. [Google Scholar] [CrossRef]
- Ghodasara, H.B.; Vaghasiya, R.G.; Patel, B.G.; Shah, V.H. Synthesis of Novel and Highly Functionalized Pyrimidine-5-carboxylate Derivatives and their Antimicrobial Evaluation. Lett. Drug Des. Discov. 2014, 11, 930–936. [Google Scholar] [CrossRef]
- Meenakshi, C.; Ramamoorthy, Y.; Muthusubramanian, S.; Sivasubramanian, S. Microvawe Assisted Synthesis of 4,6-Diarylpyridazin-3(2H)-ones in Solid State. Synth. Commun. 2001, 31, 645–651. [Google Scholar] [CrossRef]
- Abd Rabo Moustafa, M.M.; Pagenkopf, B.L. Synthesis of 5-Azaindoles via a Cycloaddition Reaction between Nitriles and Donor-Acceptor Cyclopropanes. Org. Lett. 2010, 12, 3168–3171. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Das, J.; Sarkar, A.; Ng, S.W.; Tiekink, E.R.T. Oxidation with selenium dioxide: The first report of solvent-selective steroidal aromatization, efficient access to 4b,7a-dihydroxy steroids. Tetrahedron 2012, 68, 6485–6491. [Google Scholar] [CrossRef]
- Ghosh, A.; Saha, B.; Pradhan, B.P.; Ghosh, P. Selenium dioxide oxidation of oxime derivative of lupanone and antimicrobial activity of the oxidized products. Res. J. Chem. Sci. 2013, 3, 64–68. [Google Scholar]
- Yalgudre, R.S.; Gokavi, G.S. Selenium dioxide catalysed oxidation of acetic acid hydrazide by bromate in aqueous hydrochloric acid medium. J. Chem. Sci. 2012, 124, 821–826. [Google Scholar] [CrossRef]
- Yalgudre, R.S.; Gokavi, G.S. Kinetics and mechanism of uncatalyzed and selenium dioxide catalyzed oxidation of nicotinic acid hydrazide by bromate. Indian J. Chem. Technol. 2013, 20, 70–76. [Google Scholar]
- Młochowski, J.; Giurg, M. New Trends in Chemistry and Application of Aromatic and Related Selenaheterocycles. Top. Heterocycl. Chem. 2009, 19, 287–340. [Google Scholar]
- Yamazaki, S. Three or four Heteroatoms Including at Least One Selenium or Tellurium. In Comprehensive Heterocyclic Chemistry III; Katritzky, A., Ramsden, C., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 6, pp. 512–580. [Google Scholar]
- Młochowski, J.; Kloc, K.; Lisiak, R.; Potaczek, P.; Wójtowicz, H. Developments in the chemistry of selenaheterocyclic compounds of practical importance in synthesis and medicinal biology. ARKIVOC 2007, 6, 14–46. [Google Scholar] [CrossRef]
- Al-Smadi, M.; Ratrout, S. New 1,2,3-Selenadiazole and 1,2,3-Thiadiazole Derivatives. Molecules 2004, 9, 957–967. [Google Scholar] [CrossRef] [PubMed]
- Al-Smadi, M.; Al-Momani, F. Synthesis, Characterization and Antimicrobial Activity of New 1,2,3-Selenadiazoles. Molecules 2008, 13, 2740–2749. [Google Scholar] [CrossRef] [PubMed]
- Selvam, C.; Nidhin, P.; Shanmugam, M.; Paramasivam, M.; Perumal, M.; Dharmarajan, S. A facile synthesis of carbocycle-fused mono and bis-1,2,3-selenadiazoles and their antimicrobial and antimycobacterial studies. Eur. J. Med. Chem. 2011, 46, 5465–5472. [Google Scholar]
- El-Desoky, E.I.; Badria, F.A.; Abozeid, M.A.; Kandeel, E.A.; Abdel-Rahman, A.H. Synthesis and antitumor studies of novel benzopyrano-1,2,3-selenadiazole and spiro[benzopyrano]-1,3,4- thiadiazoline derivatives. Med. Chem. Res. 2013, 22, 2105–2114. [Google Scholar] [CrossRef]
- Prabakaran, K.; Khan, F.R.N.; Jin, J.S.; Jeong, E.D.; Manivel, P. Facile synthesis of 3- aryl-1- ((4-aryl-1,2,3-selenadiazol-5-yl)sulfanyl)isoquinolines. Chem. Pap. 2011, 65, 883–889. [Google Scholar] [CrossRef]
- Saravanan, S.; Amuthavalli, A.; Muthusubramanian, S. Synthesis and characterization of 5-(2-nitro-1-arylpropyl)-4-aryl-1,2,3-selenadiazoles. Indian J. Chem. Sect. B 2009, 48B, 1144–1147. [Google Scholar]
- Zhan, P.; Liu, X.; Fang, Z.; Pannecouque, C.; de Clercq, E. 1,2,3-Selenadiazole tioacetanilides: Synthesis and anti-HIV activity evaluation. Bioorg. Med. Chem. 2009, 17, 6374–6379. [Google Scholar] [CrossRef] [PubMed]
- Padmavathi, V.; Mahesh, K.; Subbaiah, D.R.C.V.; Padmaja, A. A new class of s ulfur-linked bis-1,2,3-selenadiazoles, 1,2,3-thiadiazoles, and 2H-diazaphospholes. Heteroatom Chem. 2008, 19, 261–265. [Google Scholar] [CrossRef]
- Aitken, R.A. Product class 27: Selenazoles and tellurazoles containing one or more other heteroatoms. Sci. Synth. 2004, 13, 777–822. [Google Scholar]
- Grivas, R. 2,1,3-Benzoselenadiazoles as Valuable Synthetic Intermediates. Curr. Org. Chem. 2004, 4, 707–721. [Google Scholar] [CrossRef]
- Suzuki, T.; Tsui, F.; Okubo, T.; Okada, A.; Obana, Y.; Fukushima, T.; Miyashi, T. Preparation, Structure, and Amphoteric Redox Properties of p-Phenylenediamine-type Dyes Fused with a Chalcogenadiazole Unit. J. Org. Chem. 2001, 66, 8954–8960. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Anand, A.; Mahajan, M.P. SeO2-Mediated Oxidation of 1,3-Diazabuta-1,3-dienes: A Single-Pot Synthesis of Functionalized 4-Hydroxyimidazoles. Synlett 2006, 2199–2002. [Google Scholar]
- Shaw, A.Y.; Denning, C.R.; Hulme, C. One-pot two-step synthesis of quinoxalinones and diazepinones via a tandem oxidative amidation-deprotection-cyclization sequence. Synthesis 2013, 45, 459–462. [Google Scholar] [CrossRef]
- Zhang, Y.; Tanimoto, H.; Nishiyama, Y.; Morimoto, T.; Kakiuchi, K. Synthesis of hetarenoindanone based on selenium dioxide-promoted direct intramolecular cyclization. Heterocycles 2011, 83, 2337–2342. [Google Scholar]
- Ló, S.M.S.; Ducatti, D.R.B.; Duarte, M.E.R.; Barreira, S.M.W.; Noseda, M.D.; Gonçalves, A.G. Synthesis of meso-tetraarylporphyrins using SeO2 as oxidant. Tetrahedron Lett. 2011, 52, 1441–1443. [Google Scholar] [CrossRef]
- Kim, H.S.; Jeong, K. Synthesis of Novel 1,2-Diazepino[3,4-b]quinoxaline and Pyridazino[3,4-b]quinoxaline Derivatives. J. Korean Chem. Soc. 1999, 43, 302–306. [Google Scholar]
- Takami, S.; Oshida, A.; Tawada, Y.; Kashino, S.; Satake, K.; Kimura, M. Selenium Dioxide Oxidations of Dialkyl-3H-Azepines: The First Synthesis of 2-Azatropone from Oxidation of 2,5-Di-tert-butyl-3H-azepine. J. Org. Chem. 2000, 65, 6093–6096. [Google Scholar] [CrossRef] [PubMed]
- Brząszcz, M.; Maposah, M.; Kloc, K.; Młochowski, J. Selenium(IV) oxide catalyzed oxidation of aldehydes to carboxylic acids with hydrogen peroxide. Synth. Commun. 2000, 30, 4425–4434. [Google Scholar] [CrossRef]
- Shah, K.J.; Chandla, S.B. Liquid-phase oxidation accompanied by skeletal rearrangement of acetophenone and acetone by hydrogen peroxide in the presence of selenium dioxide. J. Chem. Technol. Biotechnol. 1993, 57, 343–347. [Google Scholar] [CrossRef]
- Rohman, M.R.; Kharkongor, I.; Rajbangshi, M.; Mecadon, H.; Laloo, B.M.; Sahu, P.R.; Kharbangar, I.; Myrboh, B. One-Pot Synthesis of Unsymmetrical Benzils by Oxidative Coupling Using Selenium Dioxide and p-Toluenesulfonic Acid Monohydrate. Eur. J. Org. Chem. 2012, 320–328. [Google Scholar] [CrossRef]
- Levchenko, K.S.; Semenova, I.S.; Yarovenko, V.N.; Shmelin, P.S.; Krayushkin, M.M. Facile syntheses of 2-substituted 3-cyanochromones. Tetrahedron Lett. 2012, 53, 3630–3632. [Google Scholar] [CrossRef]
- Vollbrecht, S.; Dobreva, G.; Cartis, I.; du Mont, W.W.; Jeske, J.; Ruthe, F.; Jones, P.G.; Ernst, L.; Grahn, W.; Papke, U.; et al. Building the “phosphoindigo” backbone by oxidative coupling of phosphindolin-3-ones with selenium dioxide. Z. Anorg. Allg. Chem. 2008, 634, 1321–1325. [Google Scholar] [CrossRef]
- Shaw, A.Y.; Denning, C.R.; Hulme, C. Selenium dioxide-mediated synthesis of α-ketoamides from arylglyoxals and secondary amines. Tetrahedron Lett. 2012, 53, 4151–4153. [Google Scholar] [CrossRef] [PubMed]
- Priewisch, B.; Rück-Braun, K. Preparation of nitrosoarenes for the synthesis of azobenzenes. J. Org. Chem. 2005, 70, 2350–2352. [Google Scholar] [CrossRef] [PubMed]
- Pansare, S.V.; Malusare, M.G. Oxidation of 1,2,5-Thiadiazolidine 1,1-Dioxides: Synthesis of Diaryl 1,2-Diketones. Synlett 1997, 671, 671–672. [Google Scholar] [CrossRef]
- Potapov, A.S.; Chernova, N.P.; Ogorodnikov, V.D.; Petrenko, T.V.; Khlebnikov, A.I. Synthesisand oxidation of some azole-containing thioethers. Beilstein J. Org. Chem. 2011, 7, 1526–1532. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; He, Q.; Zhang, Z.; Ding, N.; Hu, B. Oxidative desulfurization of dibenzothiophene with hydrogen peroxide catalyzed by selenium(IV)-containing peroxotungstate. Chem. Commun. 2011, 47, 12194–12196. [Google Scholar] [CrossRef] [PubMed]
- Kamata, K.; Hirano, T.; Kuzuya, S.; Mizuno, N. Hydrogen-Bond-Assisted Epoxidation of Homoallylic and Allylic Alcohols with Hydrogen Peroxide Catalyzed by Selenium-Containing Dinuclear Peroxotungstate. J. Am. Chem. Soc. 2009, 131, 6997–7004. [Google Scholar] [CrossRef] [PubMed]
- Ogura, F.; Otsubo, T.; Wirth, T.; Ali Khan, Z. Dimethyl Selenoxide. In e-EROS Encyclopedia of Reagents for Organic Synthesis; Fuchs, P.L., Ed.; Wiley & Sons: Chichester, UK, 2007. [Google Scholar]
- Drabowicz, J.; Łyżwa, P.; Łuczak, J.; Mikołajczyk, M.; Laur, P. New procedures for the oxidation of sulphides to sulfoxides and sulfones. Phosphorus Sulfur. Silicon Relat. Elem. 1997, 120–121, 425–426. [Google Scholar] [CrossRef]
- Goodman, M.A.; Detty, M.R. Selenoxides as catalysts for epoxidation and Baeyer-Villiger oxidation with hydrogen peroxide. Synlett 2006, 1100–1104. [Google Scholar] [CrossRef]
- Betzemeier, B.; Lhermitte, F.; Knochel, P. A selenium catalysed epoxidation in perfluorinated solvents with hydrogen peroxide. Synlett 1999, 489–491. [Google Scholar] [CrossRef]
- Ten Brink, G.-J.; Vis, J.M.; Arends, I.W.C.E.; Sheldon, R.A. Selenium catalyzed oxidations with aqueous hydrogen peroxide. Part 3. Oxidation of carbonyl compounds under mono/bi/triphasic conditions. Tetrahedron 2002, 58, 3977–3983. [Google Scholar] [CrossRef]
- Back, T.G.; Moussa, Z. Diselenides and allyl selenides as glutathione peroxidase mimetics. J. Am. Chem. Soc. 2003, 125, 13455–13460. [Google Scholar] [CrossRef] [PubMed]
- Back, T.G.; Kuzma, D.; Parvez, M. Aromatic derivatives and tellurium analogues of cyclic seleninate esters and spirodioxyselenuranes that act as glutathione peroxidase mimetics. J. Org. Chem. 2005, 70, 9230–9236. [Google Scholar] [CrossRef] [PubMed]
- Press, D.J.; Mercier, E.A.; Kuzma, D.; Back, T.G. Substituent effects upon the catalytic activity of aromatic cyclic seleninate esters and spirodioxyselenuranes that act as glutathione peroxidase mimetics. J. Org. Chem. 2008, 73, 4252–4255. [Google Scholar] [CrossRef] [PubMed]
- Press, D.J.; McNeil, N.M.R.; Hambrook, M.; Back, T.G. Effects of Methoxy Substituents on the Glutathione Peroxidase-like Activity of Cyclic Seleninate Esters. J. Org. Chem. 2014, 79, 9394–9401. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, V.; Alberto, E.E.; Tondo, D.W.; Dambrowski, D.; Detty, M.R.; Nome, F.; Braga, A.L. GPx-Like Activity of Selenides and Selenoxides: Experimental Evidence for the Involvement of Hydroxy Perhydroxy Selenane as the Active Species. J. Am. Chem. Soc. 2012, 134, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Tidei, C.; Piroddi, M.; Galli, F.; Santi, C. Oxidation of thiols promoted by PhSeZnCl. Tetrahedron Lett. 2012, 53, 232–234. [Google Scholar] [CrossRef]
- Zhang, J.; Koizumi, T. Reactivity of Chlorooxachalcogenuranes: Oxidation of Sulfides to Sulfoxides Using Chlorooxaselenuranes. Synth. Commun. 2000, 30, 979–987. [Google Scholar] [CrossRef]
- Francavilla, G.; Drake, M.D.; Bright, F.V.; Detty, M.R. Dendrimeric organochalcogen catalysts for the activation of hydrogen peroxide: Improved catalytic activity through statistical effects and and cooperativity in succesive generation. J. Am. Chem. Soc. 2001, 123, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Drake, M.D.; Bright, F.V.; Detty, M.R. Dendrimeric organochalcogen catalysts for the activation of hydrogen peroxide: Origins of the “dendrimeric effect” with catalysts terminating phenylseleno groups. J. Am. Chem. Soc. 2003, 125, 12558–12566. [Google Scholar] [CrossRef] [PubMed]
- Bennett, S.M.; Tang, Y.; McMaster, D.; Bright, F.V.; Detty, M.R. A xerogel-sequestered selenoxide catalyst for bromination with hydrogen peroxide and sodium bromide in an aqueous environment. J. Org. Chem. 2008, 73, 6849–6852. [Google Scholar] [CrossRef] [PubMed]
- Back, T.G. Benzeneseleninic acid. In e-EROS Encyclopedia of Reagents for Organic Synthesis; Fuchs, P.L., Ed.; John Wiley & Sons: Chichester, UK, 2001. [Google Scholar]
- Renga, J.M. o-Nitrobenzeneseleninic Acid. In e-EROS Encyclopedia of Reagents for Organic Synthesis; Fuchs, P.L., Ed.; John Wiley & Sons: Chichester, UK, 2001. [Google Scholar]
- Renga, J.M. 2,4-Dinitrobenzeneseleninic Acid. In e-EROS Encyclopedia of Reagents for Organic Synthesis; Fuchs, P.L., Ed.; John Wiley & Sons: Chichester, UK, 2001. [Google Scholar]
- Ninomiya, I.; Hashimoto, C.; Kiguchi, T.; Naito, T.; Barton, D.H.R.; Luchinchi, X.; Milliet, P. Dehydrogenation with Benzeneseleninic Anhydride in Total Synthesis of Ergot Alkaloids. J. Chem. Soc. Perkin. Trans. 1 1990, 707–713. [Google Scholar] [CrossRef]
- Syper, L.; Mlochowski, J. A Covenient Oxidation of Halomethylarenes and Alcohols to Aldehydes with Dimethyl Selenoxide and Potassium Seleninate. Synthesis 1984, 747–751. [Google Scholar] [CrossRef]
- Crich, D.; Zou, Y. Catalytic allylic oxidation with a recyclable, fluorous seleninic acid. Org. Lett. 2004, 6, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Crich, D.; Zou, Y. Catalytic oxidation adjacent to carbonyl groups and at benzylic positions with a fluorous seleninic acid in the presence of iodoxybenzene. J. Org. Chem. 2005, 70, 3309–3311. [Google Scholar] [CrossRef] [PubMed]
- Sheng, S.R.; Huang, X. Oxidative conversion of aldoximes into carboxylic acid esters catalysed by polystyrene-bound phenylseleninic acid. J. Chem. Res. 2003, 491–492. [Google Scholar] [CrossRef]
- Drake, M.D.; Bateman, M.A.; Detty, M.R. Substituent effects in arylseleninic acid-catalyzed bromination of organic substrates with sodium bromide and hydrogen peroxide. Organometallics 2003, 22, 4158–4162. [Google Scholar] [CrossRef]
- Goodmanm, M.A.; Detty, M.R. Selenoxides as catalysts for the activation of hydrogen peroxide. Bromination of organic substrates with sodium bromide and hydrogen peroxide. Organometallics 2004, 23, 3016–3020. [Google Scholar] [CrossRef]
- Hopf, H.; Hucker, J.; Ernst, L. On the functionalization of [2.2](1,4)phenanthrenoparacyclophane. Eur. J. Org. Chem. 2007, 1891–1904. [Google Scholar] [CrossRef]
- Dutta, D.; Datta, A.; vander Velde, D.G.; Georg, G.I. Oxidation of baccatin III at C14. A facile rearrangement of the baccatin III core. Lett. Org. Chem. 2007, 4, 151–154. [Google Scholar] [CrossRef]
- Boyer, J.; Bernardes-Génisson, V.; Nepveu, F. Access to unsymmetrical 1,2-diketone immediates via benzeneseleninic anhydride-promoted oxidation: Application to indolone-N-oxide synthesis. J. Chem. Res. Synop. 2003, 507–508. [Google Scholar] [CrossRef]
- Toki, N.; Satoh, T. Selective oxidation of alcohols at the benzylic position by benzeneseleninic anhydride. Chem. Pharm. Bull. 2004, 52, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Koepke, T.; Pink, M.; Zaleski, J.M. Elucidation of the extraordinary 4-membered pyrrole ring-contracted azeteoporphyrinoid as an intermediate in chlorin oxidation. Chem. Commun. 2006, 47, 4940–4942. [Google Scholar] [CrossRef] [PubMed]
- Jastrzebska, I.; Dobrogowska, A.; Lutostanska, E.; Morzycki, J.W. On reactions of spirostane sapogenins with benzeneseleninic anhydride. Tetrahedron 2010, 66, 5024–5029. [Google Scholar] [CrossRef]
- Mercier, E.A.; Smith, C.D.; Parvez, M.; Back, T.G. Cyclic Seleninate Esters as Catalysts for the Oxidation of Sulfides to Sulfoxides, Epoxidation of Alkenes, and Conversion of Enamines to α-Hydroxyketones. J. Org. Chem. 2012, 77, 3508–3517. [Google Scholar] [CrossRef] [PubMed]
- Byers, J.H.; Nguyen, T.; Kobayashi, Y. Diphenyl Diselenide. In e-EROS Encyclopedia of Reagents for Organic Synthesis; Fuchs, P.L., Ed.; John Wiley & Sons: Chichester, UK, 2007. [Google Scholar]
- Ścianowski, J.; Rafinski, Z.; Wojtczak, A. Syntheses and reactions of new optically active terpene dialkyl diselenides. Eur. J. Org. Chem. 2006, 3216–3225. [Google Scholar] [CrossRef]
- Syper, L.; Młochowski, J. Benzeneperoxyseleninic acids—Synthesis and properties. Tetrahedron 1987, 43, 207–213. [Google Scholar] [CrossRef]
- Van der Toorn, J.C.; Kemperman, G.; Sheldon, R.A.; Arends, I.W.C.E. Diphenyldiselenide- Catalyzed Selective Oxidation of Activated Alcohols with tert-Butyl Hydroperoxide: New Mechanistic Insights. J. Org. Chem. 2009, 74, 3085–3089. [Google Scholar] [CrossRef] [PubMed]
- Giurg, M.; Said, S.B.; Syper, L.; Młochowski, J. One-pot oxidation of azomethine compounds into arene carboxylic acids. Synth. Commun. 2001, 31, 3151–3159. [Google Scholar] [CrossRef]
- Giurg, M.; Młochowski, J.; Ambrożak, A. Hydrogen peroxide oxidation of N,N- dimethylhydrazones promoted by selenium compounds, titanosilicalites or acetonitrile. Polish. J. Chem. 2002, 76, 1713–1720. [Google Scholar] [CrossRef]
- Giurg, M.; Młochowski, J. Oxidative ring contraction of cycloalkanones: A facile method for synthesis of medium ring cycloalkane carboxylic acids. Synth. Commun. 1 1999, 29, 2281–2291. [Google Scholar] [CrossRef]
- Giurg, M.; Kowal, E.; Muchalski, H.; Syper, L.; Młochowski, J. Catalytic oxidative domino degradation of alkyl phenols towards 2-and 3-substituted muconolactones. Synth. Commun. 2009, 39, 251–256. [Google Scholar] [CrossRef]
- Giurg, M.; Muchalski, H.; Kowal, E. Oxofunctionalized Trans-2-carboxycinnamic Acids by Catalytic Domino Oxidation of Naphthols and Hydronaphthoquinones. Synth. Commun. 2012, 42, 2526–2539. [Google Scholar] [CrossRef]
- Ten Brink, G.J.; Martijn, J.M.; Vis, J.H.; Arends, I.W.C.E.; Sheldon, R.A. Selenium-catalyzed oxidations with aqueous hydrogen peroxide. 2. Baeyer-Villiger reactions in homogenous solution. J. Org. Chem. 2001, 66, 2429–2433. [Google Scholar] [CrossRef] [PubMed]
- Ten Brink, G.-J.; Fernandes, B.C.M.; van Vliet, M.C.A.; Arends, I.W.C.E.; Sheldon, R.A. Selenium catalyzed oxidations with aqueous hydrogen peroxide. Part I. Epoxidation reactions in homogenous solution. J. Chem. Soc. Perkin Trans. 1 2001, 224–228. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, J.; Yu, L.; Shi, X.; Zhang, M.; Xu, Q.; Lauters, M. Organoselenium-Catalyzed Baeyer-Villiger Oxidation of α,β-Unsaturated Ketones by Hydrogen Peroxide to Access Vinyl Esters. Adv. Synth. Catal. 2015, 357, 955–960. [Google Scholar] [CrossRef]
- De Torres, M.; Arends, I.W.C.E.; Mayoral, J.A.; Pires, E.; Jimenez-Oses, G. A highly efficient green and recoverable catalytic system for the epoxidation of fatty esters and biodiesel with H2O2 Appl. Catal. A: General 2012, 425–426, 91–96. [Google Scholar] [CrossRef]
- Ichikawa, H.; Usami, Y.; Arimoto, M. Synthesis of novel organoselenium as catalyst for Bayer-Villiger oxidation with 30% H2O2. Tetrahedron Lett. 2005, 46, 8665–8668. [Google Scholar] [CrossRef]
- Yu, L.; Wang, J.; Chen, T.; Wang, Y.; Xu, Q. Recyclable 1,2-bis[3,5-bis(trifluoromethyl)phenyl]diselane-catalyzed oxidation of cyclohexene with H2O2: A practical access to trans-1,2-cyclohexanediol. Appl. Organomet. Chem. 2014, 28, 652–656. [Google Scholar] [CrossRef]
- Miyake, Y.; Nishibayashi, Y.; Uemura, S. Asymmetric Baeyer-Villiger Oxidation of Cyclic Ketones Using Chiral Organoselenium Catalysts. Bull. Chem. Soc. Jpn. 2002, 75, 2233–2237. [Google Scholar] [CrossRef]
- Orentas, E.; Bagdziunas, G.; Berg, U.; Zilinskas, A.; Butkus, E. Enantiospecific synthesis and chiroptical properties of bicyclic enones. Eur. J. Org. Chem. 2007, 4251–4256. [Google Scholar] [CrossRef]
- Yu, L.; Li, H.; Zhang, X.; Ye, J.; Liu, J.; Xu, Q.; Lautens, M. Organoselenium-Catalyzed Mild Dehydration of Aldoximes an Unexpected Practical Method for Organonitrile Synthesis. Org. Lett. 2014, 16, 1346–1349. [Google Scholar] [CrossRef] [PubMed]
- Brodsky, B.H.; Du Bois, J. Oxaziridine-Mediated Catalytic Hydroxylation of Unactivated 3 °C-H Bonds Using Hydrogen Peroxide. J. Am. Chem. Soc. 2005, 127, 15391–15393. [Google Scholar] [CrossRef] [PubMed]
- Santoro, S.; Santi, C.; Sabatini, M.; Testaferi, L.; Tiecco, M. Eco-Friendly olefin dihydroxylation catalyzed by diphenyl diselenide. Adv. Synth. Catal. 2008, 350, 2881–2884. [Google Scholar] [CrossRef]
- Zhao, D.; Johansson, M.; Bäeckvall, J.E. In situ generation of nitroso compounds from catalytic hydrogen peroxide oxidation of primary aromatic amines and their one-pot use in hetero-Diels-Alder reactions. Eur. J. Org. Chem. 2007, 26, 4431–4436. [Google Scholar] [CrossRef]
- Alberto, E.E.; Braga, A.L.; Detty, M.R. Imidazolium-containing diselenides for catalytic oxidations with hydrogen peroxide and sodium bromide in aqueous solutions. Tetrahedron 2012, 68, 10476–10481. [Google Scholar] [CrossRef]
- Mugesh, G.; Singh, H.B. Synthetic organoselenium compounds as antioxidants: Glutathione peroxidase activity. Chem. Soc. Rev. 2000, 29, 347–357. [Google Scholar] [CrossRef]
- Mugesh, G.; Du Mont, W.W.; Sies, H. Chemistry of Biologically Important Synthetic Organoselenium Compounds. Chem. Rev. 2001, 101, 2125–2180. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Garcia, M. Organoselenium Compounds as Potential Therapeutic and Chemopreventive Agents: A Review. Curr. Med. Chem. 2004, 11, 1657–1659. [Google Scholar] [CrossRef] [PubMed]
- Antony, S.; Bayse, C.A. Modeling the Mechanism of the Glutathione Peroxidase Mimic Ebselen. Inorg. Chem. 2011, 50, 12075–12084. [Google Scholar] [CrossRef] [PubMed]
- Bhabak, K.P.; Mugesh, G. Functional Mimics of Glutathione Peroxidase: Bioinspired Synthetic Antioxidants. Acc. Chem. Res. 2010, 43, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Giurg, M.; Wiech, E.; Piekielska, K.; Gębala, M.; Młochowski, J.; Wolañski, M.; Ditkowski, P.; Peczyńska-Czoch, W. A New Approach to Synthesis of Questiomycin A: Oxidative Cyclocondensation of o-Aminophenol. Pol. J. Chem. 2006, 80, 297–306. [Google Scholar] [CrossRef]
- Giurg, M.; Piekielska, K.; Gębala, M.; Ditkowski, P.; Wolański, M.; Peczyńska-Czoch; Młochowski, J. Catalytic Oxidative Cyclocondensation of o-Aminophenols to 2-Amino-3H- phenoxazin-3-ones. Synth. Commun. 2007, 37, 1779–1789. [Google Scholar] [CrossRef]
- Wójtowicz, H.; Brząszcz, M.; Kloc, K.; Młochowski, J. Selective oxidation of aromatic aldehydes to arenecarboxylic acids using ebselen-tert-butyl hydroperoxide catalytic system. Tetrahedron 2001, 57, 9743–9748. [Google Scholar] [CrossRef]
- Sarma, B.; Mugesh, G. Antioxidant Activity of the Anti-Inflammatory Compound Ebselen: A Reversible Cyclization Pathway via Selenenic and Seleninic Acid Intermediates. Chem. -Eur. J. 2008, 14, 10603–10614. [Google Scholar] [CrossRef] [PubMed]
- Giurg, M.; Wójtowicz, H.; Młochowski, J. Hydroperoxide oxidation of azomethines and alkylarenes catalyzed by ebselen. Pol. J. Chem. 2002, 76, 537–542. [Google Scholar]
- Wójtowicz, H.; Młochowski, J.; Syper, L.; Yadav, H.S. t-Butyl hydroperoxide oxidative dealkylation of hydroquinone ethers to 1,4-quinones. Synth. Commun. 2006, 36, 1991–2000. [Google Scholar] [CrossRef]
- Balkrishna, S.J.; Prasad, C.D.; Panini, P.; Detty, M.; Chopra, D.; Kumar, S. Isoselenazolones As Catalysts for the Activation of Bromine: Bromolactonization of Alkenoic Acids and Oxidation of Alcohols. J. Org. Chem. 2012, 77, 9541–9552. [Google Scholar] [CrossRef] [PubMed]
- Wójtowicz-Młochowska, H.; Soroko, G.; Młochowski, J. New recoverable organoselenium catalyst for hydroperoxide oxidation.of organic substrates. Synth. Commun. 2008, 38, 2000–2010. [Google Scholar] [CrossRef]
- Giurg, M.; Brząszcz, M.; Młochowski, J. Hydroperoxide oxidation of different organic compounds catalyzed by silica-supported selenenamide. Pol. J. Chem. 2006, 80, 417–428. [Google Scholar] [CrossRef]
- Młochowski, J.; Kloc, K.; Syper, L.; Inglot, A.D.; Piasecki, E. Aromatic and azaaromatic diselenides, benzisoselenazolones and related compounds active in humans: Synthesis and properties. Liebigs Ann. Chem. 1993, 1239–1244. [Google Scholar] [CrossRef]
- Pfeiffer, W.D. Annulated selenazole compounds. Sci. Synth. 2001, 11, 931–940. [Google Scholar]
- Młochowski, J. 1,2-Selenazoles. In Comprehensive Heterocyclic Chemistry III; Katritzky, A., Ramsden, C., Scriven, E.F.V., Taylor, R.J.K., Eds.; Elsevier: Oxford, UK, 2008; Volume 4, pp. 755–789. [Google Scholar]
- Zade, S.S.; Panda, S.; Singh, H.B.; Wolmershaüser, G. Synthesis of diaryl selenides using the in situ reagent SeCl2. Tetrahedron Lett. 2005, 46, 665–669. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Młochowski, J.; Wójtowicz-Młochowska, H. Developments in Synthetic Application of Selenium(IV) Oxide and Organoselenium Compounds as Oxygen Donors and Oxygen-Transfer Agents. Molecules 2015, 20, 10205-10243. https://doi.org/10.3390/molecules200610205
Młochowski J, Wójtowicz-Młochowska H. Developments in Synthetic Application of Selenium(IV) Oxide and Organoselenium Compounds as Oxygen Donors and Oxygen-Transfer Agents. Molecules. 2015; 20(6):10205-10243. https://doi.org/10.3390/molecules200610205
Chicago/Turabian StyleMłochowski, Jacek, and Halina Wójtowicz-Młochowska. 2015. "Developments in Synthetic Application of Selenium(IV) Oxide and Organoselenium Compounds as Oxygen Donors and Oxygen-Transfer Agents" Molecules 20, no. 6: 10205-10243. https://doi.org/10.3390/molecules200610205
APA StyleMłochowski, J., & Wójtowicz-Młochowska, H. (2015). Developments in Synthetic Application of Selenium(IV) Oxide and Organoselenium Compounds as Oxygen Donors and Oxygen-Transfer Agents. Molecules, 20(6), 10205-10243. https://doi.org/10.3390/molecules200610205




